A Review of Cross-Species Transmission Mechanisms of Influenza Viruses
Simple Summary
Abstract
1. Introduction
2. Classification of Influenza Viruses
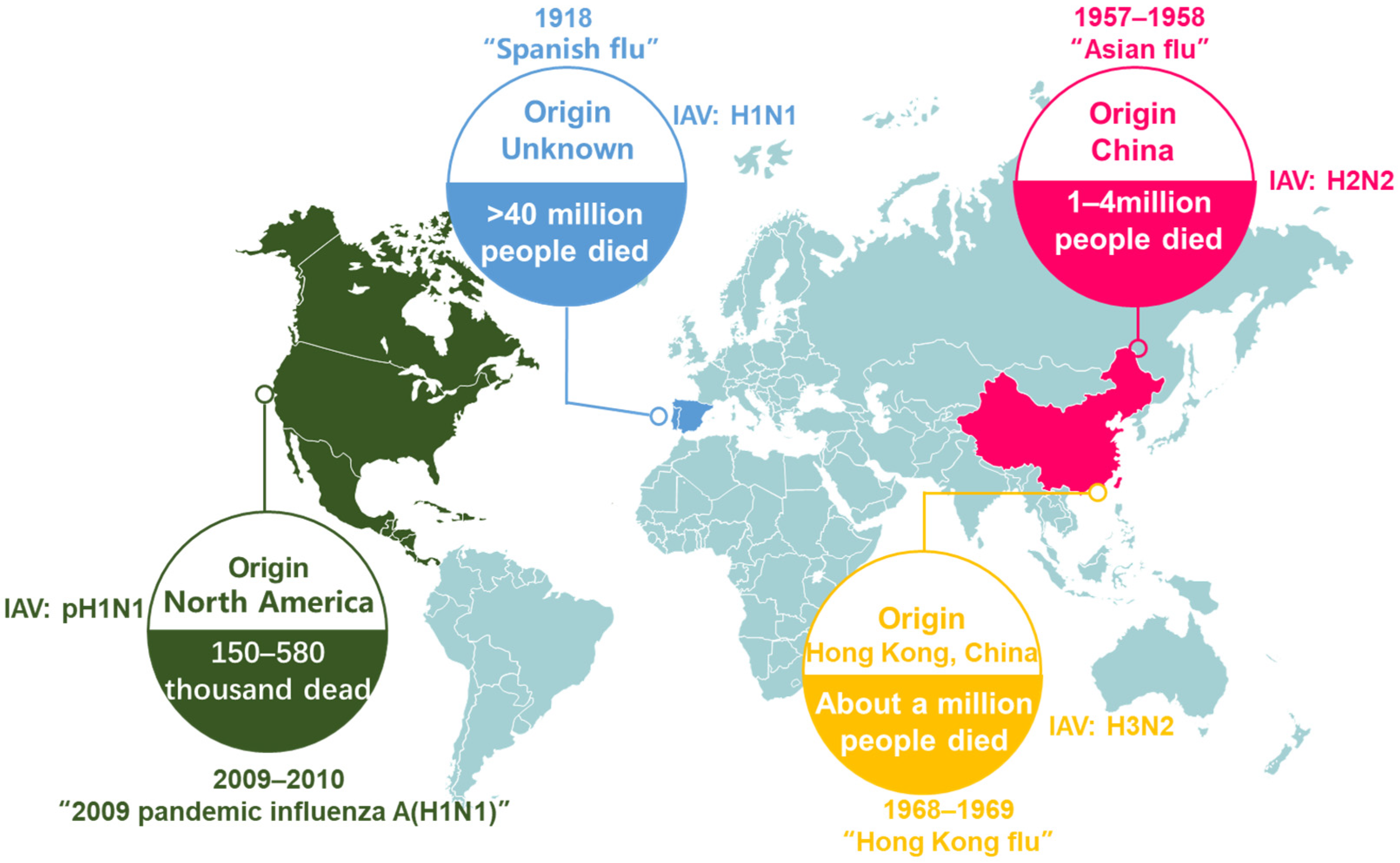
3. Epidemic Status of Influenza A Virus
4. Cross-Species Transmission Mechanism
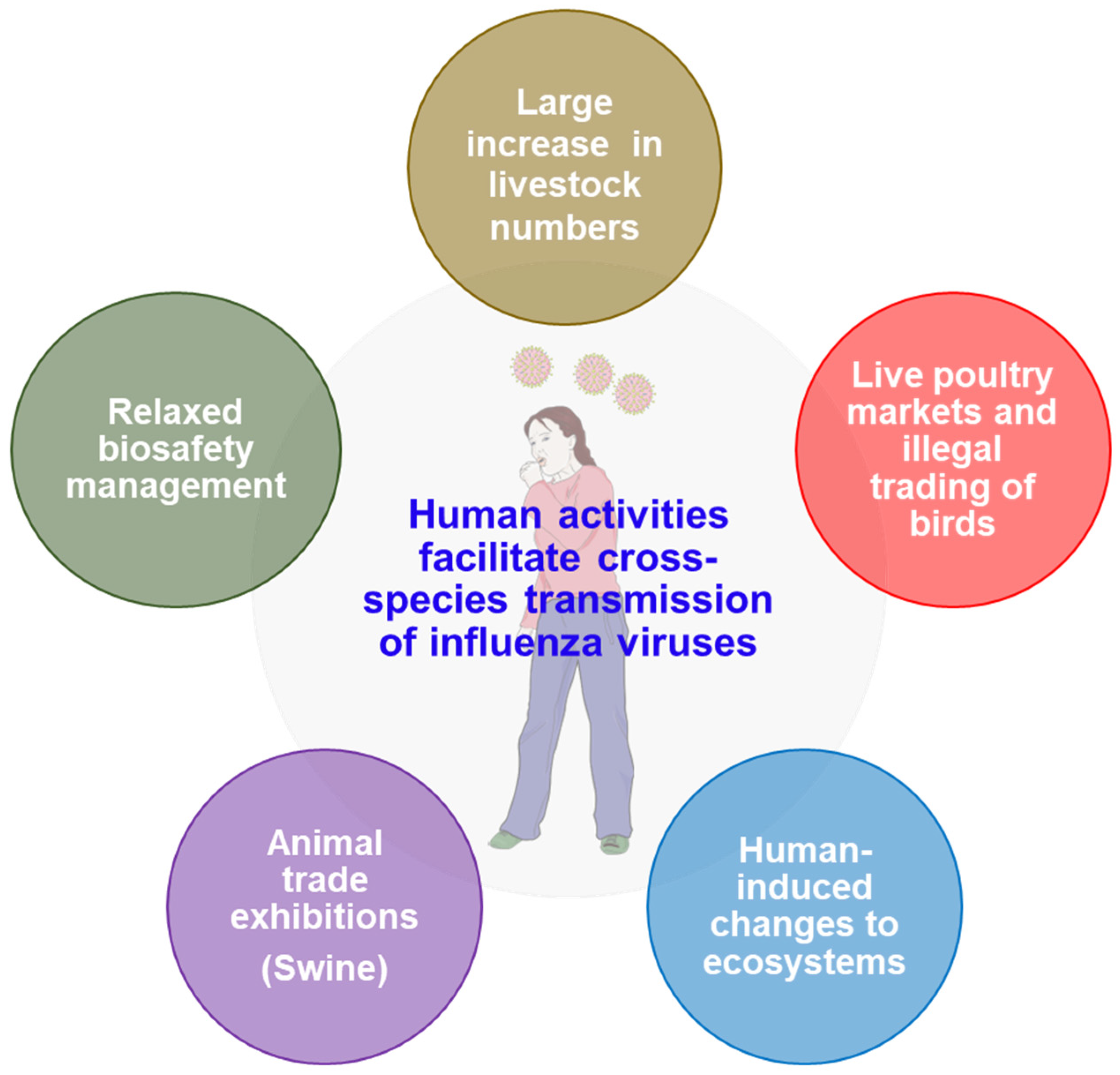
5. Human Activities and Cross-Species Transmission of Influenza Viruses
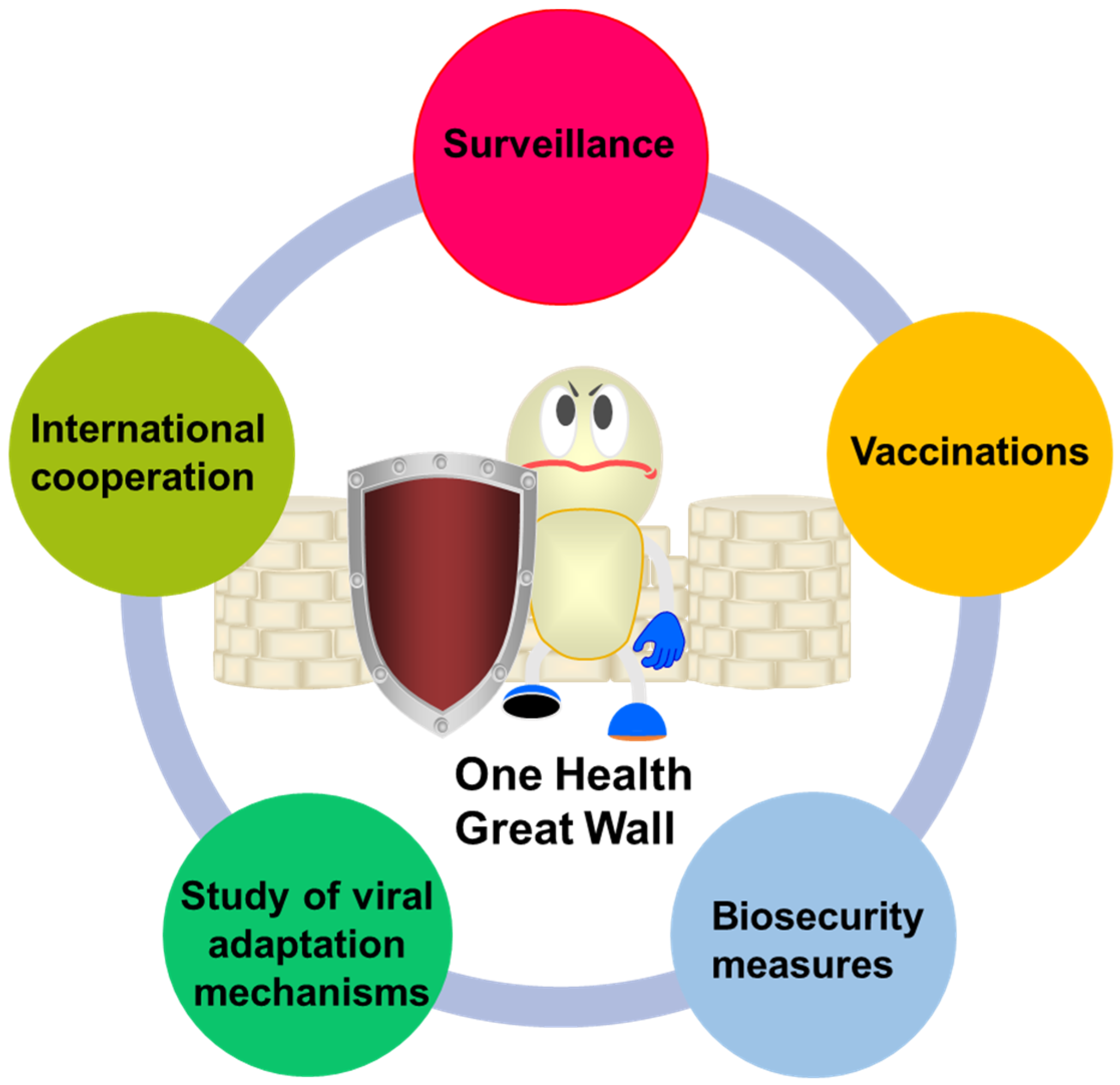
6. Strategies and Prospects for Human Response to Interspecies Transmission of Influenza Virus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, S.J.; Hsu, Y.M.; Huang, S.W.; Tsai, H.P.; Lee, L.Y.Y.; Hurt, A.C.; Barr, I.G.; Shih, S.R.; Wang, J.R. Genetic variations on 31 and 450 residues of influenza A nucleoprotein affect viral replication and translation. J. Biomed. Sci. 2020, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; McBride, R.; Dortmans, J.; Peng, W.; Bakkers, M.J.G.; de Groot, R.J.; van Kuppeveld, F.J.M.; Paulson, J.C.; de Vries, E.; de Haan, C.A.M. Mutation of the Second Sialic Acid-Binding Site, Resulting in Reduced Neuraminidase Activity, Preceded the Emergence of H7N9 Influenza A Virus. J. Virol. 2017, 91, e00049-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Z.; Wang, X. Strain-Specific Antagonism of the Human H1N1 Influenza A Virus against Equine Tetherin. Viruses 2018, 10, 264. [Google Scholar] [CrossRef]
- Huang, J.; Li, K.; Xiao, S.; Hu, J.; Yin, Y.; Zhang, J.; Li, S.; Wang, W.; Hong, J.; Zhao, Z.; et al. Global epidemiology of animal influenza infections with explicit virus subtypes until 2016: A spatio-temporal descriptive analysis. One Health 2023, 16, 100514. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, X.; Chen, P.; Deng, G.; Li, Y.; Shi, J.; Gu, C.; Kong, H.; Suzuki, Y.; Jiang, Y.; et al. Characterization of Clade 7.2 H5 Avian Influenza Viruses That Continue To Circulate in Chickens in China. J. Virol. 2016, 90, 9797–9805. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, L.; Li, J.; Zhao, Q.; Wang, J.; He, X.; Huang, S.; Wang, Q.; Zhao, Y.; Wang, G.; et al. Low Polymerase Activity Attributed to PA Drives the Acquisition of the PB2 E627K Mutation of H7N9 Avian Influenza Virus in Mammals. mBio 2019, 10, e01162-19. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Wang, F.; Qi, J.; Wu, Y.; Song, H.; Gao, F.; Bi, Y.; Zhang, Y.; Fan, Z.; et al. Structures and receptor binding of hemagglutinins from human-infecting H7N9 influenza viruses. Science 2013, 342, 243–247. [Google Scholar] [CrossRef]
- Xiong, X.; Martin, S.R.; Haire, L.F.; Wharton, S.A.; Daniels, R.S.; Bennett, M.S.; McCauley, J.W.; Collins, P.J.; Walker, P.A.; Skehel, J.J.; et al. Receptor binding by an H7N9 influenza virus from humans. Nature 2013, 499, 496–499. [Google Scholar] [CrossRef]
- de Vries, R.P.; Peng, W.; Grant, O.C.; Thompson, A.J.; Zhu, X.; Bouwman, K.M.; de la Pena, A.T.T.; van Breemen, M.J.; Ambepitiya Wickramasinghe, I.N.; de Haan, C.A.M.; et al. Three mutations switch H7N9 influenza to human-type receptor specificity. PLoS Pathog. 2017, 13, e1006390. [Google Scholar] [CrossRef]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef]
- Garcia-Sastre, A. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 2001, 279, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89 Pt 10, 2359–2376. [Google Scholar] [CrossRef]
- Mehle, A.; Doudna, J.A. Adaptive strategies of the influenza virus polymerase for replication in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 21312–21316. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C.; Mubareka, S.; Palese, P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009, 5, e1000252. [Google Scholar] [CrossRef]
- Osterhaus, A.D.; Rimmelzwaan, G.F.; Martina, B.E.; Bestebroer, T.M.; Fouchier, R.A. Influenza B virus in seals. Science 2000, 288, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Epstein, J.H.; Murray, K.A.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Ali Khan, S.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenstrom, J.; Osterhaus, A.D.; Fouchier, R.A. Global patterns of influenza a virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- de Graaf, M.; Fouchier, R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Wentworth, D.E.; Das, S.R.; Sreevatsan, S.; Killian, M.L.; Nolting, J.M.; Slemons, R.D.; Bowman, A.S. Evolutionary Dynamics of Influenza A Viruses in US Exhibition Swine. J. Infect. Dis. 2016, 213, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.S.; Walia, R.R.; Nolting, J.M.; Vincent, A.L.; Killian, M.L.; Zentkovich, M.M.; Lorbach, J.N.; Lauterbach, S.E.; Anderson, T.K.; Davis, C.T.; et al. Influenza A(H3N2) Virus in Swine at Agricultural Fairs and Transmission to Humans, Michigan and Ohio, USA, 2016. Emerg. Infect. Dis. 2017, 23, 1551–1555. [Google Scholar] [CrossRef]
- Fobian, K.; Fabrizio, T.P.; Yoon, S.W.; Hansen, M.S.; Webby, R.J.; Larsen, L.E. New reassortant and enzootic European swine influenza viruses transmit efficiently through direct contact in the ferret model. J. Gen. Virol. 2015, 96, 1603–1612. [Google Scholar] [CrossRef]
- Hause, B.M.; Collin, E.A.; Liu, R.; Huang, B.; Sheng, Z.; Lu, W.; Wang, D.; Nelson, E.A.; Li, F. Characterization of a novel influenza virus in cattle and Swine: Proposal for a new genus in the Orthomyxoviridae family. mBio 2014, 5, e00031-14. [Google Scholar] [CrossRef]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013, 9, e1003176. [Google Scholar] [CrossRef]
- Chiapponi, C.; Faccini, S.; De Mattia, A.; Baioni, L.; Barbieri, I.; Rosignoli, C.; Nigrelli, A.; Foni, E. Detection of Influenza D Virus among Swine and Cattle, Italy. Emerg. Infect. Dis. 2016, 22, 352–354. [Google Scholar] [CrossRef]
- Cox, N.J.; Subbarao, K. Global epidemiology of influenza: Past and present. Annu. Rev. Med. 2000, 51, 407–421. [Google Scholar] [CrossRef]
- Kobasa, D.; Takada, A.; Shinya, K.; Hatta, M.; Halfmann, P.; Theriault, S.; Suzuki, H.; Nishimura, H.; Mitamura, K.; Sugaya, N.; et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 2004, 431, 703–707. [Google Scholar] [CrossRef]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team; Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, J.; Wang, L.; Ran, L.; Gao, G.F. Ecology and evolution of avian influenza viruses. Curr. Biol. 2024, 34, R716–R721. [Google Scholar] [CrossRef]
- Ito, T.; Couceiro, J.N.; Kelm, S.; Baum, L.G.; Krauss, S.; Castrucci, M.R.; Donatelli, I.; Kida, H.; Paulson, J.C.; Webster, R.G.; et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998, 72, 7367–7373. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125. [Google Scholar] [CrossRef]
- Paul Glezen, W.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The burden of influenza B: A structured literature review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P. Editorial commentary: Changing epidemiology of influenza B virus. Clin. Infect. Dis. 2014, 59, 1525–1526. [Google Scholar] [CrossRef]
- Abdullahi, O.; Karani, A.; Tigoi, C.C.; Mugo, D.; Kungu, S.; Wanjiru, E.; Jomo, J.; Musyimi, R.; Lipsitch, M.; Scott, J.A. Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. J. Infect. Dis. 2012, 206, 1020–1029. [Google Scholar] [CrossRef]
- Belser, J.A.; Eckert, A.M.; Tumpey, T.M.; Maines, T.R. Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models. Microbiol. Mol. Biol. Rev. 2016, 80, 733–744. [Google Scholar] [CrossRef]
- Laurie, K.L.; Carolan, L.A.; Middleton, D.; Lowther, S.; Kelso, A.; Barr, I.G. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J. Infect. Dis. 2010, 202, 1011–1020. [Google Scholar] [CrossRef]
- Chen, R.; Holmes, E.C. The evolutionary dynamics of human influenza B virus. J. Mol. Evol. 2008, 66, 655–663. [Google Scholar] [CrossRef]
- Langat, P.; Raghwani, J.; Dudas, G.; Bowden, T.A.; Edwards, S.; Gall, A.; Bedford, T.; Rambaut, A.; Daniels, R.S.; Russell, C.A.; et al. Genome-wide evolutionary dynamics of influenza B viruses on a global scale. PLoS Pathog. 2017, 13, e1006749. [Google Scholar] [CrossRef]
- Vijaykrishna, D.; Holmes, E.C.; Joseph, U.; Fourment, M.; Su, Y.C.; Halpin, R.; Lee, R.T.; Deng, Y.M.; Gunalan, V.; Lin, X.; et al. The contrasting phylodynamics of human influenza B viruses. eLife 2015, 4, e05055. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Z.; Lin, T.; Sreenivasan, C.; Gao, R.; Thomas, M.; Druce, J.; Hause, B.M.; Kaushik, R.S.; Li, F.; et al. Genetic and antigenic characteristics of a human influenza C virus clinical isolate. J. Med. Virol. 2020, 92, 161–166. [Google Scholar] [CrossRef]
- Sederdahl, B.K.; Williams, J.V. Epidemiology and Clinical Characteristics of Influenza C Virus. Viruses 2020, 12, 89. [Google Scholar] [CrossRef]
- Kwasnik, M.; Rola, J.; Rozek, W. Influenza D in Domestic and Wild Animals. Viruses 2023, 15, 2433. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Liu, R.; Gao, R.; Guo, Y.; Hause, B.M.; Thomas, M.; Naveed, A.; Clement, T.; Rausch, D.; Christopher-Hennings, J.; et al. Influenza C and D Viruses Demonstrated a Differential Respiratory Tissue Tropism in a Comparative Pathogenesis Study in Guinea Pigs. J. Virol. 2023, 97, e0035623. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Katsushima, N.; Nagai, Y.; Shoji, M.; Itagaki, T.; Sakamoto, M.; Kitaoka, S.; Mizuta, K.; Nishimura, H. Clinical features of influenza C virus infection in children. J. Infect. Dis. 2006, 193, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Fu, X.; Li, G.; Kerlin, F.; Veit, M. Novel Influenza D virus: Epidemiology, pathology, evolution and biological characteristics. Virulence 2017, 8, 1580–1591. [Google Scholar] [CrossRef]
- Nelson, M.I.; Vincent, A.L. Reverse zoonosis of influenza to swine: New perspectives on the human-animal interface. Trends Microbiol. 2015, 23, 142–153. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Hui, X.; Cao, L.; Xu, T.; Zhao, L.; Huang, K.; Zou, Z.; Ren, P.; Mao, H.; Yang, Y.; Gao, S.; et al. PSMD12-Mediated M1 Ubiquitination of Influenza A Virus at K102 Regulates Viral Replication. J. Virol. 2022, 96, e0078622. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Worobey, M.; Han, G.Z.; Rambaut, A. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc. Natl. Acad. Sci. USA 2014, 111, 8107–8112. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, Y.; Krauss, S.; Webster, R.G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 1989, 63, 4603–4608. [Google Scholar] [CrossRef]
- Scholtissek, C.; Rohde, W.; Von Hoyningen, V.; Rott, R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 1978, 87, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Bahl, J.; Vijaykrishna, D.; Zhang, J.; Poon, L.L.; Chen, H.; Webster, R.G.; Peiris, J.S.; Guan, Y. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. USA 2009, 106, 11709–11712. [Google Scholar] [CrossRef]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 2001, 14, 129–149. [Google Scholar] [CrossRef]
- Tundup, S.; Kandasamy, M.; Perez, J.T.; Mena, N.; Steel, J.; Nagy, T.; Albrecht, R.A.; Manicassamy, B. Endothelial cell tropism is a determinant of H5N1 pathogenesis in mammalian species. PLoS Pathog. 2017, 13, e1006270. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 2005, 3, 591–600. [Google Scholar] [CrossRef]
- Dibarbora, M.; Cappuccio, J.; Olivera, V.; Quiroga, M.; Machuca, M.; Perfumo, C.; Perez, D.; Pereda, A. Swine influenza: Clinical, serological, pathological, and virological cross-sectional studies in nine farms in Argentina. Influenza Other Respir. Viruses 2013, 7 (Suppl. S4), 10–15. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Wu, W.; Liu, Z.; He, Z.; Chen, Z.; Zhao, B.; Wu, S.; Yang, C.; Qu, X.; et al. Phylogeny, Pathogenicity, Transmission, and Host Immune Responses of Four H5N6 Avian Influenza Viruses in Chickens and Mice. Viruses 2019, 11, 1048. [Google Scholar] [CrossRef]
- Chrzastek, K.; Segovia, K.; Torchetti, M.; Killian, M.L.; Pantin-Jackwood, M.; Kapczynski, D.R. Virus Adaptation Following Experimental Infection of Chickens with a Domestic Duck Low Pathogenic Avian Influenza Isolate from the 2017 USA H7N9 Outbreak Identifies Polymorphic Mutations in Multiple Gene Segments. Viruses 2021, 13, 1166. [Google Scholar] [CrossRef]
- Claas, E.C.; Osterhaus, A.D.; van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, É.; et al. Avian influenza overview December 2022–March 2023. EFSA J. 2023, 21, e07917. [Google Scholar] [CrossRef]
- Yamaji, R.; Zhang, W.; Kamata, A.; Adlhoch, C.; Swayne, D.E.; Pereyaslov, D.; Wang, D.; Neumann, G.; Pavade, G.; Barr, I.G.; et al. Pandemic risk characterisation of zoonotic influenza A viruses using the Tool for Influenza Pandemic Risk Assessment (TIPRA). Lancet Microbe 2024, 6, 100973. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hao, T.; Han, P.; Wang, H.; Zhang, X.; Li, X.; Wang, Y.; Chen, J.; Li, Y.; Jin, X.; et al. Receptor binding, structure, and tissue tropism of cattle-infecting H5N1 avian influenza virus hemagglutinin. Cell 2025, 188, 919–929.e9. [Google Scholar] [CrossRef]
- Spruit, C.M.; Zhu, X.; Tomris, I.; Rios-Carrasco, M.; Han, A.X.; Broszeit, F.; van der Woude, R.; Bouwman, K.M.; Luu, M.M.T.; Matsuno, K.; et al. N-Glycolylneuraminic Acid Binding of Avian and Equine H7 Influenza A Viruses. J. Virol. 2022, 96, e0212021. [Google Scholar] [CrossRef]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Avian flu: Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- van Riel, D.; Munster, V.J.; de Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.; Osterhaus, A.D.; Kuiken, T. H5N1 Virus Attachment to Lower Respiratory Tract. Science 2006, 312, 399. [Google Scholar] [CrossRef] [PubMed]
- Rios Carrasco, M.; Lin, T.H.; Zhu, X.; Garcia, A.G.; Uslu, E.; Liang, R.; Spruit, C.M.; Richard, M.; Boons, G.J.; Wilson, I.A.; et al. The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors. bioRxiv 2025. [Google Scholar] [CrossRef]
- Van Poucke, S.; Doedt, J.; Baumann, J.; Qiu, Y.; Matrosovich, T.; Klenk, H.D.; Van Reeth, K.; Matrosovich, M. Role of Substitutions in the Hemagglutinin in the Emergence of the 1968 Pandemic Influenza Virus. J. Virol. 2015, 89, 12211–12216. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, Y.; Yan, H.; An, Q.; Liang, C.; Liu, L.; Qian, J. Molecular Markers and Mechanisms of Influenza A Virus Cross-Species Transmission and New Host Adaptation. Viruses 2024, 16, 883. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Zhu, X.; Wang, S.; Zhang, D.; McBride, R.; Yu, W.; Babarinde, S.; Paulson, J.C.; Wilson, I.A. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science 2024, 386, 1128–1134. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Insights on influenza pathogenesis from the grave. Virus Res. 2011, 162, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef]
- Gabriel, G.; Dauber, B.; Wolff, T.; Planz, O.; Klenk, H.D.; Stech, J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. USA 2005, 102, 18590–18595. [Google Scholar] [CrossRef]
- Ganti, K.; Bagga, A.; Carnaccini, S.; Ferreri, L.M.; Geiger, G.; Joaquin Caceres, C.; Seibert, B.; Li, Y.; Wang, L.; Kwon, T.; et al. Influenza A virus reassortment in mammals gives rise to genetically distinct within-host subpopulations. Nat. Commun. 2022, 13, 6846. [Google Scholar] [CrossRef]
- Ortigoza, M.B.; Blaser, S.B.; Zafar, M.A.; Hammond, A.J.; Weiser, J.N. An Infant Mouse Model of Influenza Virus Transmission Demonstrates the Role of Virus-Specific Shedding, Humoral Immunity, and Sialidase Expression by Colonizing Streptococcus pneumoniae. mBio 2018, 9, e02359-18. [Google Scholar] [CrossRef]
- Eaton, M.D. Transmission of Epidemic Influenza Virus in Mice by Contact. J. Bacteriol. 1940, 39, 229–241. [Google Scholar] [CrossRef]
- Diavatopoulos, D.A.; Short, K.R.; Price, J.T.; Wilksch, J.J.; Brown, L.E.; Briles, D.E.; Strugnell, R.A.; Wijburg, O.L. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J. 2010, 24, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Luan, H. Cell-Autonomous and Non-Cell-Autonomous Antiviral Immunity via siRNA-Directed RNAi in Drosophila melanogaster. Immune Discov. 2025, 1, 10001. [Google Scholar] [CrossRef]
- Ip, D.K.; Lau, L.L.; Leung, N.H.; Fang, V.J.; Chan, K.H.; Chu, D.K.; Leung, G.M.; Peiris, J.S.; Uyeki, T.M.; Cowling, B.J. Viral Shedding and Transmission Potential of Asymptomatic and Paucisymptomatic Influenza Virus Infections in the Community. Clin. Infect. Dis. 2017, 64, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Memoli, M.J.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Proudfoot, K.; Fargis, S.; Stein, M.; Dunfee, R.L.; Shaw, P.A.; et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: An A(H1N1)pdm09 dose-finding investigational new drug study. Clin. Infect. Dis. 2015, 60, 693–702. [Google Scholar] [CrossRef]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Kallfass, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P. Visualizing the beta interferon response in mice during infection with influenza A viruses expressing or lacking nonstructural protein 1. J. Virol. 2013, 87, 6925–6930. [Google Scholar] [CrossRef]
- Shaman, J.; Kohn, M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar] [CrossRef]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; van den Brand, J.M.; Manz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza A virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Ma, J.; Shen, H.; Liu, Q.; Bawa, B.; Qi, W.; Duff, M.; Lang, Y.; Lee, J.; Yu, H.; Bai, J.; et al. Pathogenicity and transmissibility of novel reassortant H3N2 influenza viruses with 2009 pandemic H1N1 genes in pigs. J. Virol. 2015, 89, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Webster, R.G.; Govorkova, E.A. H5N1 influenza–continuing evolution and spread. N. Engl. J. Med. 2006, 355, 2174–2177. [Google Scholar] [CrossRef]
- Gilbert, M.; Xiao, X.; Pfeiffer, D.U.; Epprecht, M.; Boles, S.; Czarnecki, C.; Chaitaweesub, P.; Kalpravidh, W.; Minh, P.Q.; Otte, M.J.; et al. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc. Natl. Acad. Sci. USA 2008, 105, 4769–4774. [Google Scholar] [CrossRef]
- Greger, M. The human/animal interface: Emergence and resurgence of zoonotic infectious diseases. Crit. Rev. Microbiol. 2007, 33, 243–299. [Google Scholar] [CrossRef]
- Webster, R.G. Wet markets--a continuing source of severe acute respiratory syndrome and influenza? Lancet 2004, 363, 234–236. [Google Scholar] [CrossRef]
- Gilbert, M.; Golding, N.; Zhou, H.; Wint, G.R.; Robinson, T.P.; Tatem, A.J.; Lai, S.; Zhou, S.; Jiang, H.; Guo, D.; et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat. Commun. 2014, 5, 4116. [Google Scholar] [CrossRef] [PubMed]
- Kung, N.Y.; Morris, R.S.; Perkins, N.R.; Sims, L.D.; Ellis, T.M.; Bissett, L.; Chow, M.; Shortridge, K.F.; Guan, Y.; Peiris, M.J. Risk for infection with highly pathogenic influenza A virus (H5N1) in chickens, Hong Kong, 2002. Emerg. Infect. Dis. 2007, 13, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Bahl, J.; Pham, T.T.; Hill, N.J.; Hussein, I.T.; Ma, E.J.; Easterday, B.C.; Halpin, R.A.; Stockwell, T.B.; Wentworth, D.E.; Kayali, G.; et al. Ecosystem Interactions Underlie the Spread of Avian Influenza A Viruses with Pandemic Potential. PLoS Pathog. 2016, 12, e1005620. [Google Scholar] [CrossRef]
- Guan, Y.; Poon, L.L.; Cheung, C.Y.; Ellis, T.M.; Lim, W.; Lipatov, A.S.; Chan, K.H.; Sturm-Ramirez, K.M.; Cheung, C.L.; Leung, Y.H.; et al. H5N1 influenza: A protean pandemic threat. Proc. Natl. Acad. Sci. USA 2004, 101, 8156–8161. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmed, H.; Amjad, S.; Afzal, M.S.; Haider, W.; Simsek, S.; Khawaja, M.R.; Khan, D.H.; Naz, S.; Durrance-Bagale, A.; et al. Community Based Assessment of Behavior and Awareness of Risk Factors of Cystic Echinococcosis in Major Cities of Pakistan: A One Health Perspective. Front. Public Health 2021, 9, 648900. [Google Scholar] [CrossRef]
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227–234. [Google Scholar] [CrossRef]
- Fuller, T.L.; Gilbert, M.; Martin, V.; Cappelle, J.; Hosseini, P.; Njabo, K.Y.; Abdel Aziz, S.; Xiao, X.; Daszak, P.; Smith, T.B. Predicting hotspots for influenza virus reassortment. Emerg. Infect. Dis. 2013, 19, 581–588. [Google Scholar] [CrossRef]
- Bowman, A.S.; Nelson, S.W.; Page, S.L.; Nolting, J.M.; Killian, M.L.; Sreevatsan, S.; Slemons, R.D. Swine-to-human transmission of influenza A(H3N2) virus at agricultural fairs, Ohio, USA, 2012. Emerg. Infect. Dis. 2014, 20, 1472–1480. [Google Scholar] [CrossRef]
- McBride, D.S.; Nolting, J.M.; Nelson, S.W.; Spurck, M.M.; Bliss, N.T.; Kenah, E.; Trock, S.C.; Bowman, A.S. Shortening Duration of Swine Exhibitions to Reduce Risk for Zoonotic Transmission of Influenza A Virus. Emerg. Infect. Dis. 2022, 28, 2035–2042. [Google Scholar] [CrossRef]
- Nelson, M.I.; Stratton, J.; Killian, M.L.; Janas-Martindale, A.; Vincent, A.L. Continual Reintroduction of Human Pandemic H1N1 Influenza A Viruses into Swine in the United States, 2009 to 2014. J. Virol. 2015, 89, 6218–6226. [Google Scholar] [CrossRef] [PubMed]
- Netrabukkana, P.; Robertson, I.D.; Kasemsuwan, S.; Wongsathapornchai, K.; Fenwick, S. Assessing Potential Risks of Influenza A Virus Transmission at the Pig-Human Interface in Thai Small Pig Farms Using a Questionnaire Survey. Transbound. Emerg. Dis. 2016, 63, e135–e139. [Google Scholar] [CrossRef]
- Lipsitch, M.; Barclay, W.; Raman, R.; Russell, C.J.; Belser, J.A.; Cobey, S.; Kasson, P.M.; Lloyd-Smith, J.O.; Maurer-Stroh, S.; Riley, S.; et al. Viral factors in influenza pandemic risk assessment. eLife 2016, 5, e18491. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, R.; Subbarao, K. Passive immunization with influenza haemagglutinin specific monoclonal antibodies. Hum. Vaccin. Immunother. 2018, 14, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Sims, L.D. Progress in control of H5N1 highly pathogenic avian influenza and the future for eradication. Avian Dis. 2012, 56, 829–835. [Google Scholar] [CrossRef]
- Scholtissek, C.; Burger, H.; Kistner, O.; Shortridge, K.F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 1985, 147, 287–294. [Google Scholar] [CrossRef]
- Nelson, M.I.; Wentworth, D.E.; Culhane, M.R.; Vincent, A.L.; Viboud, C.; LaPointe, M.P.; Lin, X.; Holmes, E.C.; Detmer, S.E. Introductions and evolution of human-origin seasonal influenza a viruses in multinational swine populations. J. Virol. 2014, 88, 10110–10119. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, C.M.; McBride, D.S.; Trock, S.C.; Habing, G.G.; Hoet, A.E.; Nelson, S.W.; Nolting, J.M.; Bowman, A.S. Evolution of influenza A viruses in exhibition swine and transmission to humans, 2013–2015. Zoonoses Public Health 2024, 71, 281–293. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Sun, X.; Lin, X.; Zhao, L.; Chen, H.; Jin, M. Evidence for a novel mechanism of influenza A virus host adaptation modulated by PB2-627. FEBS J. 2019, 286, 3389–3400. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. Predicting the Next Influenza Pandemics. J. Infect. Dis. 2019, 219, S14–S20. [Google Scholar] [CrossRef]
- Durand, L.O.; Glew, P.; Gross, D.; Kasper, M.; Trock, S.; Kim, I.K.; Bresee, J.S.; Donis, R.; Uyeki, T.M.; Widdowson, M.A.; et al. Timing of influenza A(H5N1) in poultry and humans and seasonal influenza activity worldwide, 2004–2013. Emerg. Infect. Dis. 2015, 21, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. Advances in Influenza Virus Research: A Personal Perspective. Viruses 2018, 10, 724. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, X.; Tian, X.; Ding, S.; Gao, G.; Cui, J.; Zhang, C.; Zhao, T.; Duan, L.; Wang, H. A Review of Cross-Species Transmission Mechanisms of Influenza Viruses. Vet. Sci. 2025, 12, 447. https://doi.org/10.3390/vetsci12050447
Hui X, Tian X, Ding S, Gao G, Cui J, Zhang C, Zhao T, Duan L, Wang H. A Review of Cross-Species Transmission Mechanisms of Influenza Viruses. Veterinary Sciences. 2025; 12(5):447. https://doi.org/10.3390/vetsci12050447
Chicago/Turabian StyleHui, Xianfeng, Xiaowei Tian, Shihuan Ding, Ge Gao, Jiyan Cui, Chengguang Zhang, Tiesuo Zhao, Liangwei Duan, and Hui Wang. 2025. "A Review of Cross-Species Transmission Mechanisms of Influenza Viruses" Veterinary Sciences 12, no. 5: 447. https://doi.org/10.3390/vetsci12050447
APA StyleHui, X., Tian, X., Ding, S., Gao, G., Cui, J., Zhang, C., Zhao, T., Duan, L., & Wang, H. (2025). A Review of Cross-Species Transmission Mechanisms of Influenza Viruses. Veterinary Sciences, 12(5), 447. https://doi.org/10.3390/vetsci12050447








