1. Introduction
Since the 1950s, antibiotics have been added to animal feed for the purpose of improving the nutritional status of animals and promoting growth [
1]. However, the abuse of antibiotics has led to the emergence of drug resistance [
2], which is a threat to human and animal health and the environment. Many countries have banned low-dose antibiotics as growth promoters in livestock feed, accelerating research into suitable natural alternatives with similar or better beneficial effects. As consumers pay more and more attention to food quality and safety, the market needs to provide higher quality pork to meet the needs of consumers, and at the same time, improving the quality of meat through nutritional adjustment or changing the feed composition is one of the hot spots of pig nutrition research [
3].
Mallotus oblongifolius is a genus of Euphorbia, mainly distributed in Hainan Island.
Mallotus oblongifolius is rich in various nutrients, carbohydrates, fat, crude protein, and offers high feeding value [
4]. The total free amino acids account for 0.2%, mainly containing proline, alanine, asparagine, glutamine, and so on [
5]. The leaves of
Mallotus oblongifolius are consumed as herbal tea for clearing heat, detoxification, anti-oxidation, and digestive aid, as well as being a popular health drink [
6,
7,
8]. The main components of
Mallotus oblongifolius include polysaccharides, polyphenols, saponins, and pigments [
7,
9], which contribute to the health benefits of
Mallotus oblongifolius. It was found that with the reduction in particle size, the polysaccharide content in the powder was increased, and the polyphenols were decreased [
10,
11]. Polysaccharide has the biological activities of lowering blood sugar, reducing blood lipids, anti-oxidation, improving intestinal flora, and so on. Ultrafine grinding technology emerged in the 1980s; by the use of mechanical and fluid forces, the material particles are ground to a particle size of 1~10 μm using fine grinding technology [
10]. Compared with coarse powder, micro powder products have uniform texture, strong fluidity and adsorption, huge specific surface area and porosity, strong chemical reactivity, high solubility, and other physical and chemical properties [
12,
13]. After ultramicro-grinding, the active ingredients of
Mallotus oblongifolius are fully exposed to improve bioavailability and enhance efficacy [
11]. Research suggests MO has beneficial components and potential antioxidant and resistance roles. However, its effects on pigs remain unexplored. Thus, we included the ultrafine powder derived from
Mallotus oblongifolius (MOUP) in the feed to study its impact on the growth and meat quality of Hainan pigs during the late fattening stage.
The Hainan pig is a native endemic pig breed mainly raised on Hainan Island. It has characteristics such as heat resistance, disease resistance, and good meat characteristics, and is highly favored by consumers. Nevertheless, Hainan pigs exhibit an extended feeding cycle, sluggish growth, and suboptimal feed conversion efficiency (FCR), particularly during the latter phase of fattening.
Therefore, the objective of this study was to evaluate the effects of dietary addition of MOUP on growth performance and meat quality of Hainan pigs.
2. Materials and Methods
2.1. Preparation of MOUP
Mallotus oblongifolius was purchased from Dongmen Farmers’ Market, Qiongshan District, Haikou City. The leaves of one-year-old Mallotus oblongifolius were air-dried. An ultramicro pulverizer (LWF-6B1, Jinan Longwei Pharmaceutical Equipment Co., Ltd., Jinan, China) was used to grinds the material into powder, which was then filtered via a 300-mesh screen.
2.2. Animal and Experimental Design
A total of sixty-four healthy castrated pigs (ternary hybrid pigs, Duroc × Duroc × Tunchang) with comparable initial body weight (BW, 68.06 ± 1.03 kg, 150 days old) were allocated randomly into four groups: the control group (CONT), the antibiotic group (ANTI), the 0.1% MOUP (1 g MOUP per Kg of feed) group (PT1), and the 0.5% MOUP (5 g MOUP per Kg of feed) group (PT2). There were four replicate pens per treatment with four pigs per pen. The CONT group was given the basal diet (
Table 1) based on the nutrient requirements for swine (NRC, 2012), the ANTI group was fed the basal diet supplemented with 300 mg/kg colistin sulfate (180122757, Best Biological technology institute Co., Ltd., Changsha, China), the PT1 group was fed the basal diet supplemented with 0.1% MOUP, and the PT2 group was fed the basal diet supplemented with 0.5% MOUP. The pre-test lasted for 7 days and the formal test lasted for 70 days. The temperature of the pig house was maintained at 25–30 °C and the humidity at 60~70%. Pigs were given ad libitum access to water. During the period, the experimental pigs were fed three times a day in the morning, mid-day, and evening, and their feed intake was recorded. Disinfection and immunization were carried out according to the routine procedures. Euthanasia, necropsy, tissue sample collection, and processing pigs were euthanized with a lethal overdose of euthanal (euthanasia solution, pentobarbital sodium, and phenytoin sodium). Experimental pigs were purchased from Tunchang Tianzhihong Ecological Agriculture and Animal Husbandry Co., Ltd., Tunchang, China. The animal test was approved by the Animal Conservation and Use Committee of Hainan Academy of Agricultural Sciences (HNSYY20230203).
2.3. Slaughter and Sample Collection
At the end of the experiment period, the pigs were fasted for 12 h, and individually weighed. Pig slaughtering followed the slaughter slab procedures [
14]. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion efficiency (FCR) were calculated based on body weight and feed intake. Blood was collected from the anterior vena cava and the serum was prepared by tilting at room temperature for 30 min, centrifugation at 3000 r/min for 15 min, and stored in a −20° refrigerator for test. Six pigs from each treatment were randomly selected and slaughtered (at least 1 in each replicate). After slaughtering the pigs, the head, hooves, tail, and offal were completely removed and the hot carcass weight was recorded to calculate the slaughter rate. The longissimus thoracis (LT) muscles from the left carcass side between the 9th and 10th ribs were collected immediately after slaughter and stored at −80 °C until analysis.
2.4. Carcass Characteristics and Meat Quality
The mean backfat was measured on the first rib, last rib, and last lumbar vertebrae in the midline using a sliding caliper. The left side of the carcass was split at the 10th rib to determine the 10th-rib backfat thickness and longissimus muscle area. Based on the National Pork Producers Council, pH and flesh color CIELAB values (L*, a* and b*) of LT were determined at 45 min postmortem. A portable pH meter (FE28, METTLER TOLEDO, Shanghai, China) and chromatic aberration meter (TS7700, Sanen Shi, Shenzhen, China) were used. To determine drip loss, the meat core was weighed and tied with cotton thread (diameter 25 mm), then suspended in a sealed triangle bottle without contact to store at 4 °C for 24 h before weighing. Shear forces were determined as follows: Approximately 250 g LT per pig, refrigerated at 4 °C for 48 h, was boiled in 80 °C water to an internal temperature of 70 °C, then cooled to 25 °C. A muscle tenderness instrument (C-LM3B, Northeast Agricultural University, Heilongjiang, China) was used to cut 10 cylindrical cores (1 cm in diameter × 1 m in length) of a mature LT sample perpendicular to the direction of the muscle fibers. The average peak shear force in Newtons (N) was recorded.
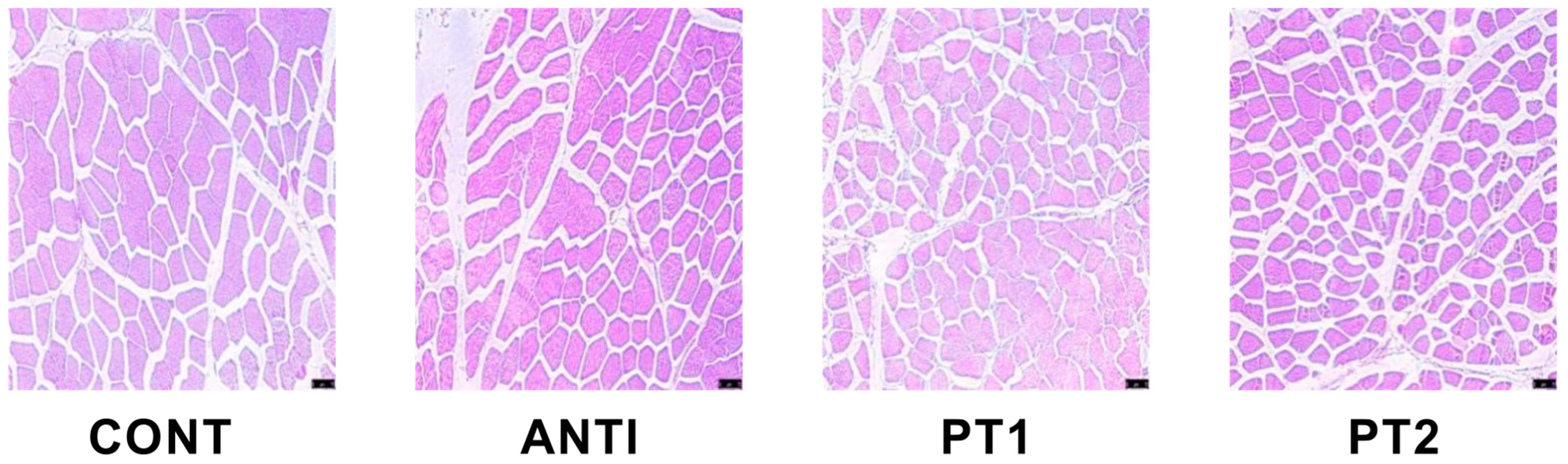
2.5. Histochemical Staining
After removing the grease and fascia from the surface, 2 cm longissimus dorsi muscle of the slaughtered pig was placed in a centrifuge tube filled with 4% paraformaldehyde solution to completely soak and fix the shape of muscle fibers. Wuhan Xyever Biotechnology Co, Ltd. (Wuhan, China). was commissioned to perform HE stain sealing. Five samples were randomly chosen from each group, and three images from each sample were analyzed using Image J software (1.30v).
2.6. Antioxidant Indices of Muscle
The total antioxidant capacity (A015-2-1), superoxide dismutase (A001-3-2), glutathione peroxidase (A005-1-2), and malondialdehyde (A003-1-2) contents of muscle were determined using the kit produced by Nanjing Jiangcheng Institute of Bioengineering according to the kit instructions.
2.7. Distribution of Amino Acids in Longissimus Pectoralis Muscle
The amino acid content in muscle was detected according to the determination method of amino acid in food (GB 5009.124-2016, China). The meat sample was freeze-dried and crushed for detection. The sample of 1 g longissimus dorsi muscle was placed in a 10 mL HCl (6 mol/L) hydrolyzed tube, sealed with nitrogen for three times after freezing, and placed in a constant temperature drying oven. Hydrolysis at 110 °C for 22 h to 24 h was performed. After cooling, the hydrolysate was transferred and 0.02 mol/L HCl was used in a 50 mL flask. After mixing, 1 mL solution was transferred to a 15 mL test tube and evaporated in a 65 °C water bath until dry. The sample was dissolved with 2 mL HCl (0.02 mol/L), filtered (0.22 μm), transferred to the instrument sample bottle, and determined by an amino acid automatic analyzer L-8900. Guangzhou Jinzhi Detection Technology Co., Ltd. (Guangzhou, China) was commissioned to carry out the analysis.
2.8. Statistical Analysis
The data collected from all experiments (n = 6) were initially summarized using Excel software, followed by analysis using one-way ANOVA conducted with SPSS 20 software (International Business Machines Co., Armonk, NY, USA). All the table data are expressed as means ± SD, and the figure data are expressed as means ± SEM. p-values < 0.05 were considered significant.
4. Discussion
The economic value of livestock and poultry is closely associated with the quality of meat, and consumers’ purchasing decisions are directly influenced by the color of the meat. The pH value and meat color serve as crucial indicators of meat freshness. The pH 45 min value provides insights into the acidity and alkalinity of the pork itself, while the pH 24 h value indicates the rate at which the pork’s acidity and alkalinity decline [
15]. Typically, within 45 min of slaughter, the muscle pH is lowered from 6.3 to 6.7, and the muscle color and hydration rate remain within normal levels [
16]. However, when the pH is lowered to 5.5, particularly under high-temperature conditions, the muscle undergoes protein deterioration, exudation increase, color paleness, and a change in texture, resulting in the formation of white muscle (PSE) meat [
17]. In the present study, the inclusion of 0.1% and 0.5% MOUP in the diet resulted in an improvement in the L* value and a decrease in the change in pH value. Notably, the inclusion of 0.5% MOUP in the diet also led to a reduction in shear force, which is an important indicator of muscle tenderness. Shear force is the current evaluation index of tenderness; the greater the shear force, the older the meat, and vice versa [
18]. The potential explanation for the observed phenomenon could be attributed to the ability of tea polyphenols to augment the antioxidant activity of cell membrane lipids, thereby safeguarding their structural integrity and stability. This protective effect mitigates the degradation of muscle proteins, leading to a gradual decline in pH levels and a reduction in the surface reflectance of meat, which contribute to the enhancement of muscle lightness [
19]. Previous studies also support our results; the supplementation of tea polyphenol compound additive in the diet of finishing pigs increased the a* value and decreased the shear force [
20]. Moreover, dietary supplementation of 400 mg/kg and 500 mg/kg tea polyphenols in finishing pigs significantly decreased muscle shear force and increased the pH, L* value, a* value, and b* value [
21]. Unlike our findings, tea polyphenol can enhance the a* and b* values of meat, likely because of its different primary functional components compared to MOUP.
To conduct a more comprehensive examination of the impact of MOUP on muscular tissues, we conducted an analysis of the morphological characteristics of muscle fibers. Muscle fibers make up 70% to 90% of muscle volume, and their morphology and characteristics are key determinants of muscle mass and pork quality [
22]. In our study, feeding 0.1% and 0.5% MOUP significantly reduced the diameter and the mean area of muscle fiber. In addition, the extract derived from MO, known for its antioxidant properties, exhibited a protective effect against ethanol-induced acute gastric mucosa injury in rats [
7]. This effect was attributed to the extract’s ability to enhance the content of SOD, CAT, and GSH-Px enzymes, while concurrently reducing the levels of ROS, MDA, and MPO content [
23]. The current investigation revealed that the application of 0.5% MOUP resulted in a significant augmentation of T-AOC and GSH-Px levels. Consequently, MOUP exhibits advantageous properties in terms of enhancing the antioxidant capacity of the longissimus dorsi muscle.
Amino acids are the basic unit of protein composition, from the perspective of nutrition; the type, content, and proportion of amino acids in protein determine the nutritional value of pork [
24,
25]. Therefore, the content of amino acids in the carcass has a great relationship with the flavor and quality of meat [
26,
27]. The presence of flavor amino acids, namely aspartic acid, glutamic acid, proline, glycine, and serine, has a direct impact on the sensory perception of pork flavor [
28]. On the other hand, lysine, threonine, tryptophan, and sarcosine influence the physicochemical characteristics of muscle tissue and overall meat quality, consequently influencing the overall quality of pork [
27].
Mallotus oblongifolius is abundant in various amino acids, such as alanine, proline, asparagine, glutamine, and other indispensable amino acids [
5]. In the present study, it was observed that the inclusion of MOUP in the diet resulted in a notable augmentation of the overall amino acid composition in the longest dorsal muscle. Our study revealed that incorporating MOUP into the diet significantly changes the amino acid profile of the longest dorsal muscle. Adding 0.1% and 0.5% MOUP increased arginine, alanine, leucine, phenylalanine, tyrosine, and TAA, while decreasing proline [
25]. In particular, 0.5% MOUP also boosted glycine levels. Notably, the dosage of 0.5% exhibited the most pronounced impact. The aforementioned evidence demonstrates that the utilization of MOUP, a superfine powder, has the potential to enhance muscle flavor and augment muscle nutrient content.
Growth performance is an important manifestation of the economic value of pigs. Improving growth performance of pigs and shortening the time of putting out pigs can increase economic income. Our results showed that dietary supplementation of 0.1% and 0.5% MOUP had no significant effect on the growth performance of Hainan pigs in the late fattening stage. Articles have indicated that the inclusion of green tea or tea in the diet does not exert any discernible impact on the production performance of pigs during the latter phases of fattening [
29,
30]. Additionally, it has been documented that the inclusion of 6% green tea powder results in a decrease in ADFI, while the incorporation of 5% tea powder leads to a reduction in ADG [
31]. The reduction in polyphenol and alkaloid content in the bitter tea following ultramicro-grinding may not have had an impact on the feed intake of pigs. Moreover, the presence of anti-nutritional factors in tea powder can diminish the digestibility of dietary protein in pigs and consequently influence their weight gain [
32].
Slaughter performance is the main indicator used to measure the economic benefits of breeding. Our results showed that the supplementation of MOUP did not affect the hot carcass weight, slaughter rate, backfat thickness, fat mass, abdominal fat rate, or lean mass of pigs. However, a recent study has demonstrated that the inclusion of 2% tea powder in pig feed yields a notable increase in lean meat percentage, as well as a significant augmentation in eye muscle area and hind leg ratio [
33]. Furthermore, another investigation has revealed that the incorporation of 4% tea powder in the diet of Tibetan fragrant pigs leads to the lowest backfat thickness and fat percentage, accompanied by the highest lean meat percentage [
30]. According to the report, the inclusion of tea by-products in the diet of fattening pigs resulted in a linear decrease in backfat thickness as the quantity of tea by-products increased [
34]. Previous studies have found that tea polyphenols can affect fat deposition by regulating lipid metabolism [
35,
36]. The findings of this research exhibit incongruity with the aforementioned studies, potentially attributable to the comparatively minimal dosage employed or the specific type of tea utilized [
10].