First Specific Detection of Mammalian Orthoreovirus from Goats Using TaqMan Real-Time RT-PCR Technology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Primers and TaqMan Probe
2.2. Construction of L1 Standard Plasmid
2.3. Virus Isolation
2.4. RNA Extraction and RT-PCR Amplification
2.5. Optimization of TaqMan qRT-PCR
2.6. Method Specificity, Sensitivity and Repeatability
2.7. MRV Detection in Clinical Samples
2.8. Statistical Analysis
3. Results
3.1. Standard Curve of qRT-PCR
3.2. Repeatability of qRT-PCR
3.3. Specificity of TaqMan RT-qPCR
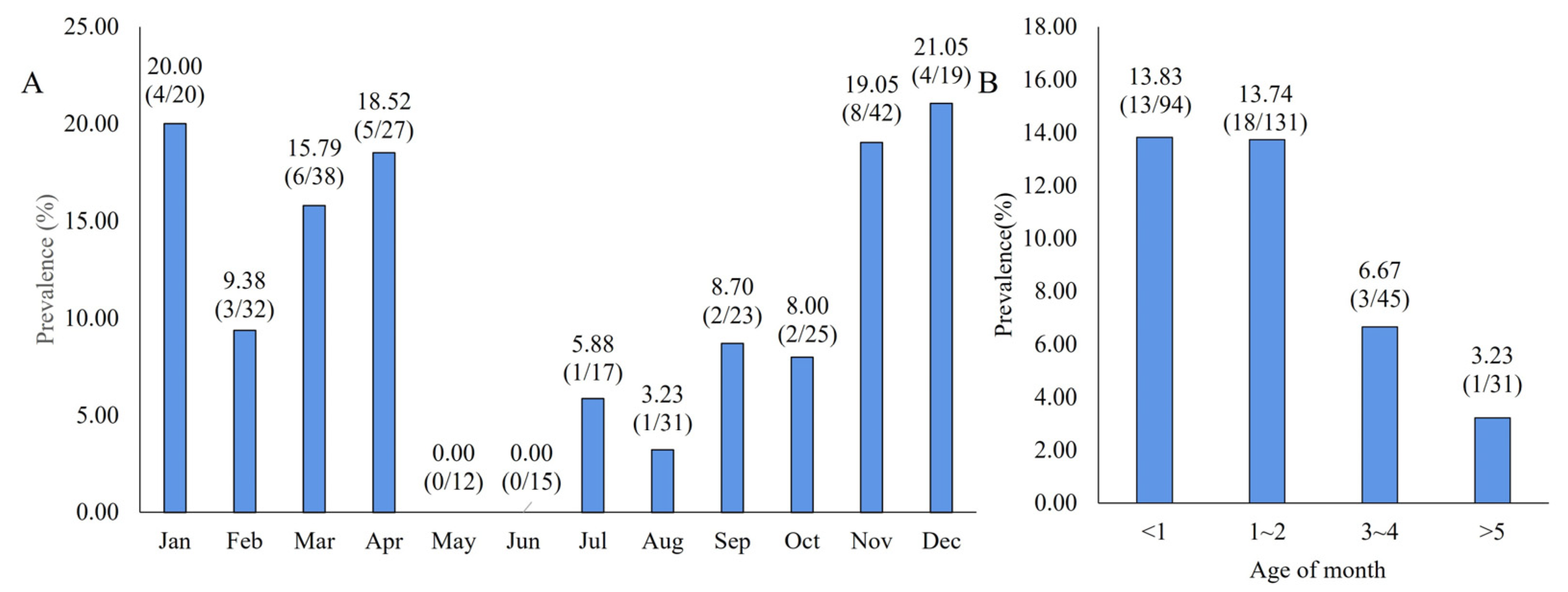
3.4. MRV Detection in Clinical Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabin, A.B. Reoviruses: A New Group of Respiratory and Enteric Viruses Formerly Classified as Echo Type 10 Is Described. Science 1959, 130, 1387–1389. [Google Scholar] [CrossRef]
- Ouattara, L.A.; Barin, F.; Barthez, M.A.; Bonnaud, B.; Roingeard, P.; Goudeau, A.; Castelnau, P.; Vernet, G.; Paranhos-Baccala, G.; Komurian-Pradel, F. Novel Human Reovirus Isolated from Children with Acute Necrotizing Encephalopathy. Emerg. Infect. Dis. 2011, 17, 1436–1444. [Google Scholar] [CrossRef]
- Thimmasandra Narayanappa, A.; Sooryanarain, H.; Deventhiran, J.; Cao, D.; Ammayappan Venkatachalam, B.; Kambiranda, D.; LeRoith, T.; Heffron, C.L.; Lindstrom, N.; Hall, K.; et al. A Novel Pathogenic Mammalian Orthoreovirus from Diarrheic Pigs and Swine Blood Meal in the United States. mBio 2015, 6, e00593-15. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Righi, F.; Filipello, V.; Trogu, T.; Lelli, D.; Bianchi, A.; Bonardi, S.; Pavoni, E.; Bertasi, B.; Lavazza, A. Mammalian Orthoreovirus (Mrv) Is Widespread in Wild Ungulates of Northern Italy. Viruses 2021, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Gutierrez-Aguire, I.; Kolenc, M.; Koren, S.; Kutnjak, D.; Pokorn, M.; Poljsak-Prijatelj, M.; Racki, N.; Ravnikar, M.; Sagadin, M.; et al. High Similarity of Novel Orthoreovirus Detected in a Child Hospitalized with Acute Gastroenteritis to Mammalian Orthoreoviruses Found in Bats in Europe. J. Clin. Microbiol. 2013, 51, 3818–3825. [Google Scholar] [CrossRef]
- Anbalagan, S.; Spaans, T.; Hause, B.M. Genome Sequence of the Novel Reassortant Mammalian Orthoreovirus Strain Mrv00304/13, Isolated from a Calf with Diarrhea from the United States. Genome. Announc. 2014, 2, e00451-14. [Google Scholar] [CrossRef]
- Lian, H.; Liu, Y.; Zhang, S.; Zhang, F.; Hu, R. Novel Orthoreovirus from Mink, China, 2011. Emerg. Infect. Dis. 2013, 19, 1985–1988. [Google Scholar] [CrossRef]
- Decaro, N.; Campolo, M.; Desario, C.; Ricci, D.; Camero, M.; Lorusso, E.; Elia, G.; Lavazza, A.; Martella, V.; Buonavoglia, C. Virological and Molecular Characterization of a Mammalian Orthoreovirus Type 3 Strain Isolated from a Dog in Italy. Vet. Microbiol. 2005, 109, 19–27. [Google Scholar] [CrossRef]
- Zhang, W.; Kataoka, M.; Doan, Y.H.; Oi, T.; Furuya, T.; Oba, M.; Mizutani, T.; Oka, T.; Li, T.C.; Nagai, M. Isolation and Characterization of Mammalian Orthoreovirus Type 3 from a Fecal Sample from a Wild Boar in Japan. Arch. Virol. 2021, 166, 1671–1680. [Google Scholar] [CrossRef]
- Besozzi, M.; Lauzi, S.; Lelli, D.; Lavazza, A.; Chiapponi, C.; Pisoni, G.; Vigano, R.; Lanfranchi, P.; Luzzago, C. Host Range of Mammalian Orthoreovirus Type 3 Widening to Alpine Chamois. Vet. Microbiol. 2019, 230, 72–77. [Google Scholar] [CrossRef]
- Li, Z.; Shao, Y.; Liu, C.; Liu, D.; Guo, D.; Qiu, Z.; Tian, J.; Zhang, X.; Liu, S.; Qu, L. Isolation and Pathogenicity of the Mammalian Orthoreovirus Mpc/04 from Masked Civet Cats. Infect. Genet. Evol. 2015, 36, 55–61. [Google Scholar] [CrossRef]
- Chua, K.B.; Voon, K.; Crameri, G.; Tan, H.S.; Rosli, J.; McEachern, J.A.; Suluraju, S.; Yu, M.; Wang, L.F. Identification and Characterization of a New Orthoreovirus from Patients with Acute Respiratory Infections. PLoS ONE 2008, 3, e3803. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Voon, K.; Yu, M.; Keniscope, C.; Abdul Rasid, K.; Wang, L.F. Investigation of a Potential Zoonotic Transmission of Orthoreovirus Associated with Acute Influenza-Like Illness in an Adult Patient. PLoS ONE 2011, 6, e25434. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Sooryanarain, H.; Yugo, D.M.; Tian, D.; Rogers, A.J.; Heffron, C.L.; Thimmasandra Narayanappa, A.; LeRoith, T.; Overend, C.; Matzinger, S.R.; et al. Evaluation of the Pathogenicity of Mammalian Orthoreovirus Type 3 (Mrv3) in Germ-Free Gnotobiotic Pigs and of the Efficacy of an Inactivated Vaccine against Mrv3 Infection in Neonatal Conventional Piglets. Vet. Microbiol. 2018, 224, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, Q.; Zhang, C.; Song, Y.; Tian, X.; Zhang, X.; Xue, C.; Xu, S.; Bi, Y.; Cao, Y. Complete Genome Sequence of a Porcine Orthoreovirus from Southern China. J. Virol. 2012, 86, 12456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.; Wang, P.; Liu, S.; Lin, W.; Hu, F.; Wu, W.; Chen, W.; Cui, S. A Potentially Novel Reovirus Isolated from Swine in Northeastern China in 2007. Virus Genes 2011, 43, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L.; Barton, E.S.; Ibach, M.L.; Robinson, C.; Campbell, J.A.; O’Donnell, S.M.; Valyi-Nagy, T.; Clarke, P.; Wetzel, J.D.; Dermody, T.S. Isolation and Molecular Characterization of a Novel Type 3 Reovirus from a Child with Meningitis. J. Infect. Dis. 2004, 189, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, D.R.; Fields, B.N. Attenuated Reovirus Type 3 Strains Generated by Selection of Haemagglutinin Antigenic Variants. Nature 1982, 297, 68–70. [Google Scholar] [CrossRef]
- Weiner, H.L.; Drayna, D.; Averill, D.R., Jr.; Fields, B.N. Molecular Basis of Reovirus Virulence: Role of the S1 Gene. Proc. Natl. Acad. Sci. USA 1977, 74, 5744–5748. [Google Scholar] [CrossRef]
- Jiang, R.D.; Li, B.; Liu, X.L.; Liu, M.Q.; Chen, J.; Luo, D.S.; Hu, B.J.; Zhang, W.; Li, S.Y.; Yang, X.L.; et al. Bat Mammalian Orthoreoviruses Cause Severe Pneumonia in Mice. Virology 2020, 551, 84–92. [Google Scholar] [CrossRef]
- Feher, E.; Kemenesi, G.; Oldal, M.; Kurucz, K.; Kugler, R.; Farkas, S.L.; Marton, S.; Horvath, G.; Banyai, K.; Jakab, F. Isolation and Complete Genome Characterization of Novel Reassortant Orthoreovirus from Common Vole (Microtus arvalis). Virus Genes 2017, 53, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Steyer, A.; Naglic, T.; Chiapponi, C.; Prosperi, A.; Faccin, F.; Sozzi, E.; Lavazza, A. Detection and Characterization of a Novel Reassortant Mammalian Orthoreovirus in Bats in Europe. Viruses 2015, 7, 5844–5854. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Cao, L.; Lei, W.; Cao, Y.; Song, J.; Tang, Q.; Zhang, H.; Feng, Y.; Yang, W.; et al. Isolation and Identification of a Natural Reassortant Mammalian Orthoreovirus from Least Horseshoe Bat in China. PLoS ONE 2015, 10, e0118598. [Google Scholar] [CrossRef] [PubMed]
- Naglič, T.; Rihtarič, D.; Hostnik, P.; Toplak, N.; Koren, S.; Kuhar, U.; Jamnikar-Ciglenečki, U.; Kutnjak, D.; Steyer, A. Identification of Novel Reassortant Mammalian Orthoreoviruses from Bats in Slovenia. BMC Vet. Res. 2018, 14, 264. [Google Scholar] [CrossRef]
- Leary, T.P.; Erker, J.C.; Chalmers, M.L.; Cruz, A.T.; Wetzel, J.D.; Desai, S.M.; Mushahwar, I.K.; Dermody, T.S. Detection of Mammalian Reovirus Rna by Using Reverse Transcription-Pcr: Sequence Diversity within the Lambda3-Encoding L1 Gene. J. Clin. Microbiol. 2002, 40, 1368–1375. [Google Scholar] [CrossRef]
- Harima, H.; Sasaki, M.; Kajihara, M.; Gonzalez, G.; Simulundu, E.; Bwalya, E.C.; Qiu, Y.; Okuya, K.; Isono, M.; Orba, Y.; et al. Characterization of Mammalian Orthoreoviruses Isolated from Faeces of Pigs in Zambia. J. Gen. Virol. 2020, 101, 1027–1036. [Google Scholar] [CrossRef]
- Wellehan, J.F., Jr.; Childress, A.L.; Marschang, R.E.; Johnson, A.J.; Lamirande, E.W.; Roberts, J.F.; Vickers, M.L.; Gaskin, J.M.; Jacobson, E.R. Consensus Nested Pcr Amplification and Sequencing of Diverse Reptilian, Avian, and Mammalian Orthoreoviruses. Vet. Microbiol. 2009, 133, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Lavazza, A.; Bresaola, M.; Canelli, E.; Boniotti, M.B.; Cordioli, P. Identification of Mammalian Orthoreovirus Type 3 in Italian Bats. Zoonoses Public Health 2013, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Castilla, D.; Escobar, V.; Ynga, S.; Llanco, L.; Manchego, A.; Lazaro, C.; Navarro, D.; Santos, N.; Rojas, M. Enteric Viral Infections among Domesticated South American Camelids: First Detection of Mammalian Orthoreovirus in Camelids. Animals 2021, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Crameri, G.; Hyatt, A.; Yu, M.; Tompang, M.R.; Rosli, J.; McEachern, J.; Crameri, S.; Kumarasamy, V.; Eaton, B.T.; et al. A Previously Unknown Reovirus of Bat Origin Is Associated with an Acute Respiratory Disease in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11424–11429. [Google Scholar] [CrossRef] [PubMed]
- Muscillo, M.; La Rosa, G.; Marianelli, C.; Zaniratti, S.; Capobianchi, M.R.; Cantiani, L.; Carducci, A. A New Rt-Pcr Method for the Identification of Reoviruses in Seawater Samples. Water Res. 2001, 35, 548–556. [Google Scholar] [CrossRef]
- Sedmak, G.; Bina, D.; Macdonald, J.; Couillard, L. Nine-Year Study of the Occurrence of Culturable Viruses in Source Water for Two Drinking Water Treatment Plants and the Influent and Effluent of a Wastewater Treatment Plant in Milwaukee, Wisconsin (August 1994 through July 2003). Appl. Environ. Microbiol. 2005, 71, 1042–1050. [Google Scholar] [CrossRef]
- Luo, Y.; Fei, L.; Yue, H.; Li, S.; Ma, H.; Tang, C. Prevalence and Genomic Characteristics of a Novel Reassortment Mammalian Orthoreovirus Type 2 in Diarrhea Piglets in Sichuan, China. Infect. Genet. Evol. 2020, 85, 104420. [Google Scholar] [CrossRef]
- Qin, P.; Li, H.; Wang, J.W.; Wang, B.; Xie, R.H.; Xu, H.; Zhao, L.Y.; Li, L.; Pan, Y.; Song, Y.; et al. Genetic and Pathogenic Characterization of a Novel Reassortant Mammalian Orthoreovirus 3 (Mrv3) from a Diarrheic Piglet and Seroepidemiological Survey of Mrv3 in Diarrheic Pigs from East China. Vet. Microbiol. 2017, 208, 126–136. [Google Scholar] [CrossRef]
- Ren, J.; Liu, W.; Sun, N.; Zhang, P.; Yin, M.; Guo, L.; Zhang, H.; Cheng, S. Isolation and Pathogenicity Analysis of Mink Orthoreoviruses. Transbound. Emerg. Dis. 2022, 69, 623–631. [Google Scholar] [CrossRef]
- Yan, X.; Sheng, J.; Zhang, C.; Li, N.; Yi, L.; Zhao, Z.; Feng, Y.; Tu, C.; He, B. Detection and Characterization of a Reassortant Mammalian Orthoreovirus Isolated from Bats in Xinjiang, China. Viruses 2022, 14, 1897. [Google Scholar] [CrossRef]
- Yang, X.L.; Tan, B.; Wang, B.; Li, W.; Wang, N.; Luo, C.M.; Wang, M.N.; Zhang, W.; Li, B.; Peng, C.; et al. Isolation and Identification of Bat Viruses Closely Related to Human, Porcine and Mink Orthoreoviruses. J. Gen. Virol. 2015, 96, 3525–3531. [Google Scholar] [CrossRef]
- Ahasan, M.S.; Subramaniam, K.; Sayler, K.A.; Loeb, J.C.; Popov, V.L.; Lednicky, J.A.; Wisely, S.M.; Campos Krauer, J.M.; Waltzek, T.B. Molecular Characterization of a Novel Reassortment Mammalian Orthoreovirus Type 2 Isolated from a Florida White-Tailed Deer Fawn. Virus Res. 2019, 270, 197642. [Google Scholar] [CrossRef]
- Coombs, K.M. Reovirus Structure and Morphogenesis. Curr. Top. Microbiol. Immunol. 2006, 309, 117–167. [Google Scholar]

| Samples | Location | Host | RT-PCR (Positive Rate) | qRT-PCR (Positive Rate) | Ct Range | Virus Isolation |
|---|---|---|---|---|---|---|
| Rectal swabs (n = 80) | Shaanxi | sheep | 2/80 (2.5%) | 9/80 (11.3%) | 29.58–31.72 | 0/9 |
| Rectal swabs (n = 93) | Jiangsu | goat | 12/93 (12.9%) | 12/93 (12.9%) | 18.44–30.61 | 12/93 (12.9%) |
| Nasal swabs (n = 26) | Jiangsu | calf | 0/26 (0) | 0/26 (0) | / | 0/26 (0) |
| Nasal swabs (n = 12) | Anhui | calf | 0/12 (0) | 0/12 (0) | / | 0/12 (0) |
| Rectal swabs (n = 128) | Xinjiang | sheep | 3/128 (2.3%) | 14/128 (10.9%) | 15.48–26.49 | 2/128 (14.3%) |
| Rectal swabs (n = 90) | Xinjiang | calf | 0/90 | 0/90 (0) | / | 0/90 (0) |
| Total | 17/429 (4.0%) | 35/429 (8.2%) | 14/429 (3.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Li, X.; Cai, X.; Li, W.; Li, J.; Yang, S.; Zhai, J.; Suolang, S.; Li, B. First Specific Detection of Mammalian Orthoreovirus from Goats Using TaqMan Real-Time RT-PCR Technology. Vet. Sci. 2024, 11, 141. https://doi.org/10.3390/vetsci11040141
Mao L, Li X, Cai X, Li W, Li J, Yang S, Zhai J, Suolang S, Li B. First Specific Detection of Mammalian Orthoreovirus from Goats Using TaqMan Real-Time RT-PCR Technology. Veterinary Sciences. 2024; 11(4):141. https://doi.org/10.3390/vetsci11040141
Chicago/Turabian StyleMao, Li, Xia Li, Xuhang Cai, Wenliang Li, Jizong Li, Shanshan Yang, Junjun Zhai, Sizhu Suolang, and Bin Li. 2024. "First Specific Detection of Mammalian Orthoreovirus from Goats Using TaqMan Real-Time RT-PCR Technology" Veterinary Sciences 11, no. 4: 141. https://doi.org/10.3390/vetsci11040141
APA StyleMao, L., Li, X., Cai, X., Li, W., Li, J., Yang, S., Zhai, J., Suolang, S., & Li, B. (2024). First Specific Detection of Mammalian Orthoreovirus from Goats Using TaqMan Real-Time RT-PCR Technology. Veterinary Sciences, 11(4), 141. https://doi.org/10.3390/vetsci11040141









