First Report on the Molecular Detection and Characterization of Rickettsia felis in Laelapidae (Acari: Mesostigmata) Mites in Malaysia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
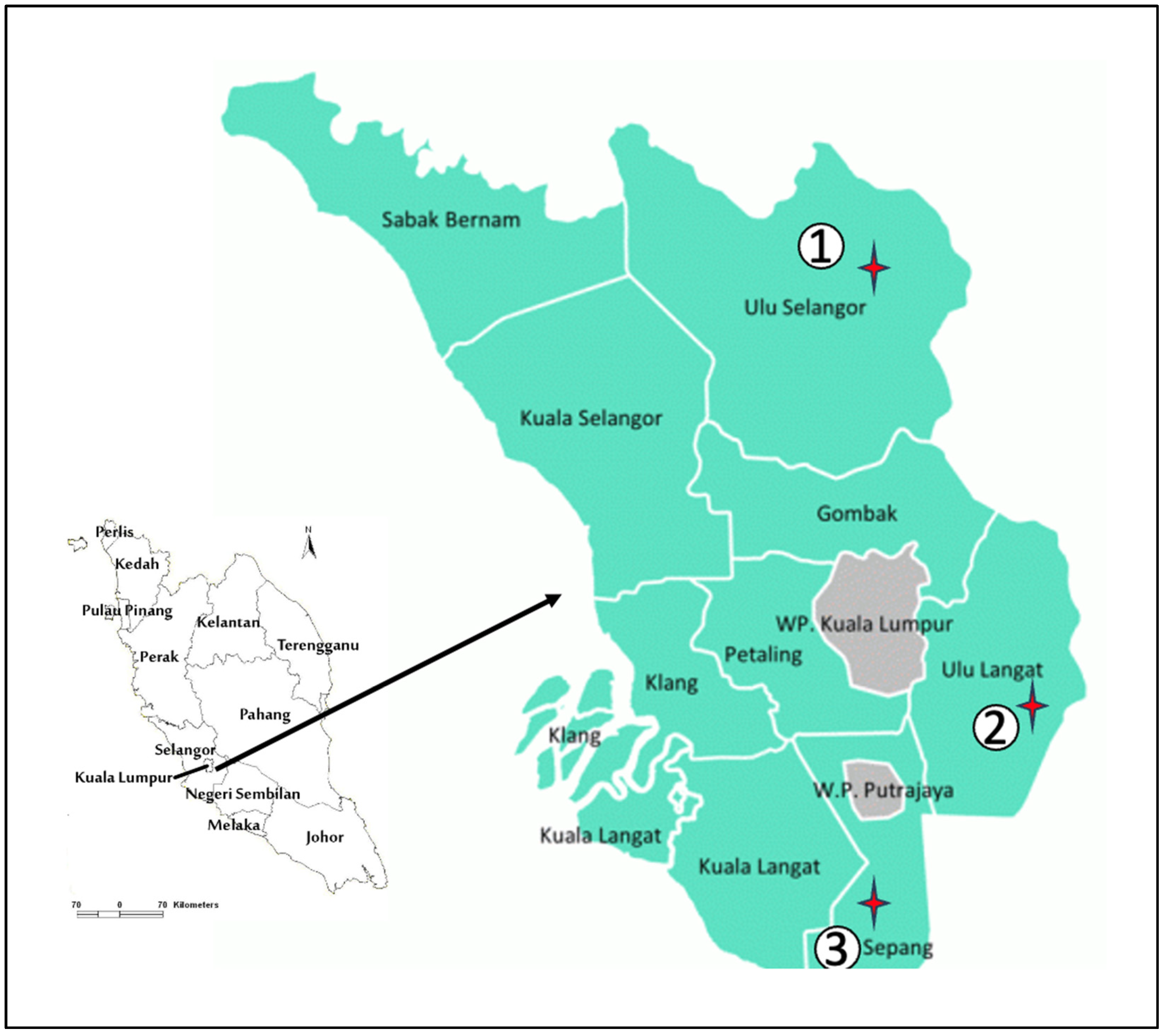
2.1. Study Area
2.2. Trapping of Small Mammals and Collection of Mites
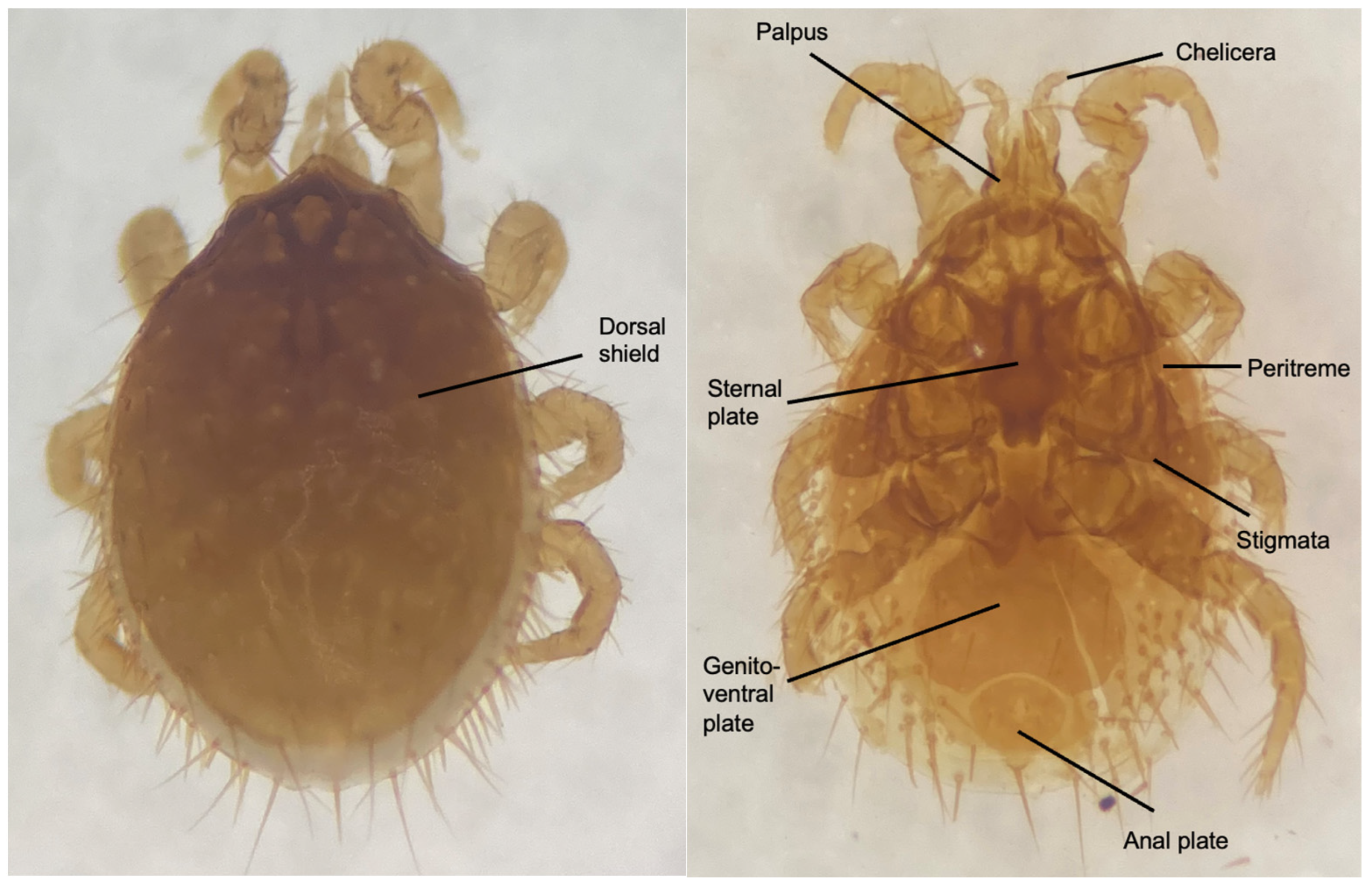
2.3. Morphological Identification of Mesostigmatid Mites
2.4. DNA Extraction of Mesostigmatid Mites
2.5. DNA Extraction from Blood
2.6. DNA Extraction from Animal Tissue
2.7. Detection of Rickettsia spp. Via Nested PCR
2.8. DNA Sequencing and Phylogenetic Analysis
2.9. Data Analyses
2.10. Statistical Analysis
3. Results
3.1. Infestation Rate of Small Mammals with Mesostigmatid Mites
3.2. Morphological Characterization
3.3. Prevalence of Rickettsia spp. in Mesostigmatid Mites and Small Mammals
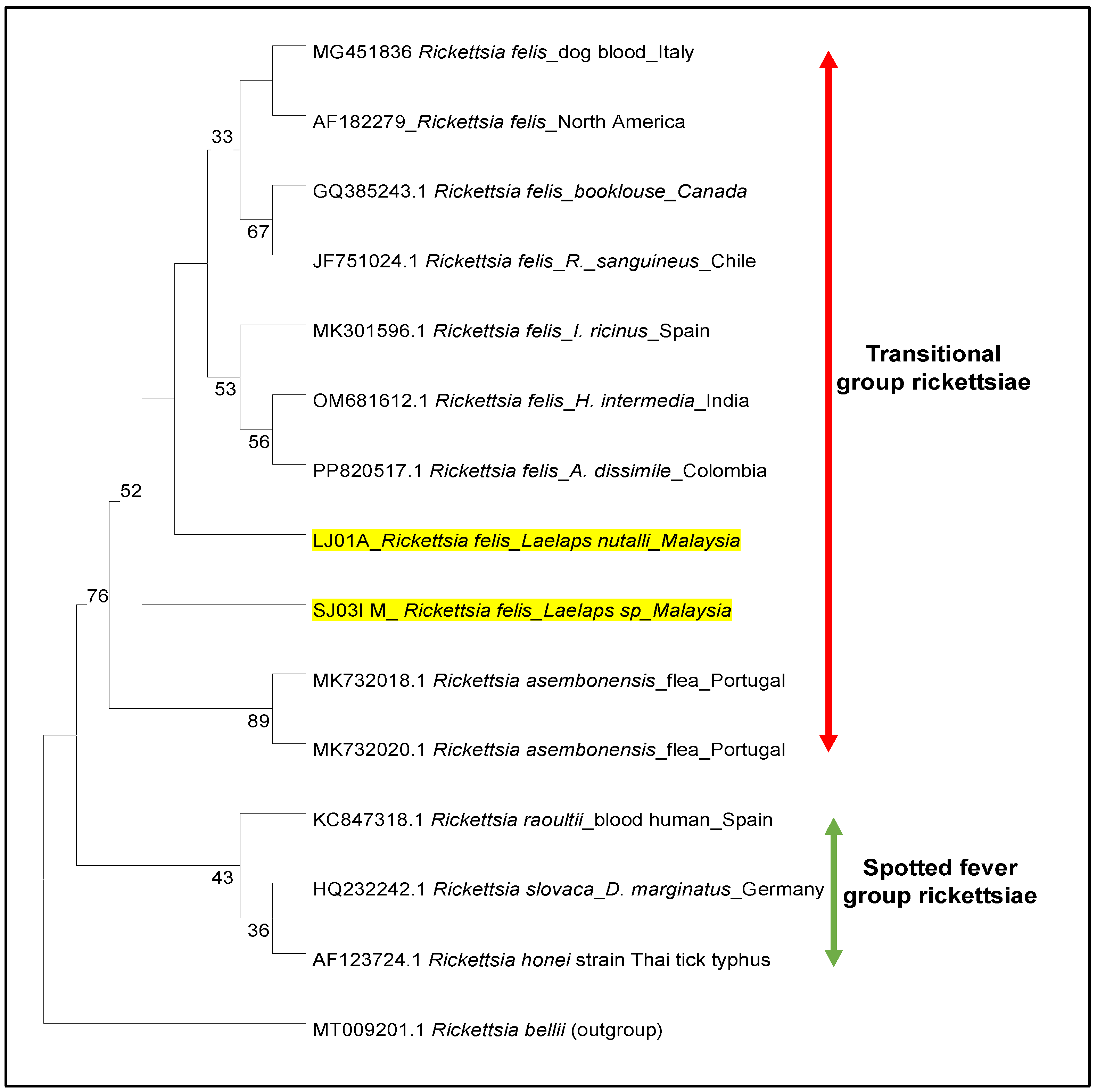
3.4. Phylogeny of Rickettsia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlgren, F.S.; Heitman, K.N.; Drexler, N.A.; Massung, R.F.; Behravesh, C.B. Human granulocytic anaplasmosis in the United States from 2008 to 2012: A summary of national surveillance data. Am. J. Trop. Med. Hyg. 2015, 93, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.J.; William, T.; Clemens, E.G.; Patel, K.; Chandna, A.; Wilkes, C.S.; Barber, B.E.; Anstey, N.M.; Dumler, J.S.; Yeo, T.W.; et al. Rickettsioses as major etiologies of unrecognized acute febrile illness, Sabah, East Malaysia. Emerg. Infect. Dis. 2020, 26, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Snowden, K.F.; Ketzis, J.K. 118—Trematodes. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2021; pp. 1528–1549. [Google Scholar] [CrossRef]
- Bang, M.S.; Kim, C.M.; Park, J.W.; Chung, J.K.; Kim, D.M.; Yun, N.R. Prevalence of Orientia tsutsugamushi, Anaplasma phagocytophilum and Leptospira interrogans in striped field mice in Gwangju, Republic of Korea. PLoS ONE 2019, 14, e0215526. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Devamani, C.S.; Prakash, J.A.J.; Alexander, N.; Stenos, J.; Schmidt, W.P. The incidence of Orientia tsutsugamushi infection in rural South India. Epidemiol. Infect. 2022, 150, e132. [Google Scholar] [CrossRef]
- Acestor, N.; Cooksey, R.; Newton, P.N.; Ménard, D.; Guerin, P.J.; Nakagawa, J.; Christophel, E.; González, I.J.; Bell, D. Mapping the Aetiology of Non-Malarial Febrile Illness in Southeast Asia through a Systematic Review-Terra Incognita Impairing Treatment Policies. PLoS ONE 2012, 7, e44269. [Google Scholar] [CrossRef]
- Radzijevskaja, J.; Kaminskiene, E.; Lipatova, I.; Mardosaite-Busaitiene, D.; Balčiauskas, L.; Stanko, M.; Paulauskas, A. Prevalence and diversity of Rickettsia species in ectoparasites collected from small rodents in Lithuania. Parasites Vectors 2018, 11, 375. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, N.; Urakami, H.; Miyamura, S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 589–591. [Google Scholar] [CrossRef]
- Minahan, N.T.; Wu, W.J.; Tsai, K.H. Rickettsia felis is an emerging human pathogen associated with cat fleas: A review of findings in Taiwan. J. Microbiol. Immunol. Infect. 2023, 56, 10–19. [Google Scholar] [CrossRef]
- Reif, K.E.; Macaluso, K.R. Ecology of Rickettsia felis: A Review. J. Med. Entomol. 2009, 46, 723–736. Available online: https://academic.oup.com/jme/article/46/4/723/937863 (accessed on 20 December 2024). [CrossRef]
- Abdad, M.Y.; Stenos, J.; Graves, S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg. Health Threat. J. 2011, 4, 7168. [Google Scholar] [CrossRef]
- Kho, K.L.; Tan, P.E.; Tay, S.T. Diversity of Rickettsiae in Feeding and Questing Ticks Collected from a Malaysian Forest Reserve Area. J. Med. Entomol. 2019, 56, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, A.; Seniczak, S.; Starý, J.; Kaczmarek, S.; Jordal, B.H.; Kowalski, J.; Roth, S.; Djursvoll, P.; Bolger, T. High diversity of mites (Acari: Oribatida, Mesostigmata) supports the high conservation value of a broadleaf forest in Eastern Norway. Forests 2021, 12, 1098. [Google Scholar] [CrossRef]
- Hoy, M.A. The predatory mite Metaseiulus occidentalis: Mitey small and mitey large genomes. BioEssays 2009, 31, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Farahi, S.; Shishehbor, P.; Nemati, A.; Perotti, M.A. Mesostigmata diversity by manure type: A reference study and new datasets from southwestern Iran. Exp. Appl. Acarol. 2022, 86, 517–534. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lee, P.L.; Wang, H.C. Molecular detection of Rickettsia species and host associations of Laelaps mites (Acari: Laelapidae) in Taiwan. Exp. Appl. Acarol. 2020, 81, 547–559. [Google Scholar] [CrossRef]
- Tay, S.T.; Ho, T.M.; Rohani, M.Y.; Devi, S. Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 280–284. [Google Scholar] [CrossRef]
- Kho, K.L.; Koh, F.X.; Hasan, L.I.M.; Wong, L.P.; Kisomi, M.G.; Bulgiba, A.; Nizam, Q.N.H.; Tay, S.T. Rickettsial seropositivity in the indigenous community and animal farm workers, and vector surveillance in Peninsular Malaysia. Emerg. Microbes Infect. 2017, 6, e18–e19. [Google Scholar] [CrossRef]
- Tappe, D.; Gross, Y.; Ngui, R.; Rauch, J.; Tay, S.T.; Lim, Y.A.L. High seroprevalence against typhus group and spotted fever group rickettsiae in rural indigenous populations of peninsular Malaysia. Vector-Borne Zoonotic Dis. 2019, 19, 323–327. [Google Scholar] [CrossRef]
- Medway, L. The Wild Mammals of Malaya (Peninsular Malaysia and Singapore); Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Francis, C.M. Field Guide to the Mammals of South-East Asia, 2nd ed.; Bloomsbury Wildl: London, UK, 2019; p. 416. [Google Scholar]
- Payne, J.; Francis, C.M.; Phillips, K. A Field Guide to the Mammals of Borneo; Sabah Society: Kota Kinabalu, Malaysia, 2005; p. 332. [Google Scholar]
- Savchenko, E.; Lareschi, M. A new species of Laelaps Koch, 1836 (Mesostigmata: Laelapidae) parasitic of the sigmodontine rodent Oligoryzomys flavescens Waterhouse, 1837 (Rodentia: Cricetidae): Molecular and morphological characterization. Acta Trop. 2019, 199, 105146. [Google Scholar] [CrossRef]
- Nadchatram, M.; Dohany, A.L. A pictorial key to the subfamilies, genera and subgenera of Southeast Asian chiggers. Bull. Inst. Med. Res. Malays. 1974, 16, 1–67. [Google Scholar]
- Chien, K.C.; Shu, P.Y.; Mu, J.J.; Wang, H.C. High Prevalence of Rickettsia spp. Infections in Small Mammals in Taiwan. Vector-Borne Zoonotic Dis. 2015, 15, 13–20. [Google Scholar]
- Mohd-Taib, F.S.; Asyikha, R.; Nor, S.M. Small mammal assemblages and their ectoparasite prevalence (Acarina) in mangrove forests of Peninsular Malaysia. Trop. Zool. 2021, 34, 24–43. [Google Scholar] [CrossRef]
- Ernieenor, F.C.L.; NorJaiza, M.J.; Fadillah, A.; Canedy, J.; Mariana, A. Screening and Genotyping of Orientia tsutsugamushi from Field-Collected on-Host Chiggers (Acari: Prostigmata) Recovered from a Positive Scrub Typhus Locality in Kelantan, Malaysia. Exp. Appl. Acarol. 2021, 84, 171–182. [Google Scholar] [CrossRef]
- Shilereyo, M.T.; Magige, F.J.; Ogutu, J.O.; Røskaft, E. Small-mammal abundance and species diversity: Land use and seasonal influences in the Serengeti Ecosystem, Tanzania. Front. Conserv. Sci. 2023, 4, 981424. [Google Scholar] [CrossRef]
- Cao, C.; Shuai, L.Y.; Xin, X.P.; Liu, Z.T.; Song, Y.L.; Zeng, Z.G. Effects of cattle grazing on small mammal communities in the Hulunber meadow steppe. PeerJ 2016, 2016, e2349. [Google Scholar] [CrossRef]
- Shilereyo, M.T.; Magige, F.J.; Ogutu, J.O.; Røskaft, E. Land use and habitat selection by small mammals in the Tanzanian Greater Serengeti Ecosystem. Glob. Ecol. Conserv. 2021, 27, e01606. [Google Scholar] [CrossRef]
- Reeves, W.K.; Dowling AP, G.; Dasch, G.A. Rickettsial agents from parasitic Dermanyssoidea (Acari: Mesostigmata). Exp. Appl. Acarol. 2006, 38, 181–188. [Google Scholar] [CrossRef]
- Park, J.W.; Chung, J.K.; Kim, S.H.; Cho, S.J.; Ha, Y.D.; Jung, S.H.; Park, H.J.; Song, H.J.; Lee, J.Y.; Kim, D.M.; et al. Seroepidemiological Survey of Zoonotic Diseases in Small Mammals with PCR Detection of Orientia tsutsugamushi in Chiggers, Gwangju, Korea. Korean J. Parasitol. 2016, 54, 307–313. [Google Scholar] [CrossRef]
- Ishak, S.N.; Shiang, L.F.; Jing, K.J.; Mohd-Taib, F.S. Screening of Rickettsia sp. In ticks (acari: Ixodidae) collected from small mammals in three recreational forests in Selangor, Malaysia. Serangga 2019, 24, 116–125. [Google Scholar]
- Shilereyo, M.; Magige, F.; Ranke, P.S.; Ogutu, J.O.; Røskaft, E. Ectoparasite load of small mammals in the Serengeti Ecosystem: Effects of land use, season, host species, age, sex and breeding status. Parasitol. Res. 2022, 121, 823–838. [Google Scholar] [CrossRef]
- Changbunjong, T.; Weluwanarak, T.; Chamsai, T.; Sedwisai, P.; Ngamloephochit, S.; Suwanpakdee, S.; Yongyuttawichai, P.; Wiratsudakul, A.; Chaichoun, K.; Ratanakorn, P. Occurrence of ectoparasites on rodents in Sukhothai Province, northern Thailand. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1324. [Google Scholar] [PubMed]
- Razali, N.B.; Shamsudin, N.; Mohd Jobran, R.A.; Yaakop, S.; Khoo, J.J.; Mohd Taib, F.S. Ectoparasites (ticks and mites) prevalence on small to medium-sized mammals associated with habitat condition in Kemasul, Pahang. Serangga 2018, 23, 72–88. [Google Scholar]
- Burhanuddin, M.; Noor, H.M.; Salim, H.; Asrif, N.A.; Jamian, S.; Azhar, B. Bait Preferences and Morphology of The Greater Bandicoot Rat Bandicota indica in a Ricefield, Kedah, Malaysia. Agric. Rep. 2023, 2, 1–11. [Google Scholar]
- Madinah, A.; Abang, F.; Ahamad, M.; Tajuddin Abdullah, M. Ectoparasites of small mammals in four localities of wildlife reserves in Peninsular Malaysia. Southeast Asian J. Trop. Med. Public Health 2011, 42, 803. Available online: https://www.researchgate.net/publication/221800392 (accessed on 15 October 2024).
- Madinah, A.; Abang, F.; Mariana, A.; Abdullah, M.T.; Mohd-Azlan, J. Interaction of ectoparasites-small mammals in tropical rainforest of Malaysia. Community Ecol. 2014, 15, 113–120. [Google Scholar] [CrossRef]
- Mohd-Azami, S.N.I.; Loong, S.K.; Khoo, J.J.; Husin, N.A.; Lim, F.S.; Mahfodz, N.H.; Ishak, S.N.; Mohd-Taib, F.S.; Makepeace, B.L.; AbuBakar, S. Molecular Surveillance for Vector-Borne Bacteria in Rodents and Tree Shrews of Peninsular Malaysia Oil Palm Plantations. Trop. Med. Infect. Dis. 2023, 8, 74. [Google Scholar] [CrossRef]
- Tay, S.T.; Rohani, M.Y.; Devi, S. Isolation and PCR detection of rickettsiae from clinical and rodent samples in Malaysia. Southeast Asian J. Trop. Med. Public Health 2002, 33, 772–779. [Google Scholar] [PubMed]
- Chareonviriyaphap, T.; Leepitakrat, W.; Lerdthusnee, K.; Chao, C.C.; Ching, W.M. Dual exposure of Rickettsia typhi and Orientia tsutsugamushi in the field-collected Rattus rodents from Thailand. J. Vector Ecol. 2014, 39, 182–189. [Google Scholar] [CrossRef]
- Ibrahim, I.N.; Okabayashi, T.; Ristiyanto; Lestari, E.W.; Yanase, T.; Muramatsu, Y.; Ueno, H.; Morita, C. Serosurvey of wild rodents for Rickettsioses (spotted fever, murine typhus and Q fever) in Java Island, Indonesia. Eur. J. Epidemiol. 1999, 15, 89–93. [Google Scholar] [CrossRef]
- Rungrojn, A.; Chaisiri, K.; Paladsing, Y.; Morand, S.; Junjhon, J.; Blacksell, S.D.; Ekchariyawat, P. Prevalence and molecular characterization of Rickettsia spp. from wild small mammals in public parks and urban areas of Bangkok metropolitan, Thailand. Trop. Med. Infect. Dis. 2021, 6, 199. [Google Scholar] [CrossRef]
- Mardosaitė-Busaitienė, D.; Radzijevskaja, J.; Balčiauskas, L.; Paulauskas, A. First detection of Rickettsia helvetica in small mammals in Lithuania. New Microbes New Infect. 2018, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.T.; Mokhtar, A.S.; Low, K.C.; Mohd Zain, S.N.; Jeffery, J.; Abdul Aziz, N.; Kho, K.L. Identification of rickettsiae from wild rats and cat fleas in Malaysia. Med. Vet. Entomol. 2014, 28 (Suppl. 1), 104–108. [Google Scholar] [CrossRef] [PubMed]
- Phomjareet, S.; Chaveerach, P.; Suksawat, F.; Jiang, J.; Richards, A.L. Spotted fever group Rickettsia infection of cats and cat fleas in Northeast Thailand. Vector-Borne Zoonotic Dis. 2020, 20, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Chaorattanakawee, S.; Tachavarong, W.; Hananantachai, H.; Bunsermyos, W.; Chanarat, N.; Promsathaporn, S.; Tippayachai, B.; Sakolvaree, J.; Pitaksajjakul, P.; Benjathummarak, S.; et al. Seasonal pattern of questing ticks and prevalence of pathogenic Rickettsia and Anaplasmataceae in Khao Yai National Park, Thailand. Travel Med. Infect. Dis. 2024, 58, 102696. [Google Scholar] [CrossRef]
- Prasetyo, D.B.; Fiorenzano, J.M.; Nop, D.; Noch, N.; Huot, B.; Mom, S.; Prum, S.; Chhe, V.; Dul, S.; Heang, V.; et al. Molecular detection of Rickettsia species in ectoparasites collected from two southern provinces of Cambodia. PLoS Neglected Trop. Dis. 2024, 18, e0012544. [Google Scholar] [CrossRef]
- Alkathiry, H.; Al-Rofaai, A.; Ya’cob, Z.; Cutmore, T.S.; Mohd-Azami, S.N.I.; Husin, N.A.; Lim, F.S.; Koosakulnirand, S.; Mahfodz, N.H.; Ishak, S.N.; et al. Habitat and season drive chigger mite diversity and abundance on small mammals in Peninsular Malaysia. Pathogens 2022, 11, 1087. [Google Scholar] [CrossRef]
| Locality | Host Species (n Examined) | Mite Species | No. of Hosts Infested (%) | Total No. of Individual Mites | Infestation Rate (%) | Mean Intensity |
|---|---|---|---|---|---|---|
| Sungai Kedondong (SK) | Maxomys whiteheadi (3) | Laelaps spp. | 3 (7.32) | 126 | 7.32 | 42 |
| Bandicota indica (2) | Laelaps spp. | 2 (4.88) | 201 | 4.88 | 100.5 | |
| Rattus tiomanicus (1) | - | 0 (0) | 0 | 0 | ||
| Total | 6 | 5 (12.20) | 327 | 12.20 | ||
| Beranang (BR) | Rattus tiomanicus (13) | Laelaps spp. | 2 (4.88) | 26 | 4.88 | 13 |
| Rattus rattus (1) | - | 0 (0) | ||||
| Tupaia glis (7) | - | 0 (0) | ||||
| Total | 21 | 2 (4.88) | 26 | 4.88 | ||
| Bagan Lalang (BL) | Rattus tiomanicus (1) | 0 (0) | 0 | 0 | ||
| Rattus rattus (7) | Laelaps spp. | 2 (4.88) | 10 | 4.88 | 5 | |
| Tupaia glis (6) | - | 0 (0) | 0 | 0 | ||
| Total | 14 | 2 (4.88) | 10 | 4.88 | ||
| Total location | 41 | 9 (21.95) | 363 | 21.96 |
| Sample Type | No. of Samples/Pools Examined | Target Gene | No. of Positives | Positivity Rate (%) | |
|---|---|---|---|---|---|
| gltA | ompB | ||||
| Blood | 36 | 0 | 0 | 0 | 0 (0/36) |
| Spleen | 41 | 0 | 0 | 0 | 0 (0/41) |
| Kidney | 41 | 0 | 0 | 0 | 0 (0/41) |
| Liver | 41 | 0 | 0 | 0 | 0 (0/41) |
| Laelaps spp. | 60 | 0 | 2 | 2 | 3.33 (2/60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Tahir, H.; Ernieenor, F.C.L.; Abidin, S.Z.; Narainasamy, V.V.; Ahamad, M. First Report on the Molecular Detection and Characterization of Rickettsia felis in Laelapidae (Acari: Mesostigmata) Mites in Malaysia. Vet. Sci. 2025, 12, 443. https://doi.org/10.3390/vetsci12050443
Mohamad Tahir H, Ernieenor FCL, Abidin SZ, Narainasamy VV, Ahamad M. First Report on the Molecular Detection and Characterization of Rickettsia felis in Laelapidae (Acari: Mesostigmata) Mites in Malaysia. Veterinary Sciences. 2025; 12(5):443. https://doi.org/10.3390/vetsci12050443
Chicago/Turabian StyleMohamad Tahir, Hiryahafira, Faraliana Che Lah Ernieenor, Suhaili Zainal Abidin, Vishalani Vishnu Narainasamy, and Mariana Ahamad. 2025. "First Report on the Molecular Detection and Characterization of Rickettsia felis in Laelapidae (Acari: Mesostigmata) Mites in Malaysia" Veterinary Sciences 12, no. 5: 443. https://doi.org/10.3390/vetsci12050443
APA StyleMohamad Tahir, H., Ernieenor, F. C. L., Abidin, S. Z., Narainasamy, V. V., & Ahamad, M. (2025). First Report on the Molecular Detection and Characterization of Rickettsia felis in Laelapidae (Acari: Mesostigmata) Mites in Malaysia. Veterinary Sciences, 12(5), 443. https://doi.org/10.3390/vetsci12050443







