Spontaneous Lesions of Endangered Geriatric Julia Creek Dunnarts (Sminthopsis douglasi, Archer 1979) with Emphasis in Reproductive Pathology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Data Collection
2.2. Gross Examination, Sample Collection and Processing
2.3. Ancillary Testing
3. Results
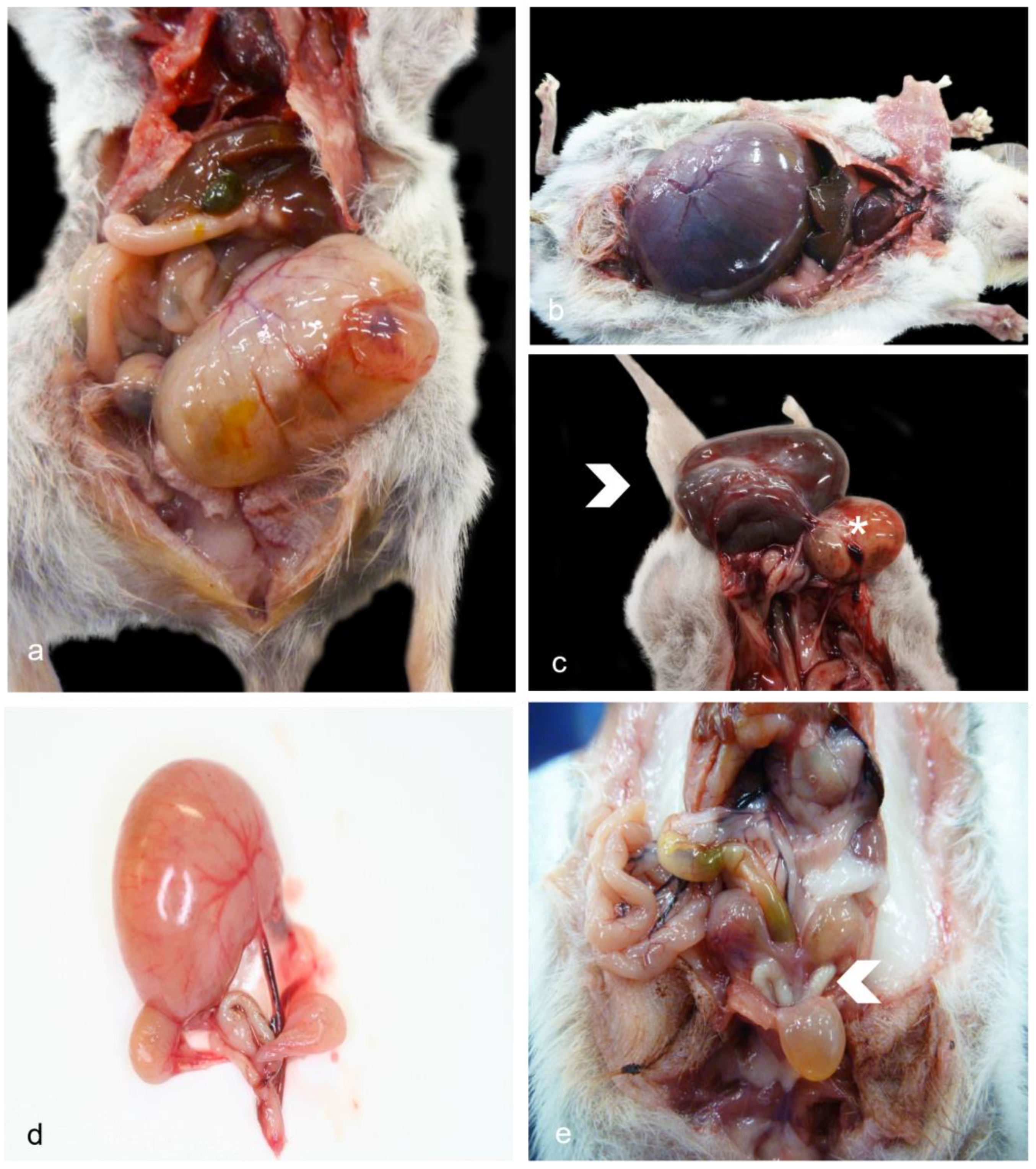
3.1. Gross Pathology—Female Reproductive Tract
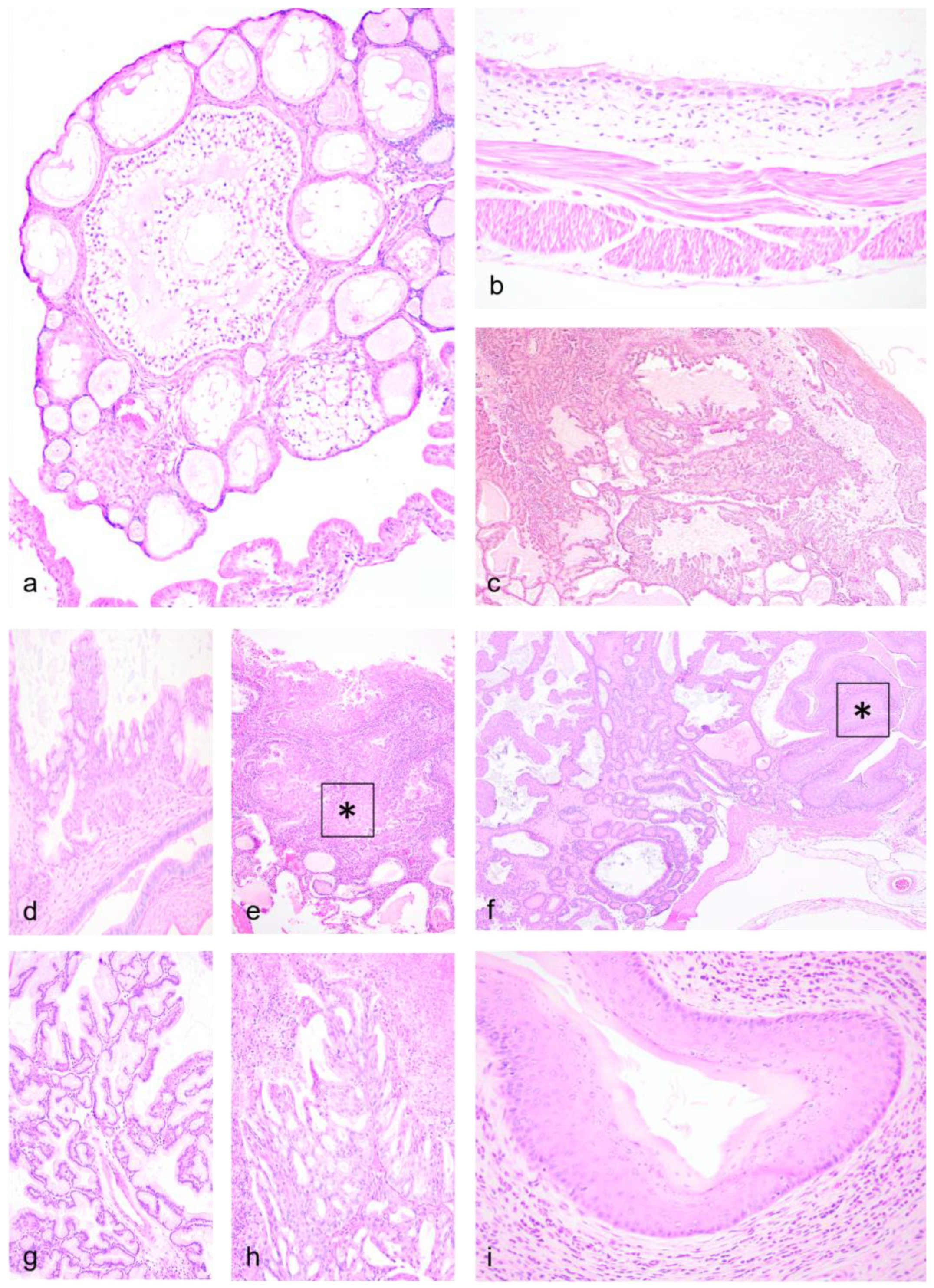
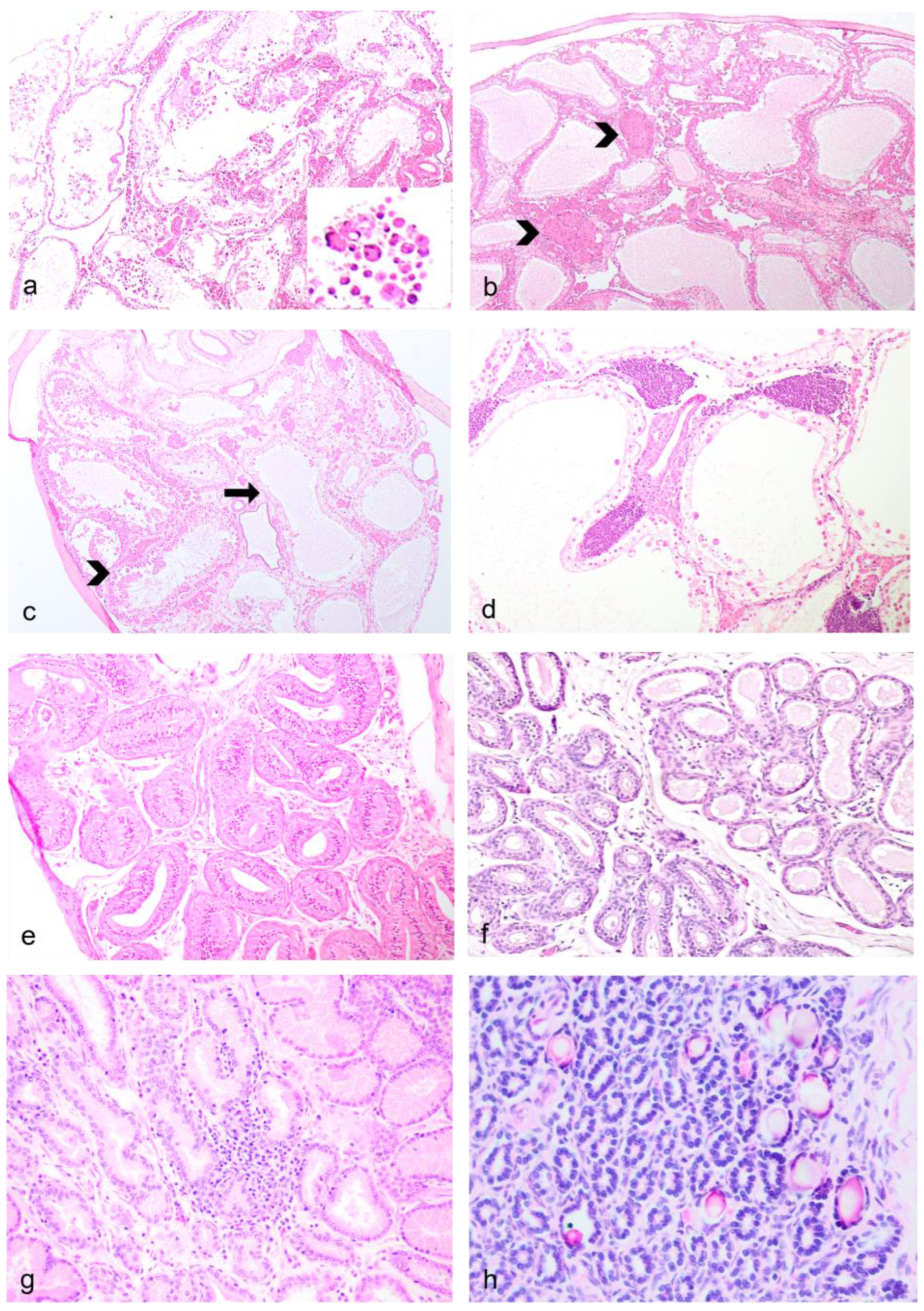
3.2. Histopathology—Female Reproductive Tract
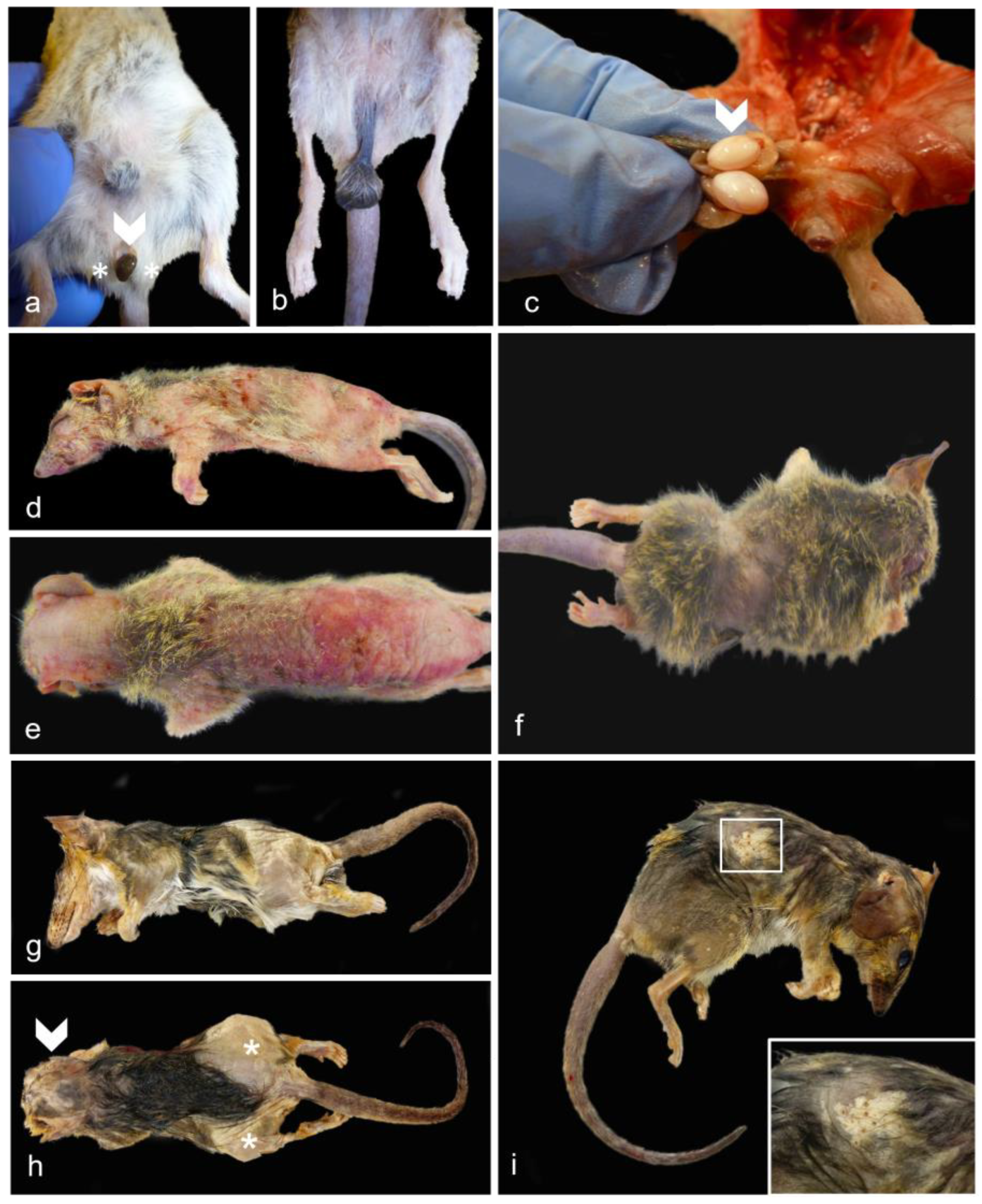
3.3. Additional Significant Non-Reproductive Disease—Female Dunnarts
3.4. Gross Pathology—Male Reproductive Tract
3.5. Histopathology—Male Reproductive Tract
3.6. Additional Significant Non-Reproductive Disease—Male Dunnarts
3.7. Histochemistry and Immunohistochemistry (IHC)
3.8. Non-Reproductive, Non-Clinically Significant Findings—Geriatric Male and Female Dunnarts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleiman, D.G.; Beck, B.B.; Dietz, J.M.; Dietz, L.A.; Ballou, J.D.; Coimbra-Filho, A.F. Conservation Program for the Golden Lion Tamarin: Captive Research and Management, Ecological Studies, Educational Strategies, and Reintroduction. In Primates; Benirschke, K., Ed.; Proceedings in Life Sciences; Springer: New York, NY, USA, 1986; pp. 959–979. ISBN 978-1-4612-9360-6. [Google Scholar]
- Ostrowski, S.; Bedin, E.; Lenain, D.M.; Abuzinada, A.H. Ten Years of Arabian Oryx Conservation Breeding in Saudi Arabia–Achievements and Regional Perspectives. Oryx 1998, 32, 209–222. [Google Scholar] [CrossRef]
- USFW. California Condor Recovery Program; U.S. Fish & Wildlife Service: Washington, DC, USA, 2021.
- Misfud, G. Ecology of the Julia Creek Dunnart Sminthopsis douglasi (Marsupialia: Dasyuridae). Master’s Thesis, La Trobe University, Melbourne, VIC, Australia, 1999. [Google Scholar]
- Department of Agriculture, Water and the Environment. Sminthopsis douglasi—Julia Creek Dunnart; Species Profile and Threats Database; Australian Government: Canberra, Australia, 2020.
- Woolley, P. New Records of the Julia Creek Dunnart, Sminthopsis douglasi (Marsupialia: Dasyuridae). Wildl. Res. 1992, 19, 779. [Google Scholar] [CrossRef]
- McAlpine, C.; Howes, A. Identification and Mapping of Critical Habitat for the Julia Creek Dunnart (Sminthopsis douglasi); Queensland Environment Protection Agency: Brisbane, Australia, 2005.
- Queensland Department of Environment and Resource Management Enhancing Biodiversity Hotspots along Western Queensland Stock Routes; 2009.
- DERM. National Recovery Plan for the Julia Creek Dunnart (Sminthopsis douglasi); Department of Environment and Resource Management: City East, QLD, Australia, 2009.
- Canfield, P.J.; Hartley, W.J.; Reddacliff, G.L. Spontaneous Proliferations in Australian Marsupials–A Survey and Review. 2. Dasyurids and Bandicoots. J. Comp. Pathol. 1990, 103, 147–158. [Google Scholar] [CrossRef]
- Survana, K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-7020-6887-4. [Google Scholar]
- Bjursell, A. Identifying Reproductive State of the Julia Creek Dunnart Sminthopsis douglasi by Behavioural Observations. Master’s Thesis, The University of Queensland, Brisbane, Australia, 2005. [Google Scholar]
- Cohen, A.A. Aging across the Tree of Life: The Importance of a Comparative Perspective for the Use of Animal Models in Aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2680–2689. [Google Scholar] [CrossRef]
- Holz, P. Dasyurids. In Medicine of Australian Mammals; CSIRO Publishing: Clayton, Australia, 2008; pp. 359–382. [Google Scholar]
- Montali, R.J. An Overview of Tumors in Zoo Animals. In Comparative Pathology of Zoo Animals; Smithsonian Institution Press: Washington, DC, USA, 1980; pp. 531–542. [Google Scholar]
- Setyo, L.; Donahoe, S.; Rose, K.; Spielman, D. Mammary Neoplasia in Common Brushtail Possums: Case Report and Retrospective Identification of Additional Cases. Aust. Vet. J. 2020, 98, 243–246. [Google Scholar] [CrossRef]
- Holz, P.H.; Little, P.B. Degenerative Leukoencephalopathy and Myelopathy in Dasyurids. J. Wildl. Dis. 1995, 31, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Pye, R.J.; Woods, G.M.; Kreiss, A. Devil Facial Tumor Disease. Vet. Pathol. 2016, 53, 726–736. [Google Scholar] [CrossRef]
- Gonzalez-Astudillo, V.; Henning, J.; Valenza, L.; Knott, L.; McKinnon, A.; Larkin, R.; Allavena, R. A Necropsy Study of Disease and Comorbidity Trends in Morbidity and Mortality in the Koala (Phascolarctos cinereus) in South-East Queensland, Australia. Sci. Rep. 2019, 9, 17494. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.S.; Howes, A.L.; Price, B.; McAlpine, C.A. Using a Bayesian Belief Network to Predict Suitable Habitat of an Endangered Mammal–The Julia Creek Dunnart (Sminthopsis douglasi). Biol. Conserv. 2007, 139, 333–347. [Google Scholar] [CrossRef]
- Every, A.L.; Selwood, L.; Castano-Rodriguez, N.; Lu, W.; Windsor, H.M.; Wee, J.L.; Swierczak, A.; Marshall, B.J.; Kaakoush, N.O.; Mitchell, H.M.; et al. Did Transmission of Helicobacter Pylori from Humans Cause a Disease Outbreak in a Colony of Stripe-Faced Dunnarts (Sminthopsis macroura)? Vet. Res. 2011, 42, 26. [Google Scholar] [CrossRef] [PubMed]
- Attwood, H.D.; Woolley, P.A. Spontaneous Malignant Neoplasms in Dasyurid Marsupials. J. Comp. Pathol. 1973, 83, 569–581. [Google Scholar] [CrossRef]
- Ladds, P. Pathology of Australian Native Wildlife; CSIRO Publishing: Collingwood, VIC, Australia, 2009. [Google Scholar]
- Goldschmidt, M.H.; Pena, L.; Zappulli, V. Tumors of the Mammary Gland. In Tumors in Domestic Animals; John Wiley & Sons, Inc.: San Francisco, CA, USA, 2017; pp. 723–772. [Google Scholar]
- Scheelings, T.; Dobson, E. Retrospective Analysis of Causes of Death in Mountain Pygmy-Possums (Burramys parvus) at Healesville Sanctuary, Victoria, Australia. Aust. Vet. J. 2015, 93, 424–429. [Google Scholar] [CrossRef]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Pathology of Laboratory Rodents and Rabbits, 4th ed.; John Wiley & Sons, Inc.: Aimes, IA, USA, 2016. [Google Scholar]
- Veiga, G.A.L.; Miziara, R.H.; Angrimani, D.S.R.; Papa, P.C.; Cogliati, B.; Vannucchi, C.I. Cystic Endometrial Hyperplasia-Pyometra Syndrome in Bitches: Identification of Hemodynamic, Inflammatory, and Cell Proliferation Changes. Biol. Reprod. 2017, 96, 58–69. [Google Scholar] [CrossRef]
- Bertram, C.A.; Müller, K.; Klopfleisch, R. Genital Tract Pathology in Female Pet Rabbits (Oryctolagus cuniculus): A Retrospective Study of 854 Necropsy Examinations and 152 Biopsy Samples. J. Comp. Pathol. 2018, 164, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.B.; Mahaney, M.C.; Gleiser, C.A.; Taylor, D.E.; VandeBerg, J.L. Spontaneous Pathology of the Gray Short-Tailed Opossum (Monodelphis domestica). Lab. Anim. Sci. 1997, 47, 19–26. [Google Scholar] [PubMed]
- Pope, J.P.; Donnell, R.L. Spontaneous Neoplasms in Captive Virginia Opossums (Didelphis virginiana): A Retrospective Case Series (1989–2014) and Review of the Literature. J. Vet. Diagn. Investig. 2017, 29, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.D.; Boever, W.J. Diseases of Exotic Animals. Medical and Surgical Management; WB Saunders Co.: Philadelphia, PA, USA, 1983. [Google Scholar]
- Barrie, M.T.; Snyder, R.L. Multiple Primary Neoplasms in an Opossum. J. Am. Vet. Med. Assoc. 1986, 189, 1160–1161. [Google Scholar] [PubMed]
- Kim, D.Y.; Mitchell, M.A.; De las Heras, M.; Taylor, H.W.; Cho, D.-Y. Spontaneous Squamous Cell Carcinoma of the Tongue and Multiple Bronchioloalveolar Carcinomas in a Virginia Opossum (Didelphis virginiana). J. Comp. Pathol. 2002, 126, 226–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walter, B.; Poth, T.; Böhmer, E.; Braun, J.; Matis, U. Uterine Disorders in 59 Rabbits. Vet. Rec. 2010, 166, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Künzel, F.; Grinninger, P.; Shibly, S.; Hassan, J.; Tichy, A.; Berghold, P.; Fuchs-Baumgartinger, A. Uterine Disorders in 50 Pet Rabbits. J. Am. Anim. Hosp. Assoc. 2015, 51, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Elsinghorst, T.A.M.; Timmermans, H.J.F.; Hendriks, H.G.C.J.M. Comparative Pathology of Endometrial Carcinoma. Vet. Q. 1984, 6, 200–208. [Google Scholar] [CrossRef]
- Adams, C.E. Ageing and Reproduction in the Female Mammal with Particular Reference to the Rabbit. J. Reprod. Fertil. Suppl. 1970, 12, 1–16. [Google Scholar]
- Johnson-Delaney, C.A.; Lennox, A.M. Reproductive Disorders of Marsupials. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; Alison, R.; Bach, U.; Colman, K.; Foley, G.L.; Harleman, J.H.; Haworth, R.; Herbert, R.; Heuser, A.; Long, G.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Female Reproductive System. J. Toxicol. Pathol. 2014, 27, 1S–107S. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Hughes, R. Reproduction in the Macropod Marsupial Potorous Tridactylus (Kerr). Aust. J. Zool. 1962, 10, 193. [Google Scholar] [CrossRef]
- Hughes, R.; Rodger, J. Studies on the Vaginal Mucus of the Marsupial Trichosurus vulpecula. Aust. J. Zool. 1971, 19, 19. [Google Scholar] [CrossRef]
- Foster, R.A. Male Genital System. In Jubb, Kennedy, Palmer’s Pathology of Domestic Animals; Elsevier, Inc.: St. Louis, MI, USA, 2016; Volume 3, pp. 465–510. [Google Scholar]
- Clegg, E.D.; Cook, J.C.; Chapin, R.E.; Foster, P.M.; Daston, G.P. Leydig Cell Hyperplasia and Adenoma Formation: Mechanisms and Relevance to Humans. Reprod. Toxicol. 1997, 11, 107–121. [Google Scholar] [CrossRef]
- Rodger, J.C. The Testis and Its Excurrent Ducts in American Caenolestid and Didelphid Marsupials. Am. J. Anat. 1982, 163, 269–282. [Google Scholar] [CrossRef]
- Taggart, D.A.; Johnson, J.; Temple-Smith, P.D. Testicular and Epididymal Development in the Brown Marsupial Mouse, Antechinus stuartii (Dasyuridae, Marsupialia). Anat. Embryol. 1993, 188, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.M.; Shaw, G.; Clark, J.; Renfree, M.B. The Functional Development of Leydig Cells in a Marsupial. J. Anat. 2008, 212, 55–66. [Google Scholar] [CrossRef]
- Barker, I.K.; Carbonell, P.L.; Bradley, A.J. Cytomegalovirus Infection of the Prostate in the Dasyurid Marsupials, Phascogale tapoatafa and Antechinus stuartii. J. Wildl. Dis. 1981, 17, 433–441. [Google Scholar] [CrossRef]
- Munday, B.L.; Obendorf, D.L. Cytomegalic Lesions in Australian Marsupials. J. Wildl. Dis. 1983, 19, 132–135. [Google Scholar] [CrossRef]
- Fabijan, J.; Woolford, L.; Lathe, S.; Simmons, G.; Hemmatzadeh, F.; Trott, D.J.; Speight, N. Lymphoma, Koala Retrovirus Infection and Reproductive Chlamydiosis in a Koala (Phascolarctos cinereus). J. Comp. Pathol. 2017, 157, 188–192. [Google Scholar] [CrossRef]
- Butler, M.D.; Griffin, K.; Brewster, C.D.; Kapuscinski, M.L.; Stenglein, M.D.; Tripp, D.W.; Quackenbush, S.L.; Fox, K.A. A Novel Retrovirus (Gunnison’s Prairie Dog Retrovirus) Associated with Thymic Lymphoma in Gunnison’s Prairie Dogs in Colorado, USA. Viruses 2020, 12, 606. [Google Scholar] [CrossRef]
- Goodnight, A.L.; Couto, C.G.; Green, E.; Barrie, M.; Myers, G. Chemotherapy and Radiotherapy for Treatment of Cutaneous Lymphoma in a Ground Cuscus (Phalanger gymnotis). J. Zoo Wildl. Med. 2008, 39, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Higbie, C.T.; Carpenter, J.W.; Choudhary, S.; DeBey, B.; Bagladi-Swanson, M.; Eshar, D. Cutaneous Epitheliotropic T-Cell Lymphoma with Metastases in a Virginia Opossum (Didelphis virginiana). J. Zoo Wildl. Med. 2015, 46, 409–413. [Google Scholar] [CrossRef]
- Scheelings, T.F.; Dobson, E.C.; Hooper, C. Cutaneous T-Cell Lymphoma in Two Captive Tasmanian Devils (Sarcophilus harrisii). J. Zoo Wildl. Med. 2014, 45, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. Immunophenotypic Characterization of Cutaneous Lymphoid Neoplasia in the Dog and Cat. J. Comp. Pathol. 1995, 112, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Frimberger, A.E.; Moore, A.S. Clinical Outcome and Prognosis of Dogs with Histopathological Features Consistent with Epitheliotropic Lymphoma: A Retrospective Study of 148 Cases (2003–2015). Vet. Dermatol. 2018, 29, 154-e59. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.O.; Kiupel, M.; Bienzle, D. Hematopoietic System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Elsevier, Inc.: St. Louis, MI, USA, 2016; Volume 3, pp. 102–268. [Google Scholar]
- Santoro, D.; Marsella, R.; Hernandez, J. Investigation on the Association between Atopic Dermatitis and the Development of Mycosis Fungoides in Dogs: A Retrospective Case-Control Study. Vet. Dermatol. 2007, 18, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Costas, L.; de Sanjosé, S.; Infante-Rivard, C. Reproductive Factors and Non-Hodgkin Lymphoma: A Systematic Review. Crit. Rev. Oncol. Hematol. 2014, 92, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.F.; Taylor, R.; Williamson, P. Demographic Risk Factors for Lymphoma in Australian Dogs: 6201 Cases. J. Vet. Intern. Med. 2018, 32, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.M.; Ginther, O.J. Circulating Nitric Oxide Metabolites during Luteolysis and the Effect of Luteinizing Hormone on Circulating Nitric Oxide Metabolites in Heifers. Theriogenology 2015, 83, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ascoli, M. Lutropin/Choriogonadotropin Stimulate the Proliferation of Primary Cultures of Rat Leydig Cells through a Pathway That Involves Activation of the Extracellularly Regulated Kinase 1/2 Cascade. Endocrinology 2007, 148, 3214–3225. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A. Possible Relationship between Long-Term Adverse Health Effects of Gonad-Removing Surgical Sterilization and Luteinizing Hormone in Dogs. Animals 2020, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.M.; Gust, S.K.; Kutzler, M.A. Luteinizing Hormone Receptor Expression by Nonneoplastic and Neoplastic Canine Lymphocytes. Am. J. Vet. Res. 2019, 80, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A. Understanding the Effects of Sustained Supraphysiologic Concentrations of Luteinizing Hormone in Gonadectomized Dogs: What We Know and What We Still Need to Learn. Theriogenology 2023, 196, 270–274. [Google Scholar] [CrossRef]
- Webster, J.D.; Solon, M.; Gibson-Corley, K.N. Validating Immunohistochemistry Assay Specificity in Investigative Studies: Considerations for a Weight of Evidence Approach. Vet. Pathol. 2021, 58, 829–840. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chang, A.-M.; Tsai, M.-S.; Huang, Y.-H.; Pei, K.J.-C.; Li, Y.-C. Association between Stress and Bilateral Symmetrical Alopecia in Free-Ranging Formosan Macaques in Mt. Longevity, Taiwan. Sci. Rep. 2021, 11, 11189. [Google Scholar] [CrossRef]
- Martin, C.O.; Wolters, M.S. Alopecia in a Population of Rafinesque’s Big-Eared Bats (Corynorhinus rafinesquii). Southwest. Nat. 2023, 67, 59–62. [Google Scholar] [CrossRef]
- Bajwa, J. Cutaneous Hyperpigmentation in Dogs. Can. Vet. J. 2022, 63, 85–88. [Google Scholar]
- Kurien, B.T.; Gross, T.; Scofield, R.H. Barbering in Mice: A Model for Trichotillomania. BMJ 2005, 331, 1503–1505. [Google Scholar] [CrossRef]
- Lutz, C.K.; Coleman, K.; Worlein, J.; Novak, M.A. Hair Loss and Hair-Pulling in Rhesus Macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 454–457. [Google Scholar] [PubMed]
- Hadinger, U.; Haymerle, A.; Knauer, F.; Schwarzenberger, F.; Walzer, C. Faecal Cortisol Metabolites to Assess Stress in Wildlife: Evaluation of a Field Method in Free-ranging Chamois. Methods Ecol. Evol. 2015, 6, 1349–1357. [Google Scholar] [CrossRef]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Hermes, R.; Hildebrandt, T.B.; Göritz, F. Reproductive Problems Directly Attributable to Long-Term Captivity–Asymmetric Reproductive Aging. Anim. Reprod. Sci. 2004, 82–83, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Dehnhard, M.; Pribbenow, S.; Silinski-Mehr, S.; Hofer, H.; Wachter, B. Asymmetric Reproductive Aging in Cheetah (Acinonyx jubatus) Females in European Zoos. J. Zoo Aquar. Res. 2019, 7, 87–93. [Google Scholar] [CrossRef]
- Fletcher, T. Luteinizing Hormone in the Kowari, Dasyuroides Byrnei (Marsupialia: Dasyuridae), during the Oestrous Cycle and Pregnancy, and the Effects of Gonadectomy in Male and Female. Reprod. Fertil. Dev. 1989, 1, 55. [Google Scholar] [CrossRef]
- Mills, H.R.; Bradshaw, F.J.; Lambert, C.; Bradshaw, S.D.; Bencini, R. Reproduction in the Marsupial Dibbler, Parantechinus apicalis; Differences between Island and Mainland Populations. Gen. Comp. Endocrinol. 2012, 178, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Woolley, P. Reproductive Pattern of Captive Boullanger Island Dibbers, Parantechinus apicalis (Marsupialia: Dasyuridae). Wildl. Res. 1991, 18, 157. [Google Scholar] [CrossRef]
- Taggart, D.A.; Shimmin, G.A.; McCloud, P.; Temple-Smith, P.D. Timing of Mating, Sperm Dynamics, and Ovulation in a Wild Population of Agile Antechinus (Marsupialia: Dasyuridae). Biol. Reprod. 1999, 60, 283–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woolley, P. Reproduction in the Ningbing Antechinus (Marsupialia, Dasyuridae)-Field and Laboratory Observations. Wildl. Res. 1988, 15, 149. [Google Scholar] [CrossRef]
- Penfold, L.M.; Powell, D.; Traylor-Holzer, K.; Asa, C.S. “Use It or Lose It”: Characterization, Implications, and Mitigation of Female Infertility in Captive Wildlife. Zoo Biol. 2014, 33, 20–28. [Google Scholar] [CrossRef]
- Witt, R.R.; Rodger, J.C. Recent Advances in Tools and Technologies for Monitoring and Controlling Ovarian Activity in Marsupials. Theriogenology 2018, 109, 58–69. [Google Scholar] [CrossRef]
- Pollock, K.; Booth, R.; Wilson, R.; Keeley, T.; Grogan, K.; Kennerley, P.; Johnston, S.D. Oestrus in the Julia Creek Dunnart (Sminthopsis douglasi) Is Associated with Wheel Running Behaviour but Not Necessarily Changes in Body Weight, Food Consumption or Pouch Morphology. Anim. Reprod. Sci. 2010, 117, 135–146. [Google Scholar] [CrossRef]
| Diagnoses | Dunnart ID | Age (Months) |
|---|---|---|
| Ovaries | ||
| Cystic ovary (possibly bursal) | 6 | 25 |
| Age-related ovarian atrophy | 8 | 25 |
| Age-related ovarian atrophy | 10 | 42 |
| Oviduct | ||
| Cystic oviduct | 10 | 42 |
| Uterus | ||
| Cystic glandular hyperplasia, squamous metaplasia | 3 | 27 |
| Cystic glandular hyperplasia/dysplasia, squamous metaplasia | 7 | 25 |
| Cystic glandular hyperplasia with mucin | 8 | 25 |
| Cystic glandular hyperplasia | 2 | 24 |
| Cystic glandular hyperplasia | 10 | 42 |
| Cystic glandular hyperplasia | 24 | 24 |
| Cystic glandular hyperplasia, polyp (glandular) | 27 | 24 |
| Cystic glandular hyperplasia, squamous metaplasia | 11 | 20 |
| Cystic glandular hyperplasia, squamous metaplasia, polyp (glandular) | 14 | 42 |
| Endometrial polyp (glandular) | 23 | 12 |
| Endometrial polyp (glandular), cystic | 30 | 24 |
| Endometrial polyp (glandular), cystic | 32 | 24 |
| Endometrial adenoma, papillary, focal squamous metaplasia | 13 | 42 |
| Cervix and vagina | ||
| Neutrophilic infiltrates in cervix and vagina with fibrosis | 8 | 25 |
| Neutrophilic infiltrates in vagina | 10 | 42 |
| Skin | ||
| Round cell infiltrates, superficial dermis, non-epitheliotropic | 30 | 24 |
| Round cell infiltrates, mid-to-deep dermal, epitheliotropic | 33 | 12 |
| Diagnoses | Dunnart ID | Age (Months) |
|---|---|---|
| Testes | ||
| Testicular tubular degeneration/atrophy with aspermatogenesis | 4 | 42 |
| Testicular tubular degeneration/atrophy with aspermatogenesis and metastatic lymphoma | 5 | 42 |
| Testicular tubular degeneration/atrophy | 9 | 42 |
| Prostate | ||
| Prostatic mineralization, multifocal | 9 | 42 |
| Lymphoplasmacytic prostatitis | 12 | 42 |
| Skin | ||
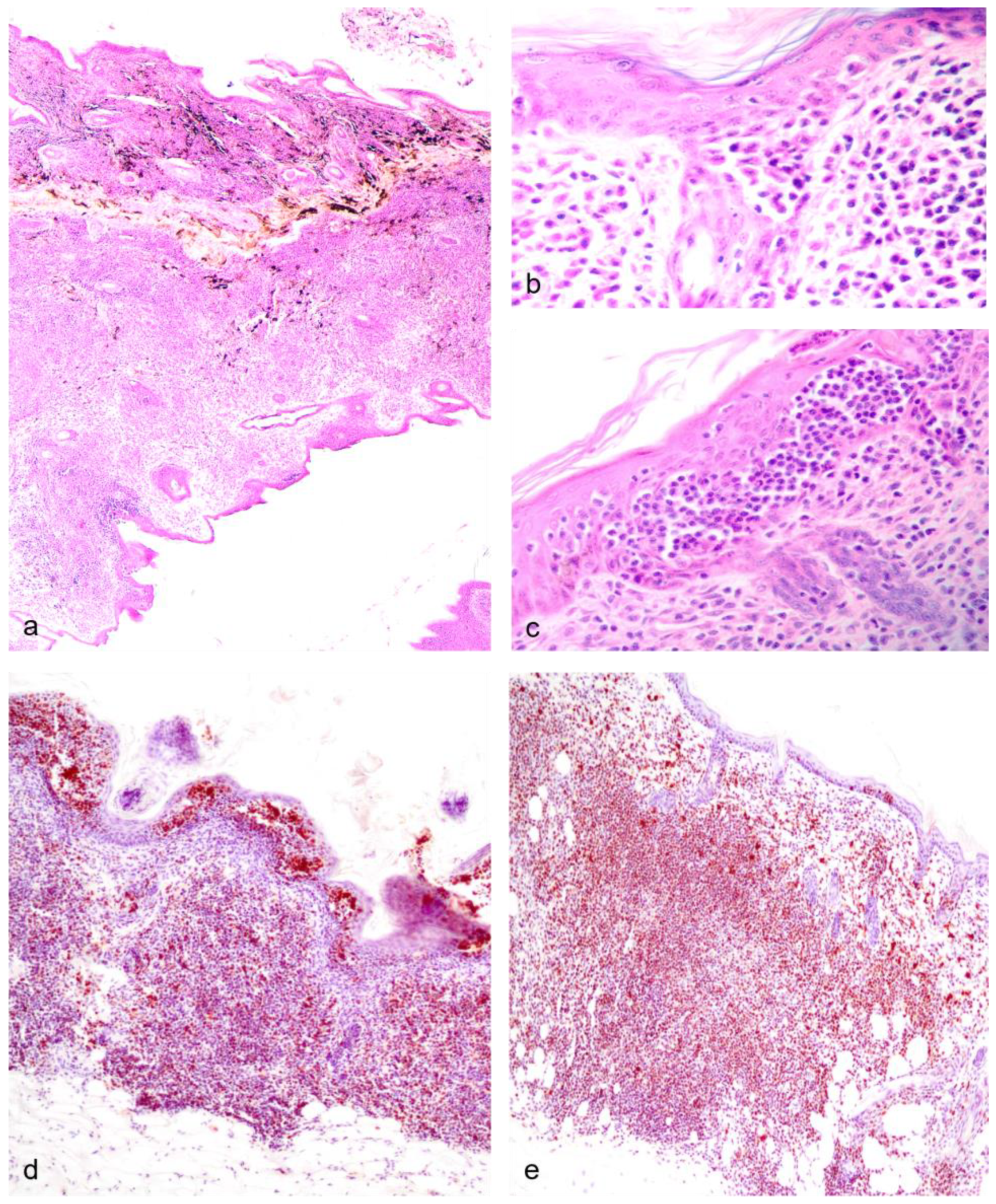
| Cutaneous epitheliotropic lymphoma (mycosis fungoides) | 1 | 24 |
| Cutaneous epitheliotropic lymphoma (mycosis fungoides), with splenic and pulmonary metastasis | 4 | 42 |
| Cutaneous epitheliotropic lymphoma (mycosis fungoides), with splenic, pulmonary and testicular metastasis | 5 | 42 |
| Cutaneous round cell infiltrates, telogen alopecia | 25 | 48 |
| Cutaneous round cell infiltrates | 31 | 48 |
| Telogen alopecia, hyperkeratosis | 20 | 36 |
| Telogen alopecia | 28 | 24 |
| Telogen alopecia | 29 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Astudillo, V.; Schaffer-White, A.; Noble, L.; O’Hara, P.; Murray, P.; Barnes, T.S.; Allavena, R. Spontaneous Lesions of Endangered Geriatric Julia Creek Dunnarts (Sminthopsis douglasi, Archer 1979) with Emphasis in Reproductive Pathology. Vet. Sci. 2024, 11, 142. https://doi.org/10.3390/vetsci11040142
Gonzalez-Astudillo V, Schaffer-White A, Noble L, O’Hara P, Murray P, Barnes TS, Allavena R. Spontaneous Lesions of Endangered Geriatric Julia Creek Dunnarts (Sminthopsis douglasi, Archer 1979) with Emphasis in Reproductive Pathology. Veterinary Sciences. 2024; 11(4):142. https://doi.org/10.3390/vetsci11040142
Chicago/Turabian StyleGonzalez-Astudillo, Viviana, Andrea Schaffer-White, Lawrence Noble, Patricia O’Hara, Peter Murray, Tamsin S. Barnes, and Rachel Allavena. 2024. "Spontaneous Lesions of Endangered Geriatric Julia Creek Dunnarts (Sminthopsis douglasi, Archer 1979) with Emphasis in Reproductive Pathology" Veterinary Sciences 11, no. 4: 142. https://doi.org/10.3390/vetsci11040142
APA StyleGonzalez-Astudillo, V., Schaffer-White, A., Noble, L., O’Hara, P., Murray, P., Barnes, T. S., & Allavena, R. (2024). Spontaneous Lesions of Endangered Geriatric Julia Creek Dunnarts (Sminthopsis douglasi, Archer 1979) with Emphasis in Reproductive Pathology. Veterinary Sciences, 11(4), 142. https://doi.org/10.3390/vetsci11040142








