Morphological and Ultrastructural Insights into the Goldfish (Carassius auratus) Spleen: Immune Organization and Cellular Composition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Semithin Sections and (Transmission Electron Microscopy) TEM Preparations
2.3. Tissue Sampling Considerations
3. Results
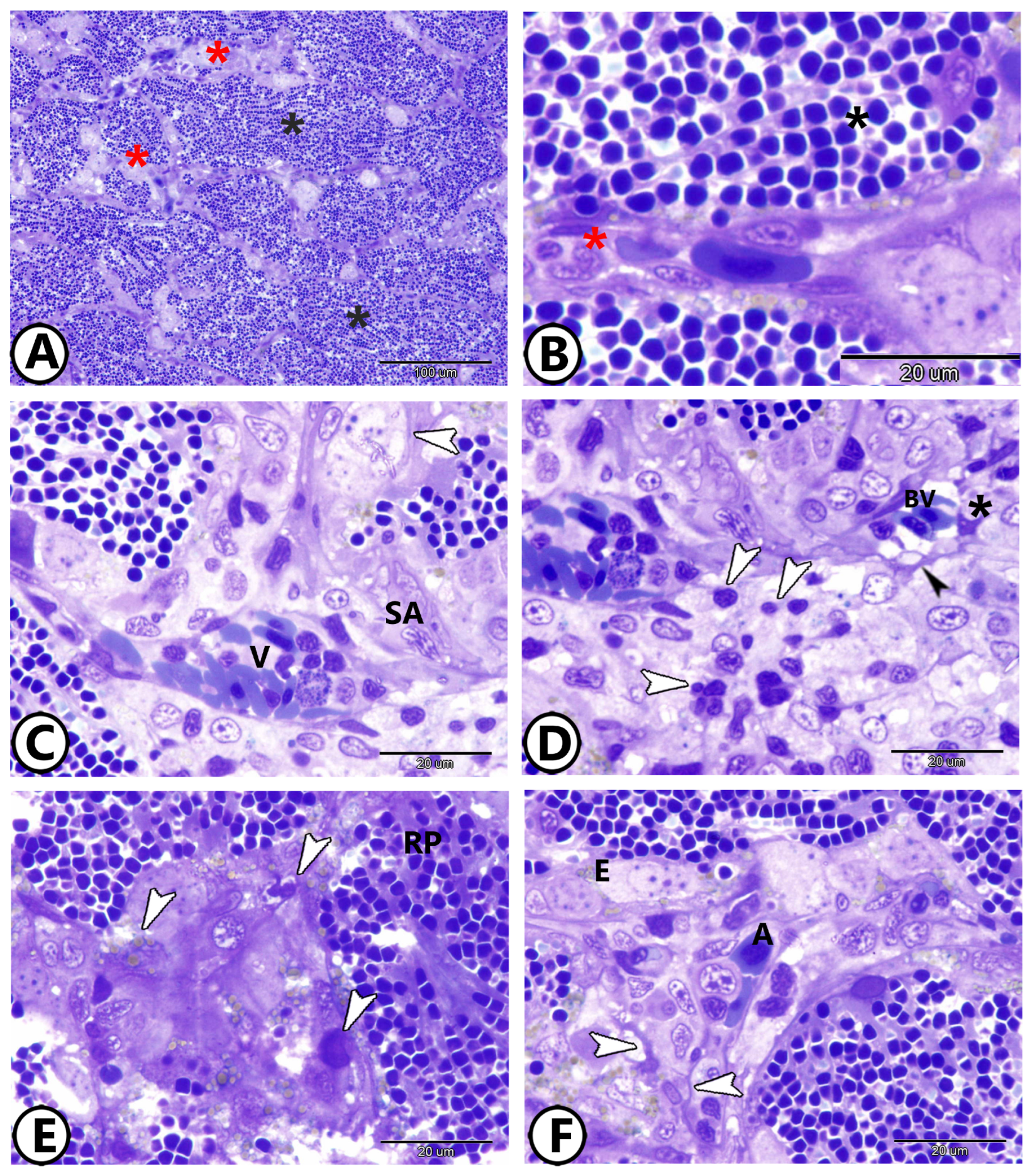
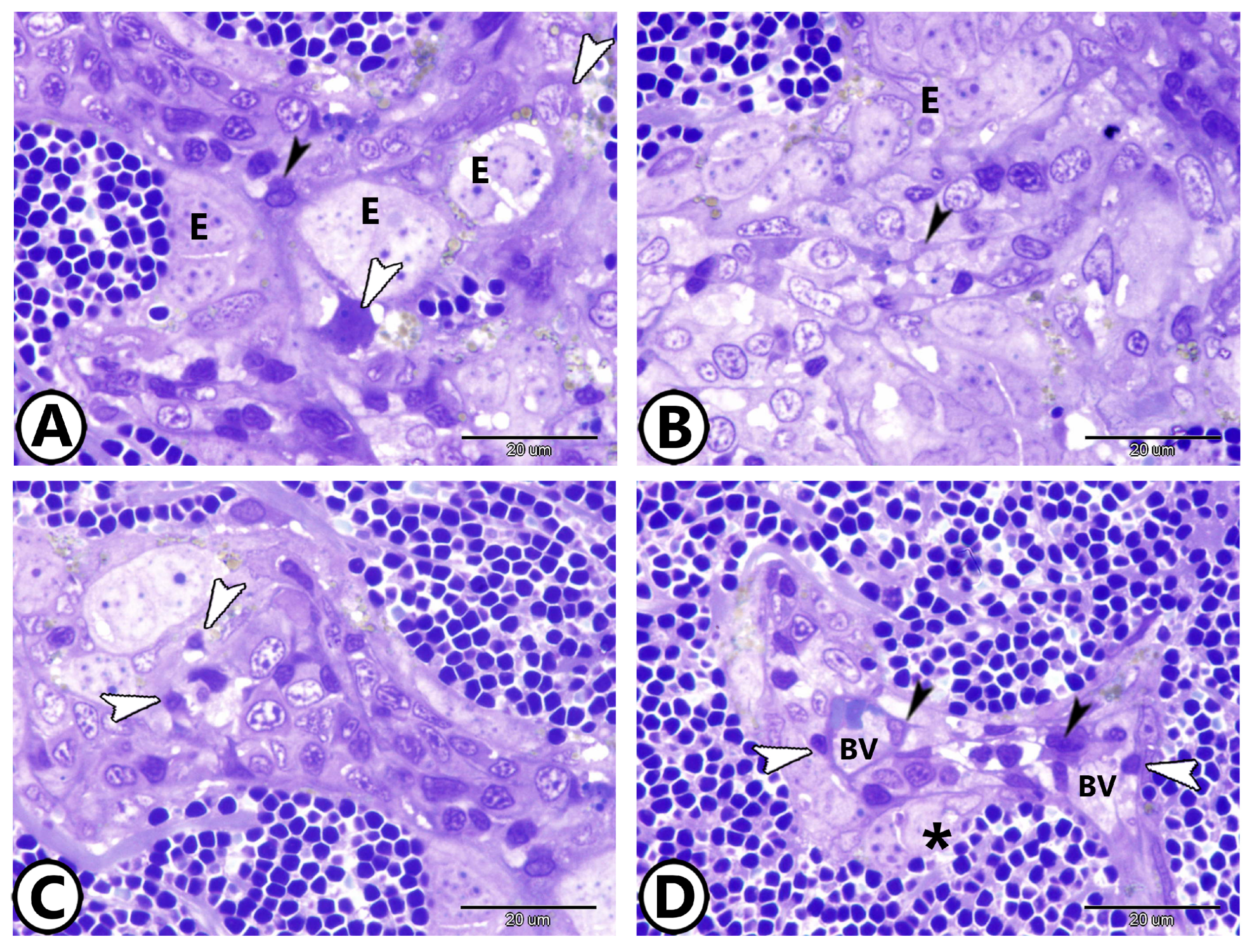
3.1. Light Microscopy
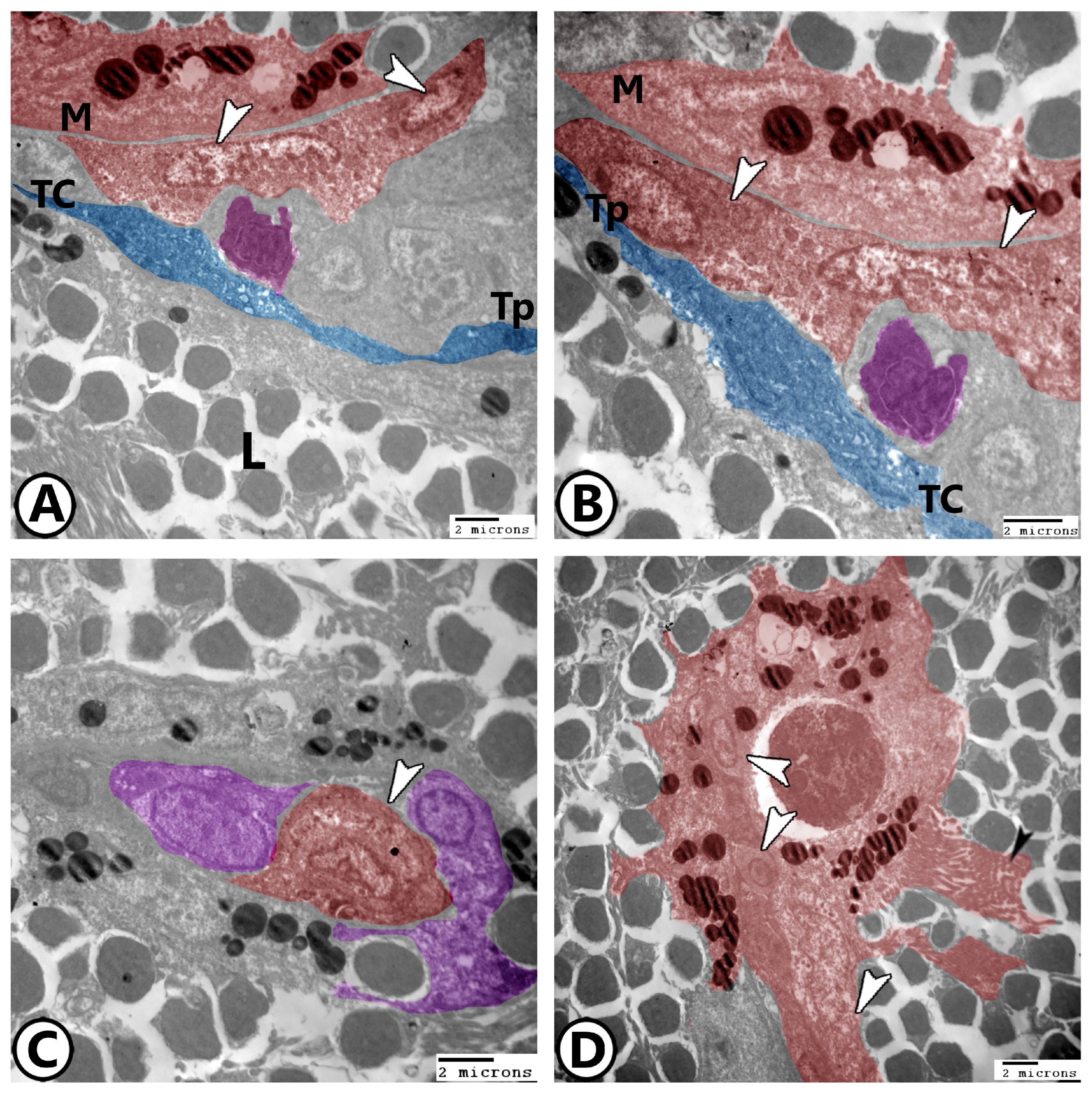
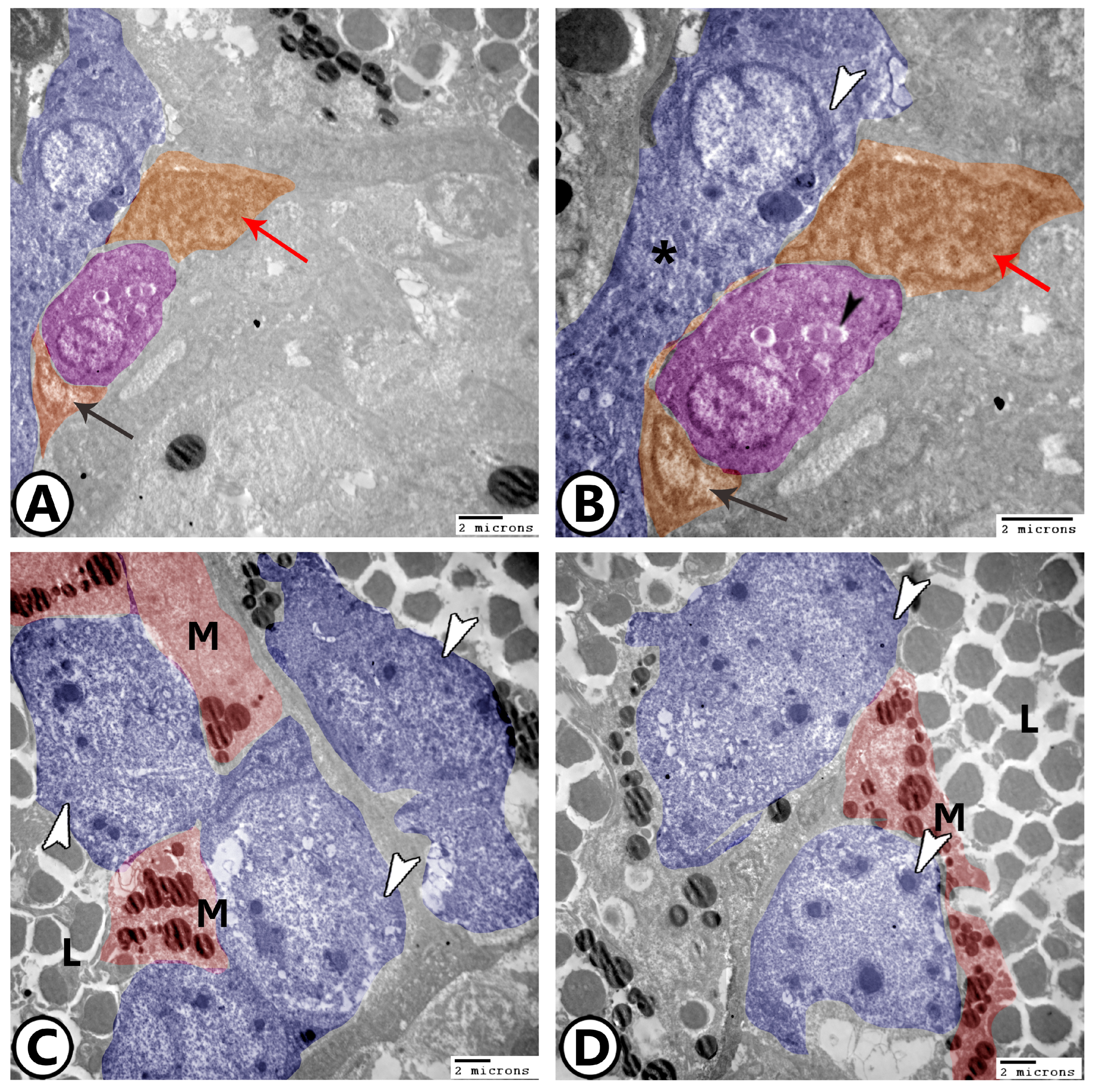
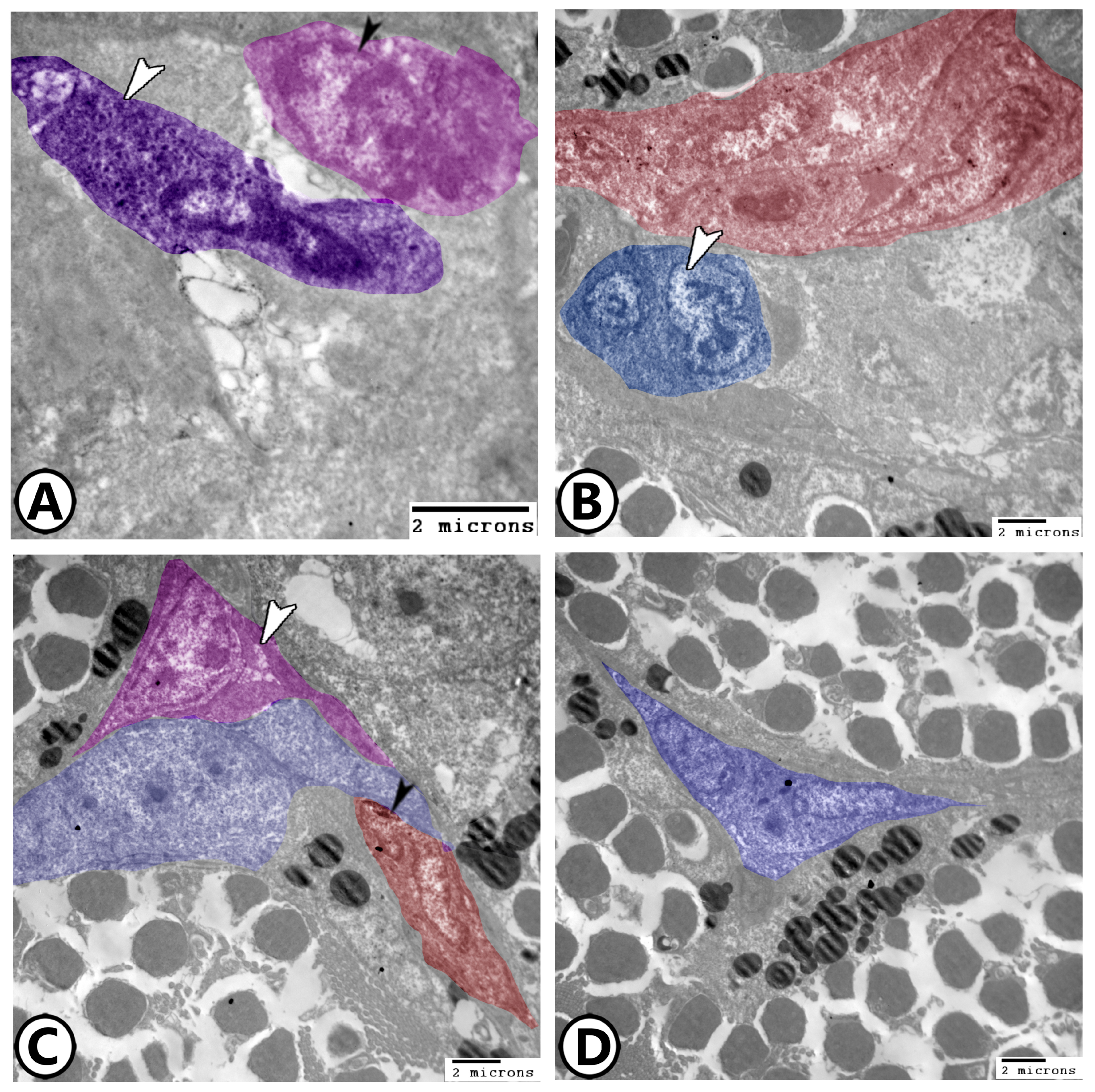
3.2. Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fänge, R.; Nilsson, S. The fish spleen: Structure and function. Experientia 1985, 41, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.G. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopathol. 2021, 36, 399–414. [Google Scholar]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Manrique, W.G.; Pereira Figueiredo, M.A.; Charlie-Silva, I.; Antonio de Andrade Belo, M.; Dib, C.C. Spleen Melanomacrophage centers response of Nile tilapia during Aeromanas hydrophila and Mycobacterium marinum infections. Fish Shellfish Immunol. 2019, 95, 514–518. [Google Scholar] [CrossRef]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.A.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef]
- Bassity, E.; Clark, T.G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Espenes, A.; Press, C.M.L.; Dannevig, B.H.; Landsverk, T. Investigation of the structural and functional features of splenic ellipsoids in rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 1995, 279, 469–474. [Google Scholar] [CrossRef]
- Sørby, R.; Wien, T.N.; Husby, G.; Espenes, A.; Landsverk, T. Filter function and immune complex trapping in splenic ellipsoids. J. Comp. Pathol. 2005, 132, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hanington, P.C.; Barreda, D.R.; Belosevic, M. A novel hematopoietic granulin induces proliferation of goldfish (Carassius auratus L.) macrophages. J. Biol. Chem. 2006, 281, 9963–9970. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Wiley-Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 6th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Posner, L.P.; Scott, G.N.; Law, J.M. Repeated exposure of goldfish (Carassius auratus) to tricaine methanesulfonate (MS-222). J. Zoo Wildl. Med. 2013, 44, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. Cell Biol. 1965, 27, 137A. [Google Scholar]
- Reynolds, E.S. The use of lead citrates at high pH as an electron-opaque stain in electron microscopy. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutiérrez-De Frías, C.; Cortés, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 12, 525–539. [Google Scholar] [CrossRef]
- Press, C.M.; Dannevig, B.H.; Landsverk, T. Comparative Immune and enzyme histochemical phenotypes of lymphoid and nonlymphoid cells within the spleen of Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 1994, 4, 79–93. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Press, C.M.; Evensen, O. The Morphology of the Immune System in Teleost Fishes. Fish Shellfish Immunol. 1999, 9, 309–318. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yang, P.; Zhang, L.; Liu, Y.; Ullah, S.; Wu, L.; Waqas, Y.; Le, Y.; Chen, W. Identification and structural composition of the blood–spleen barrier in chickens. Vet. J. 2015, 204, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.J.; Li, M.Y.; Wang, J.; Qin, J.H.; Xu, C.S.; Hei, N.N.; Yang, P.; Gandahi, J.; Chen, Q.S. Architecture of the blood-spleen barrier in the soft-shelled turtle, Pelodiseus sinensis. Anat. Rec. 2009, 292, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dai, X.; Fan, W.; Wang, J.; Qin, C.; Chen, J.; Cao, X. Location of blood-spleen barrier of Pelteobagrus vachelli by Trypan blue staining. South China Fish. Sci. 2021, 17, 44–50. [Google Scholar]
- Fulop, G.M.; McMillan, D.B. Phagocytosis in the spleen of the sunfish Lepomis spp. J. Morphol. 1984, 179, 175–195. [Google Scholar] [CrossRef]
- Udroiu, I.; Sgura, A. The phylogony of the spleen. Q. Rev. Biol. 2017, 92, 411–443. [Google Scholar] [CrossRef]
- Bach, R.; Chen, P.K.; Chapman, G.B. Changes in the spleen of the channel catfish Ictalurus punetatus Rafinesque induced by infection with Aeromonas hydrophila. J. Fish. Disease 1978, 1, 205–217. [Google Scholar] [CrossRef]
- Maas, M.G.; Bootsma, R. Uptake of bacterial antigens in the spleen of carp (Cyprinus carpio L.). Dev. Comp. Immunol. 1982, 2, 47–52. [Google Scholar]
- Furukawa, T.; Nakamura, O.; Suzuki, Y.; Atsuta, S.; Nakamura, H.; Watanabe, T. Entrapment and transport of foreign material in the spleen and kidney of Japanese conger Conger myriaster. Fish. Sci. 2002, 68, 1219–1225. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Characterization of the fish ovarian stroma during the spawning season: Cytochemical, immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2019, 94, 566–579. [Google Scholar] [CrossRef]
- Agius, C.; Roberts, R.J. Melano-macrophage centers and their role in fish pathology. J. Fish. Dis. 2003, 26, 499–561. [Google Scholar] [CrossRef]
- Diaz-Satizabal, L.; Magor, B.G. Isolation and cytochemical characterization of melanomacrophages and melanomacrophage clusters from goldfish (Carassius auratus L.). Dev. Comp. Immunol. 2015, 48, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Manrique, W.G.; da Silva Claudiano, G.; Petrillo, T.R.; Pardi de Castro, M.; Pereira Figueiredo, M.A.; de Andrade Belo, M.A.; Engracia de Moraes, J.R.; de Moraes, F.R. Response of splenic melanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758) to inflammatory stimuli by BCG and foreign bodies. J. Appl. Ichthyol. 2014, 30, 1001–1006. [Google Scholar] [CrossRef]
- Ribeiro, F.B.; Lanna, E.A.T.; Bomfim, M.A.D.; Donzele, J.L.; Quadros, M.; Cunha, P.D.S.L. True and apparent digestibility of protein and amino acids of feed in Nile tilapia. Rev. Bras. Zootec. 2011, 40, 939–946. [Google Scholar] [CrossRef]
- King, R.; Beso, A.J.G.; Candelaria, V.Y.; Dela Cruz, J.G.; Margie, S.; Tameta, A.D.C.; Espinosa, A.A. Effects of Unleaded Petroleum on the Macrophage Aggregates (MA) formation in Red King tilapia (Oreochromis sp.) Fingerlings. bioRxiv 2016, 044537. [Google Scholar] [CrossRef]
- Wolke, R.E. Piscine macrophage aggregates: A review. Annu. Rev. Fish Dis. 1992, 2, 91–108. [Google Scholar] [CrossRef]
- Micale, V.; Perdichizzi, F. A quantitative and histochemical study on melano-macrophage centers in the spleen of the teleost fish Diplodus annularis L. J. Fish Biol. 1990, 37, 191–197. [Google Scholar] [CrossRef]
- Agius, C. The Melano-Macrophage Centres of Fish: A Review. In Fish Immunology; Academic Press Inc.: Cambridge, MA, USA, 1985; pp. 85–105. [Google Scholar]
- Thorsen, J.; Høyheim, B.; Koppang, E.O. Isolation of the Atlantic salmon tyrosinase gene family reveals heterogenous transcripts in a leukocyte cell line. Pigment. Cell Res. 2006, 19, 327–336. [Google Scholar] [CrossRef]
- Weiss, L.; Geduldig, U.; Weidanz, W. Mechanisms of splenic control of murine malaria: Reticular cell activation and the development of a blood-spleen barrier. Am. J. Anat. 1986, 176, 251–285. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Yang, S.; Sun, X.; Tarique, I.; Yang, P.; Chen, Q. Morphological characterization of postembryonic development of blood–spleen barrier in duck. Poult. Sci. 2020, 99, 3823–3830. [Google Scholar] [CrossRef]
- Del Portillo, H.A.; Ferrer, M.; Brugat, T.; Martin-Jaular, L.; Langhorne, J.; Lacerda, M.V. The role of the spleen in malaria. Cell. Microbiol. 2012, 14, 343–355. [Google Scholar] [CrossRef]
- Meseguer, J.; López-Ruiz, A.; Garcí-Ayala, A. Reticulo-endothelial stroma of the head-kidney from the seawater teleost gilthead seabream (Sparus aurata L.): An ultrastructural and cytochemical study. Anat. Rec. 1995, 241, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Havixbeck, J.J.; Barreda, D.R. Neutrophil Development, Migration, and Function in Teleost Fish. Biology 2015, 4, 715–734. [Google Scholar] [CrossRef]
- Purelku, M.; Sahin, H.; Erkanli Senturk, G.; Tanriverdi, G. Distribution and morphologic characterization of telocytes in rat ovary and uterus: Insights from ultrastructural and immunohistochemical analysis. Histochem. Cell Biol. 2024, 162, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.T.; Zaccone, G.; Albano, M.; Alesci, A.; Marino, S.; Alonaizan, R.; Mokhtar, D.M. Serotonin Signaling and Macrophage Subsets in Goldfish Gills: Unraveling the Neuroimmune Network for Gill Homeostasis. Life 2025, 15, 751. [Google Scholar] [CrossRef]
- Aleksandrovych, V.; Gil, A.; Poniatowski, A. Notes about telocytes and immunity. Folia Med. Cracov. 2022, 62, 101–109. [Google Scholar] [PubMed]
- Huang, Y.; Zhang, F.; Tang, X.; Yang, X. Telocytes enhance M1 differentiation and phagocytosis while inhibits mitochondria-mediated apoptosis via activation of NF-κB in macrophages. Cell Transplant. 2021, 30, 09636897211002762. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Zaccone, G.; Hussein, M.T. Morphological and Ultrastructural Insights into the Goldfish (Carassius auratus) Spleen: Immune Organization and Cellular Composition. Vet. Sci. 2025, 12, 517. https://doi.org/10.3390/vetsci12060517
Mokhtar DM, Zaccone G, Hussein MT. Morphological and Ultrastructural Insights into the Goldfish (Carassius auratus) Spleen: Immune Organization and Cellular Composition. Veterinary Sciences. 2025; 12(6):517. https://doi.org/10.3390/vetsci12060517
Chicago/Turabian StyleMokhtar, Doaa M., Giacomo Zaccone, and Manal T. Hussein. 2025. "Morphological and Ultrastructural Insights into the Goldfish (Carassius auratus) Spleen: Immune Organization and Cellular Composition" Veterinary Sciences 12, no. 6: 517. https://doi.org/10.3390/vetsci12060517
APA StyleMokhtar, D. M., Zaccone, G., & Hussein, M. T. (2025). Morphological and Ultrastructural Insights into the Goldfish (Carassius auratus) Spleen: Immune Organization and Cellular Composition. Veterinary Sciences, 12(6), 517. https://doi.org/10.3390/vetsci12060517






