Risk Factors and Spatial Distribution of Gastrointestinal Parasites in Backyard Poultry Production Systems in Central Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size and Study Area
2.1.1. Sample Size
2.1.2. Study Area
2.1.3. Sampling Method
2.2. Parasitological Examination for Gastrointestinal Parasite Identification
2.2.1. Flotation Technique
2.2.2. McMaster Counting Technique
2.3. Data Management and Risk Factors and Spatial Analysis
2.3.1. Questionnaire Survey
2.3.2. Multivariable Regression Analysis
2.3.3. Spatial Analysis
3. Results
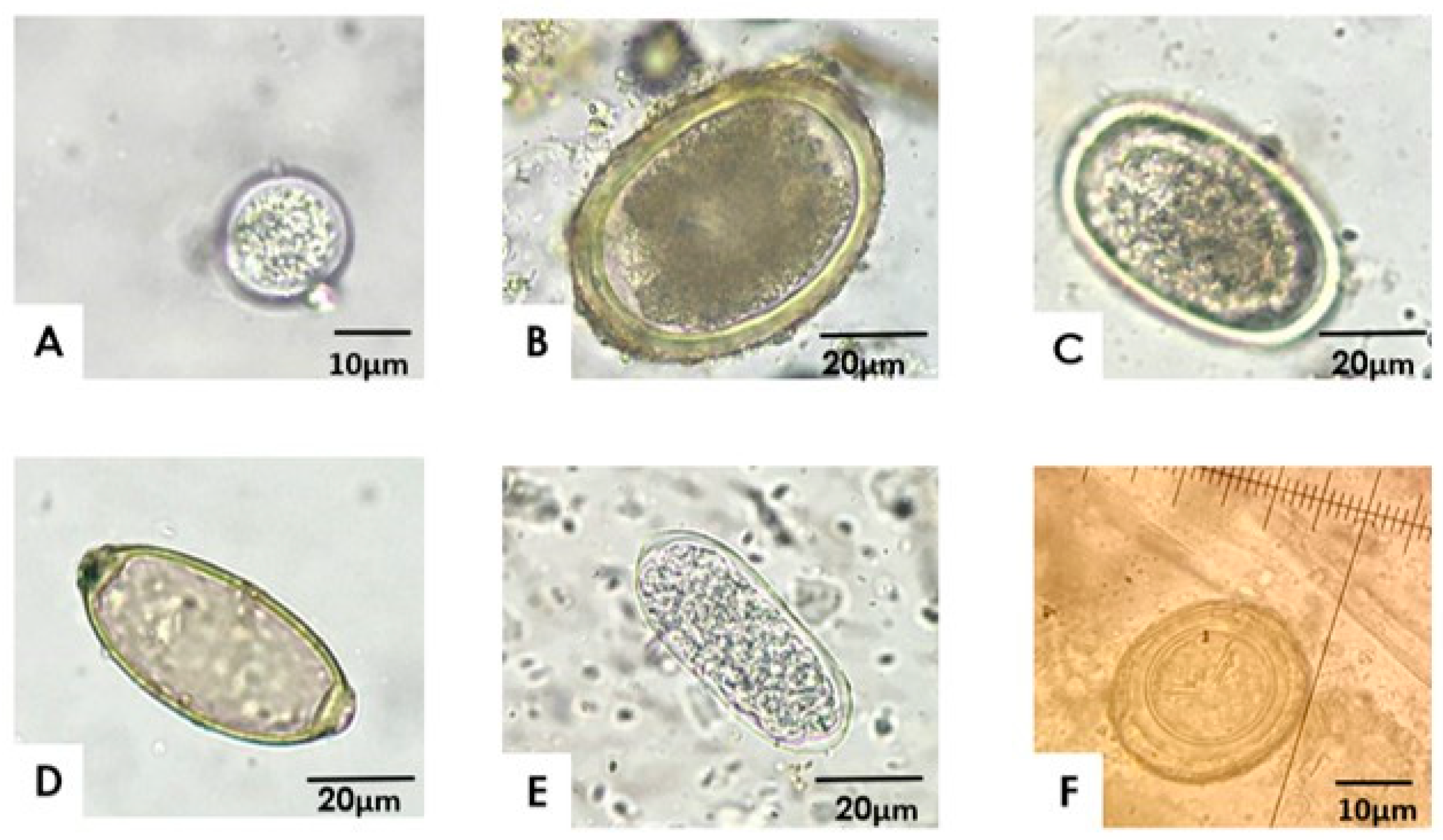
3.1. Frequency of Gastrointestinal Parasites in BPPS in Central Chile
3.2. Estimated Gastrointestinal Parasite Burden in BPPS in Central Chile
3.3. Risk Factors for Gastrointestinal Parasites in BPPS in Central Chile
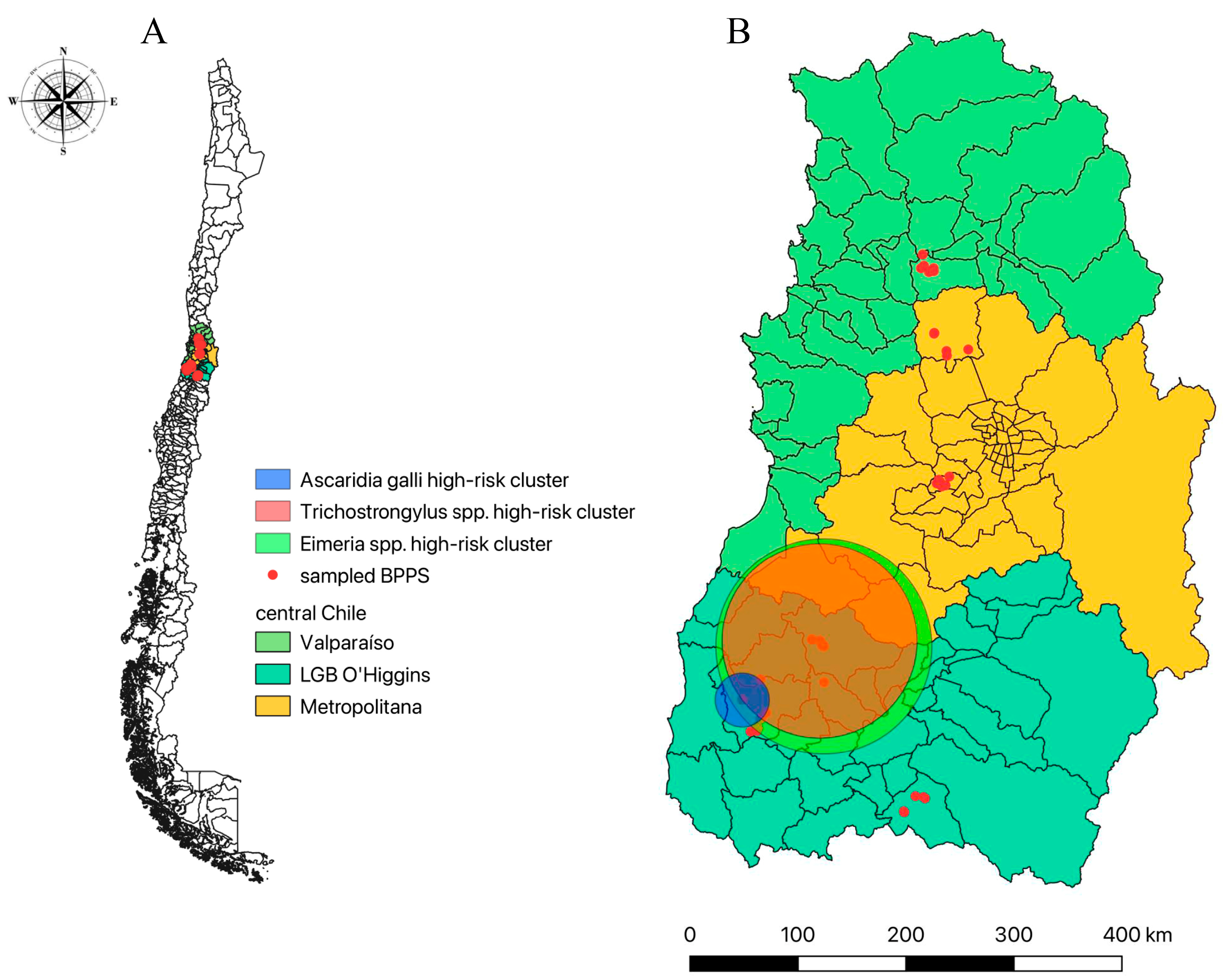
3.4. Spatial Analysis for Gastrointestinal Parasitosis in BPPS in Central Chile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-Gómez, V.; Ma, T.; Li, Y.; Rasmussen, P.; Torgerson, P.R. Global and regional prediction of coccidiosis and ascaridiosis prevalence in extensive backyard chickens in low-income and middle-income countries. Vet. Parasitol. 2024, 331, 110268. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, N.A. Backyard poultry production and its importance. Acta Sci. Vet. Sci. 2021, 3, 23–28. Available online: https://actascientific.com/ASVS/pdf/ASVS-03-0121.pdf (accessed on 9 January 2025).
- Gentile, N.; Carrasquer, F.; Marco-Fuertes, A.; Marin, C. Backyard poultry: Exploring non-intensive production systems. Poult. Sci. 2024, 103, 103284. [Google Scholar] [CrossRef] [PubMed]
- Correia-Gomes, C.; Henry, M.K.; Auty, H.K.; Gunn, G.J. Exploring the role of small-scale livestock keepers for national biosecurity—The pig case. Prev. Vet. Med. 2017, 145, 7–15. [Google Scholar] [CrossRef]
- Correia-Gomes, C.; Sparks, N. Exploring the attitudes of backyard poultry keepers to health and biosecurity. Prev. Vet. Med. 2020, 174, 104812. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. An updated insight into the gastrointestinal helminthoses of poultry: A review. Ann. Parasitol. 2022, 68, 645–656. [Google Scholar] [CrossRef]
- Shifaw, A.; Feyera, T.; Walkden-Brown, S.W.; Sharpe, B.; Elliott, T.; Ruhnke, I. Global and regional prevalence of helminth infection in chickens over time: A systematic review and meta-analysis. Poult. Sci. 2021, 100, 101082. [Google Scholar] [CrossRef]
- Alcaino, H.; Gorman, T. Parasitos de Los Animales Domesticos en Chile. Parasitol. Al Día 1999, 23, 33–41. [Google Scholar] [CrossRef]
- Mares, M.M.; Al-Quraishy, S.; Abdel-Gaber, R.; Murshed, M. Morphological and Molecular Characterization of Eimeria spp. Infecting Domestic Poultry Gallus gallus in Riyadh City, Saudi Arabia. Microorganisms 2023, 11, 795. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Höglund, J. Environmental tolerance of free-living stages of the poultry roundworm Ascaridia galli. Vet. Parasitol. 2015, 209, 101–107. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, P.; Singla, L.D.; Kashyap, N.; Bal, M.S. Assessment of risk factors associated with prevalence of gastrointestinal parasites in poultry of central plain zone of Punjab, India. Vet World 2021, 14, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Yadav, A.; Sofi, O.M.; Kushwaha, A.; Yadav, V.; Rafiqi, S.I.; Godara, R.; Katoch, R. Prevalence and molecular characterization of Eimeria species affecting backyard poultry of Jammu region, North India. Trop. Anim. Health Prod. 2022, 54, 296. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Naeini, H.R.; Ko, H.; Goo, D.; Choppa, V.S.R.; Gudidoddi, S.R.; Katha, H.R.; Kim, W.K. Synergistic impact of Salmonella typhimurium and Eimeria spp. coinfection on turkey poults: Growth performance, salmonella colonization, and ceca microbiota insights. Poult. Sci. 2025, 104, 104568. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Martin, W.; Stryhn, H. Methods in Epidemiologic Research; VER Inc.: Charlottetown, PE, Canada, 2012; Available online: https://projects.upei.ca/mer/ (accessed on 17 November 2023).
- Tomazic, M.L.; Britez, J.D.; Pisón Martínez, M.L.; Barbano, P.; Canet, Z.; Trangoni, M.D.; Poklepovich, T.J.; Cubas, F.; Alegría-Morán, R.; Ramírez-Toloza, G.; et al. Chicken Coccidiosis in Peri-Urban Family Farming in Two South American Countries: Prevalence and Circulating Eimeria spp. Animals 2025, 15, 982. [Google Scholar] [CrossRef]
- Alegría-Morán, R.; Pastenes, Á.; Cabrera, G.; Fredes, F.; Ramírez-Toloza, G. Urban public squares as potential hotspots of dog-human contact: A spatial analysis of zoonotic parasites detection in Gran Santiago, Chile. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100579. [Google Scholar] [CrossRef]
- Soulsby, E.J.L. Helminths, arthropods and protozoa of domesticated animals (7th edition): E. J. L. Soulsby, 1982. London: Baillière Tindall, 809 pp., illus. ISBN 0-7020-0820-6. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 329. [Google Scholar] [CrossRef]
- Rinaldi, L.; Levecke, B.; Bosco, A.; Ianniello, D.; Pepe, P.; Charlier, J.; Cringoli, G.; Vercruysse, J. Comparison of individual and pooled faecal samples in sheep for the assessment of gastrointestinal strongyle infection intensity and anthelmintic drug efficacy using McMaster and Mini-FLOTAC. Vet. Parasitol. 2014, 205, 216–223. [Google Scholar] [CrossRef]
- Cheru, H.; Tamrat, H.; Hailemelekot, M.; Cassini, R.; Belayneh, N. Epidemiology and identification of Eimeria species affecting poultry in East Gojjam Zone, North West Ethiopia. Vet. Med. Sci. 2023, 9, 2160–2167. [Google Scholar] [CrossRef]
- Tiersch, K.M.; Daş, G.; von Samson-Himmelstjerna, G.; Gauly, M. Artificial infection of chickens with Capillaria obsignata eggs embryonated in different media. Vet. Parasitol. 2014, 200, 139–146. [Google Scholar] [CrossRef]
- Wickware, A.B. Differential Blood Picture in Chickens: Before and after Administration of Embryonated Eggs of Heterakis Gallinae with Notes on Pathogenicity. Can. J. Comp. Med. Vet. Sci. 1947, 11, 78–83. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1661218/ (accessed on 4 September 2023).
- Ikeme, M.M. Observations on the pathogenicity and pathology of Ascaridia galli. Parasitology 1971, 63, 169–179. [Google Scholar] [CrossRef] [PubMed]
- McDougald, L.R. Internal Parasites. In Diseases of Poultry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1157–1191. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 13 March 2023).
- Team, R. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 13 March 2023).
- Costa, M.A.; Kulldorff, M. Applications of spatial scan statistics: A review. In Scan Statistics. Statistics for Industry and Technology; Glaz, J., Pozdnyakov, V., Wallenstein, S., Eds.; Birkhäuser: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Rao, H.; Shi, X.; Zhang, X. Using the Kulldorff’s scan statistical analysis to detect spatio-temporal clusters of tuberculosis in Qinghai Province, China, 2009–2016. BMC Infect. Dis. 2017, 17, 578. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Rand, K.; Williams, G. SaTScan: Software for the Spatial and Space-Time Scan Statistics; Information Management Services Inc.: Silver Spring, MD, USA, 2022; Available online: https://www.satscan.org/ (accessed on 13 March 2023).
- Quantum, G. QGIS Development Team–QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2018. Available online: https://qgis.org/project/ (accessed on 13 March 2023).
- Covacevic, G.; Esnaola, V. Production of Eggs: Current Situation and Perspectives; Office of Agrarian Studies and Policies O: Santiago, Chile, 2008. [Google Scholar]
- Lockhart, C.Y.; Stevenson, M.A.; Rawdon, T.G. A cross-sectional study of ownership of backyard poultry in two areas of Palmerston North, New Zealand. N. Z. Vet. J. 2010, 58, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Abebe, B.; Zewdie, T. Study on prevalence of ectoparasites of poultry in and around Jimma town. Eur. J. Biol. Sci. 2017, 9, 18–26. [Google Scholar] [CrossRef]
- Rashid, M.; Akbar, H.; Bakhsh, A.; Rashid, M.I.; Hassan, M.A.; Ullah, R.; Hussain, T.; Manzoor, S.; Yin, H. Assessing the prevalence and economic significance of coccidiosis individually and in combination with concurrent infections in Pakistani commercial poultry farms. Poult. Sci. 2019, 98, 1167–1175. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Attree, E.; Sanchez-Arsuaga, G.; Jones, M.; Xia, D.; Marugan-Hernandez, V.; Blake, D.; Tomley, F. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric. Biosci. 2021, 2, 37. [Google Scholar] [CrossRef]
- Belli, S.I.; Smith, N.C.; Ferguson, D.J.P. The coccidian oocyst: A tough nut to crack! Trends Parasitol. 2006, 22, 416–423. [Google Scholar] [CrossRef]
- Alcaíno, H.; González, J.P.; Fredes, F.; Gorman, T. Coccidias aviares de gallineros industriales de Chile. Parasitol. Latinoam. 2002, 57, 34–39. [Google Scholar] [CrossRef]
- Torres, P.; Cerna, O.; Rubilar, A.; Subiabre, Á.; Oyarzún, P. Blastocystosis y otras infecciones intestinales por parásitos eucarióticos en Gallus gallus domesticus en localidades del sur de Chile. Rev. De Investig. Vet. Del Perú 2020, 31, e19041. [Google Scholar] [CrossRef]
- Bolfa, P.; Callanan, J.J.; Ketzis, J.; Marchi, S.; Cheng, T.; Huynh, H.; Lavinder, T.; Boey, K.; Hamilton, C.; Kelly, P. Infections and pathology of free-roaming backyard chickens on St. Kitts, West Indies. J. Vet. Diagn. Investig. 2019, 31, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Montes-Vergara, D.E.; Cardona-Alvarez, J.; Pérez-Cordero, A. Prevalence of gastrointestinal parasites in three groups of domestic poultry managed under backyard system in the Savanna subregion, Department of Sucre, Colombia. J. Adv. Vet. Anim. Res. 2021, 8, 606–611. [Google Scholar] [CrossRef]
- Carrisosa, M.; Jin, S.; McCrea, B.A.; Macklin, K.S.; Dormitorio, T.; Hauck, R. Prevalence of Select Intestinal Parasites in Alabama Backyard Poultry Flocks. Animals 2021, 11, 939. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; van-Heerden, K.; Mohnl, M.; Barrett, N.W.; Dalloul, R.A. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 2016, 47, 111. [Google Scholar] [CrossRef]
- Kim, W.H.; Chaudhari, A.A.; Lillehoj, H.S. Involvement of T Cell Immunity in Avian Coccidiosis. Front. Immunol. 2019, 10, 2732. [Google Scholar] [CrossRef]
- Adem, D.M.; Ame, M. Prevalence of poultry coccidiosis and its associated risk factors in and around Haramaya District, Ethiopia. Vet. Med. Open J. 2023, 8, 9–17. [Google Scholar] [CrossRef]
- McDougald, L.R.; Cervantes, H.M.; Jenkins, M.C.; Hess, M.; Beckstead, R. Protozoal Infections. In Diseases of Poultry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1192–1254. [Google Scholar]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Mousa, M.R.; Attia, M.M.; Salem, H.M.; Al-Hoshani, N.; Thabit, H.; Ibrahim, M.A.; Albohiri, H.H.; Khan, S.A.; El-Saadony, M.T.; El-Tarabily, K.A.; et al. Coinfection of the gut with protozoal and metazoal parasites in broiler and laying chickens. Poult. Sci. 2024, 103, 103227. [Google Scholar] [CrossRef]
- Bautista-Vanegas, A.; Esteban-Mendoza, M.; Cala-Delgado, D. Ascaridia galli: A report of erratic migration in eggs for human consumption in Bucaramanga, Colombia-case report. Arq. Bras. De Med. Veterinária E Zootec. 2023, 75, 122–126. [Google Scholar] [CrossRef]
- Burrell, A.; Tomley, F.M.; Vaughan, S.; Marugan-Hernandez, V. Life cycle stages, specific organelles and invasion mechanisms of Eimeria species. Parasitology 2020, 147, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.K.; Maharana, B.R. Control of poultry coccidiosis: Changing trends. J. Parasit. Dis. 2011, 35, 10–17. [Google Scholar] [CrossRef]
- Makouloutou-Nzassi, P.; Longo-Pendy, N.M.; Nguema, L.K.A.; Lendzele, S.S.; Bangueboussa, F.; Bouchedi, B.; Maganga, G.D.; Boundenga, L. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Vet. J. 2024, 14, 3232–3240. [Google Scholar] [CrossRef] [PubMed]
- Calderón, E.G.Q.; Colima, A.B.G.; Rojas, Z.C. Los Factores de Riesgo Asociados a Parásitos Gastrointestinales en Animales de Producción. Cult. Científica Y Tecnológica 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Alcala-Canto, Y.; Figueroa-Castillo, J.A.; Ibarra-Velarde, F.; Vera-Montenegro, Y.; Cervantes-Valencia, M.E.; Alberti-Navarro, A. First database of the spatial distribution of Eimeria species of cattle, sheep and goats in Mexico. Parasitol. Res. 2020, 119, 1057–1074. [Google Scholar] [CrossRef]
- Cornell, K.A.; Smith, O.M.; Crespo, R.; Jones, M.S.; Crossley, M.S.; Snyder, W.E.; Owen, J.P. Prevalence Patterns for Enteric Parasites of Chickens Managed in Open Environments of the Western United States. Avian Dis. 2022, 66, 60–68. [Google Scholar] [CrossRef]
- Coroian, M.; Fábián-Ravasz, T.-Z.; Dobrin, P.R.; Györke, A. Occurrence of Eimeria spp. and Intestinal Helminths in Free-Range Chickens from Northwest and Central Romania. Animals 2024, 14, 563. [Google Scholar] [CrossRef]
- Urzúa-Encina, C.; Fernández-Sanhueza, B.; Pavez-Muñoz, E.; Ramírez-Toloza, G.; Lujan-Tomazic, M.; Rodríguez, A.E.; Alegría-Morán, R. Epidemiological Characterization of Isolates of Salmonella enterica and Shiga Toxin-Producing Escherichia coli from Backyard Production System Animals in the Valparaíso and Metropolitana Regions. Animals 2023, 13, 2444. [Google Scholar] [CrossRef]
- You, M.-J. Suppression of Eimeria tenella Sporulation by Disinfectants. Korean J. Parasitol. 2014, 52, 435–438. [Google Scholar] [CrossRef]
- Qin, Z.R.; Arakawa, A.; Baba, E.; Fukata, T.; Miyamoto, T.; Sasai, K.; Withanage, G.S.K. Eimeria tenella Infection Induces Recrudescence of Previous Salmonella enteritidis Infection in Chickens. Poult. Sci. 1995, 74, 1786–1792. [Google Scholar] [CrossRef]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: A review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef]
| Topics | Number of Questions |
|---|---|
| Environmental conditions and management systems in animal production | 13 |
| Knowledge about coccidial disease | 8 |
| Economic and financial aspects of the operation | 5 |
| Characteristics of the chicken and production model | 5 |
| Sanitary management and animal welfare in poultry production | 5 |
| Biosecurity and cleaning protocols in animal production | 4 |
| Responsibility and management of farm personnel | 4 |
| Animal welfare and health monitoring | 4 |
| Environmental characteristics and property access | 3 |
| Basic services | 3 |
| Government support | 2 |
| Parasite | Frequency of Findings | Percentage (%) | 95% CI |
|---|---|---|---|
| Eimeria spp. | 37 | 72.5 | 58.0–83.7 |
| Heterakis gallinarum | 13 | 25.4 | 14.8–39.9 |
| Ascaridia galli | 25 | 49.0 | 35.0–63.2 |
| Capillaria spp. | 26 | 50.9 | 36.8–65.0 |
| Trichostrongylus spp. | 13 | 25.4 | 14.8–39.9 |
| Cestode-like egg | 1 | 1.96 | 0.1–11.8 |
| Parasite | Intensity | ||
|---|---|---|---|
| Low | Medium | High | |
| For coccidian (OPG *) | (<1800) | (1800–6000) | (>6000) |
| Eimeria spp. | 88.3% (45/51) | 7.8% (4/51) | 3.9% (2/51) |
| For nematodes (EPG +) | (<500 EPG +) | (500–2000) | (>2000) |
| Heterakis gallinarum | 98.0% (50/51) | 2.0% (1/51) | 0.0% (0/51) |
| Ascaridia galli | 100.0% (51/51) | 0.0% (0/51) | 0.0% (0/51) |
| Capillaria spp. | 100.0% (51/51) | 0.0% (0/51) | 0.0% (0/51) |
| Trichostrongylus spp. | 94.1% (48/51) | 3.9% (2/51) | 2.0% (1/51) |
| Model | Variables | Category | p-Value | OR | CI 95% | |
|---|---|---|---|---|---|---|
| Lower | Superior | |||||
| Eimeria spp. | (Intercept) | 0.343 | 0.195 | 0.007 | 5.712 | |
| Presence of Capillaria spp. | no | reference | ||||
| yes | 0.045 * | 18.415 | 1.065 | 318.329 | ||
| Access restriction | control | reference | ||||
| no control | 0.097 | 15.24 | 0.61 | 381.023 | ||
| Ventilation system | no | reference | ||||
| yes | 0.009 * | 0.033 | 0.003 | 0.429 | ||
| Trichostrongylus spp. | (Intercept) | 0.88 | 1.155 | 0.179 | 7.445 | |
| Market products at fairs | sometimes | reference | ||||
| frequently | 0.599 | 0.381 | 0.01 | 13.934 | ||
| no | 0.026 * | 0.073 | 0.007 | 0.733 | ||
| Producer applies treatments | no | reference | ||||
| yes | 0.066 | 5.165 | 0.898 | 29.712 | ||
| Capillaria spp. | (Intercept) | 0.347 | 2.931 | 0.312 | 27.5 | |
| Presence of Eimeria spp. | no | reference | ||||
| yes | 0.047 * | 9.727 | 1.029 | 91.993 | ||
| Access restriction | control | reference | ||||
| no control | 0.018 * | 0.026 | 0.001 | 0.542 | ||
| Medicines | no | reference | ||||
| yes | 0.097 | 3.21 | 0.809 | 12.741 | ||
| Ascaridia galli | (Intercept) | 0.01 | 22.702 | 2.137 | 241.137 | |
| Water | no potable | reference | ||||
| potable | 0.036 * | 0.221 | 0.054 | 0.905 | ||
| Street sale of products | frequently | reference | ||||
| no | 0.021 * | 0.086 | 0.011 | 0.691 | ||
| Adult person handles the birds | no | reference | ||||
| yes | 0.069 | 0.18 | 0.028 | 0.988 | ||
| Parasite Specie | Cluster nº | Coordinates | Radius (km) | Relative Risk (RR) | p-Value | |
|---|---|---|---|---|---|---|
| Latitude | Longitude | |||||
| Eimeria spp. | 1 | −34.177200 | −71.398790 | 41.29 | 2.60 | <0.0001 * |
| Heterakis gallinarum | 1 | −33.612541 | −70.929228 | 3.48 | 4.29 | 0.269 |
| 2 | −34.301510 | −71.398110 | 13.81 | 5.44 | 0.828 | |
| Trichostrongylus spp. | 1 | −34.158290 | −71.416580 | 37.3 | 5.85 | 0.050 |
| Capillaria spp. | 1 | −33.146020 | −70.797880 | 127.21 | 1.95 | 0.940 |
| Ascaridia galli | 1 | −34.362870 | −71.738540 | 10.33 | 2.93 | 0.021 * |
| 2 | −33.151530 | −70.888650 | 8.27 | 2.53 | 0.912 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantin-Rosas, B.; Tomazic, M.L.; Rodríguez, A.E.; Enciso, N.; Brante-Bernier, J.; Honores, P.; Godoy-Alfaro, C.; Abarca, C.; Alegría-Morán, R.; Ramirez-Toloza, G. Risk Factors and Spatial Distribution of Gastrointestinal Parasites in Backyard Poultry Production Systems in Central Chile. Vet. Sci. 2025, 12, 448. https://doi.org/10.3390/vetsci12050448
Cantin-Rosas B, Tomazic ML, Rodríguez AE, Enciso N, Brante-Bernier J, Honores P, Godoy-Alfaro C, Abarca C, Alegría-Morán R, Ramirez-Toloza G. Risk Factors and Spatial Distribution of Gastrointestinal Parasites in Backyard Poultry Production Systems in Central Chile. Veterinary Sciences. 2025; 12(5):448. https://doi.org/10.3390/vetsci12050448
Chicago/Turabian StyleCantin-Rosas, Bruno, Mariela Luján Tomazic, Anabel Elisa Rodríguez, Nikita Enciso, Juliette Brante-Bernier, Patricia Honores, Catalina Godoy-Alfaro, Claudio Abarca, Raúl Alegría-Morán, and Galia Ramirez-Toloza. 2025. "Risk Factors and Spatial Distribution of Gastrointestinal Parasites in Backyard Poultry Production Systems in Central Chile" Veterinary Sciences 12, no. 5: 448. https://doi.org/10.3390/vetsci12050448
APA StyleCantin-Rosas, B., Tomazic, M. L., Rodríguez, A. E., Enciso, N., Brante-Bernier, J., Honores, P., Godoy-Alfaro, C., Abarca, C., Alegría-Morán, R., & Ramirez-Toloza, G. (2025). Risk Factors and Spatial Distribution of Gastrointestinal Parasites in Backyard Poultry Production Systems in Central Chile. Veterinary Sciences, 12(5), 448. https://doi.org/10.3390/vetsci12050448










