The Efficacy of Encapsulated Phytase Based on Recombinant Yarrowia lipolytica on Quails’ Zootechnic Features and Phosphorus Assimilation
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Experimental Description and Quail Husbandry
2.2. Dietary Plan
2.3. The Phytase Activity Assay
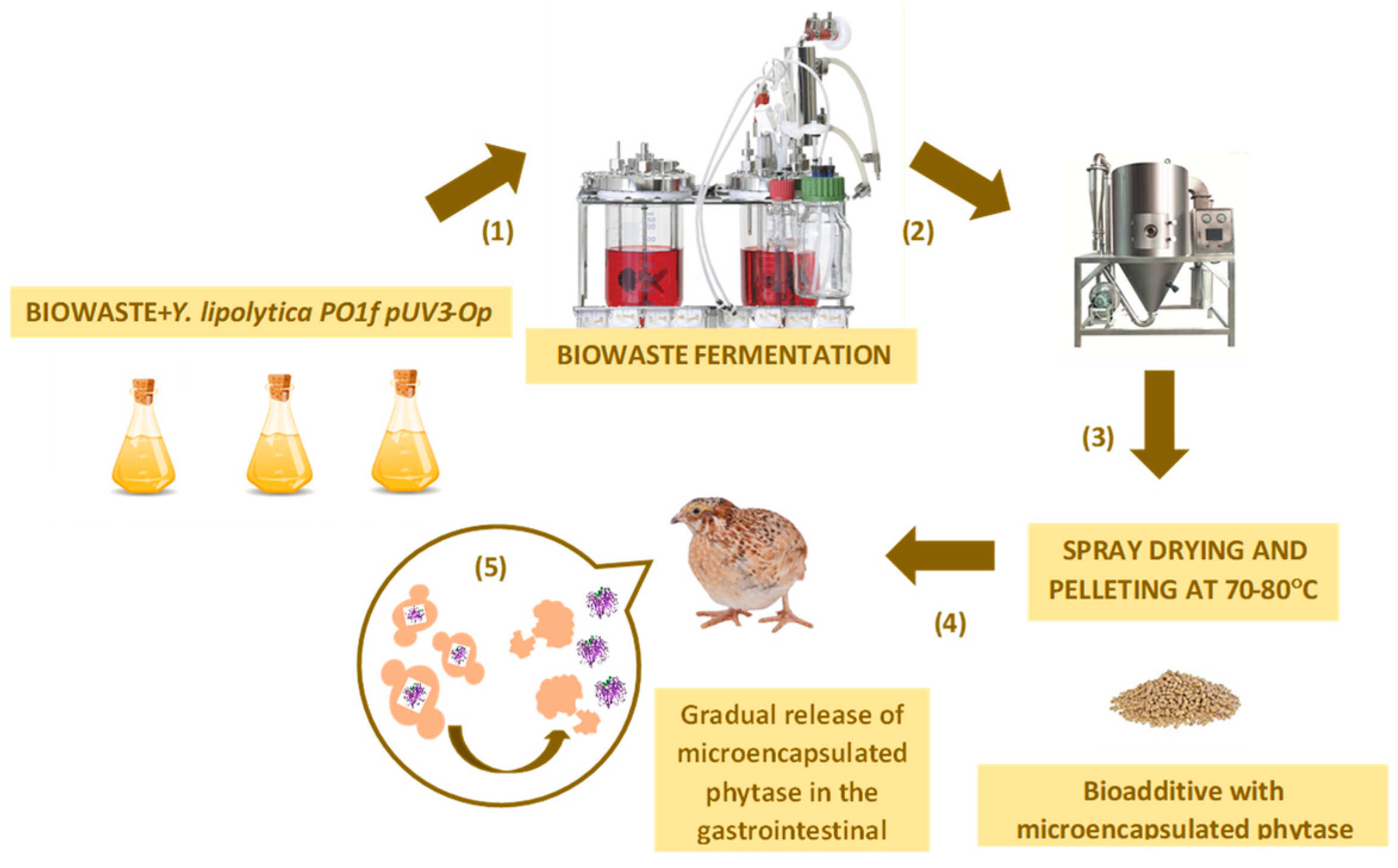
2.4. Feed Supplement Preparation Using the Yeast Strain Cultivation
2.5. Sample Collection and Processing
2.6. The Assay of The Experimental Feeds and the Quail Excreta Composition
2.7. Feed Conversion Ratio (FCR)
2.8. Assay of Phosphorus and Calcium Level in the Tibiae of the Quails
2.9. Statistics
3. Results
3.1. Stage 1
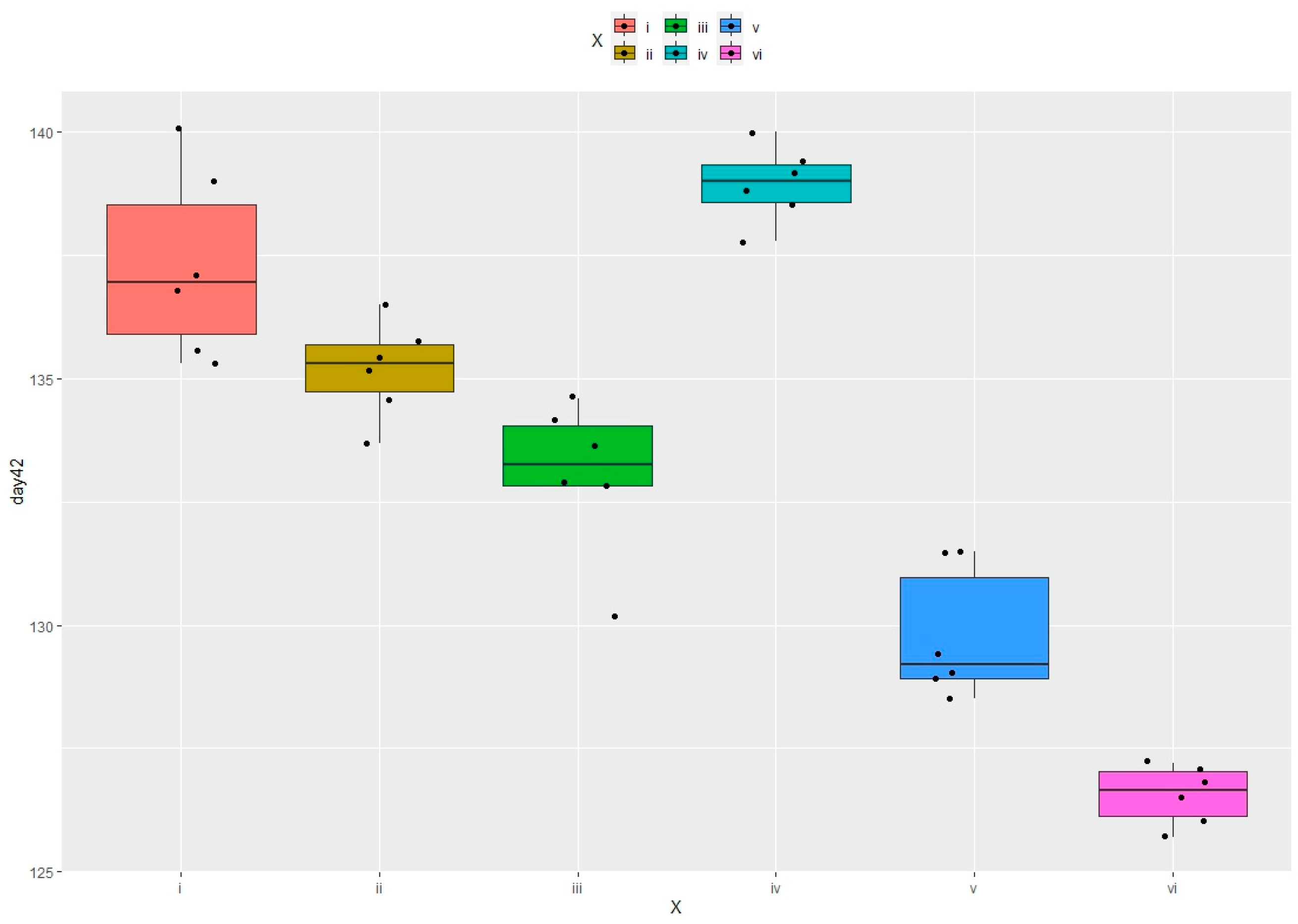
3.1.1. Growth Performance at the Age of 42 Days
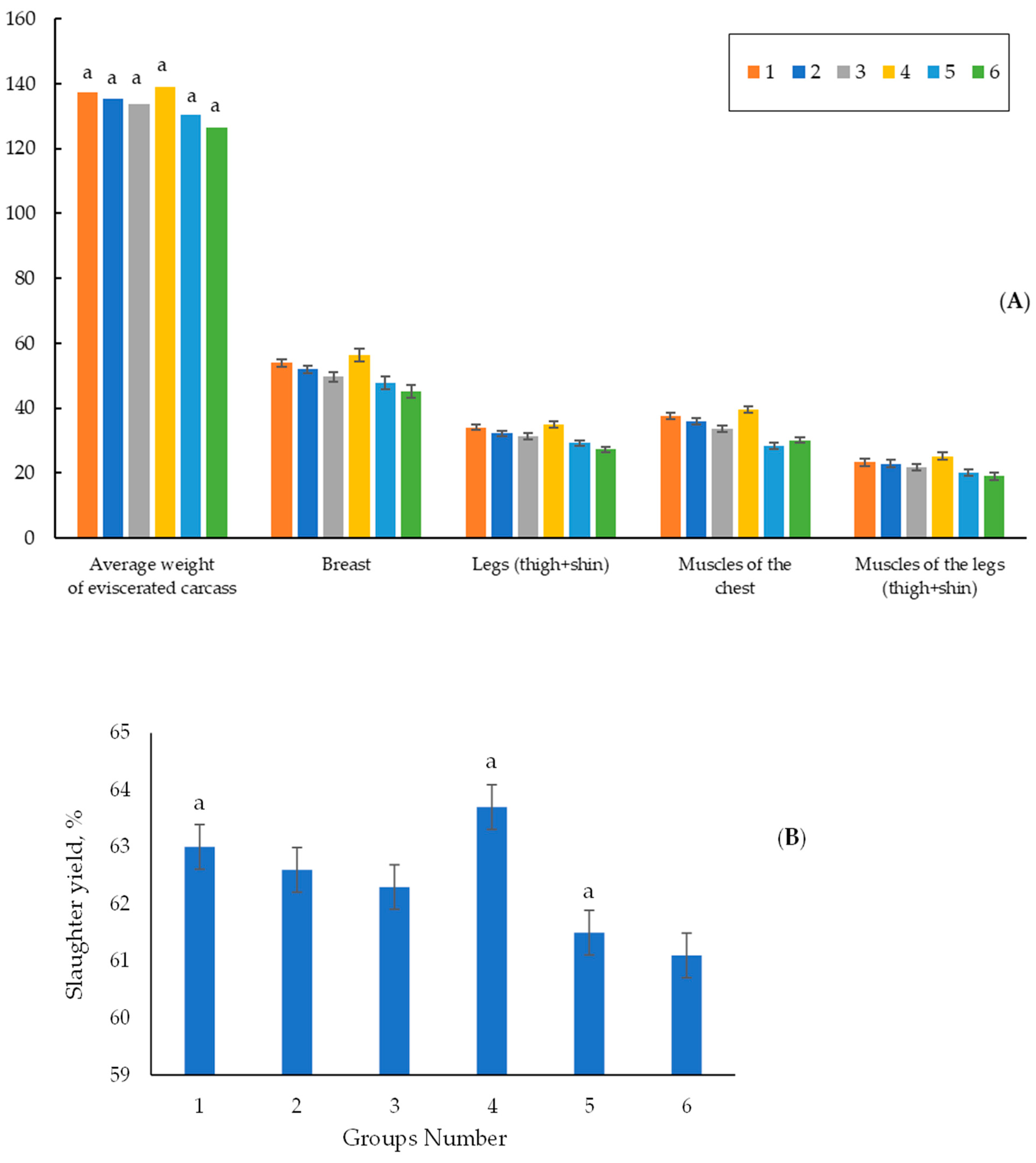
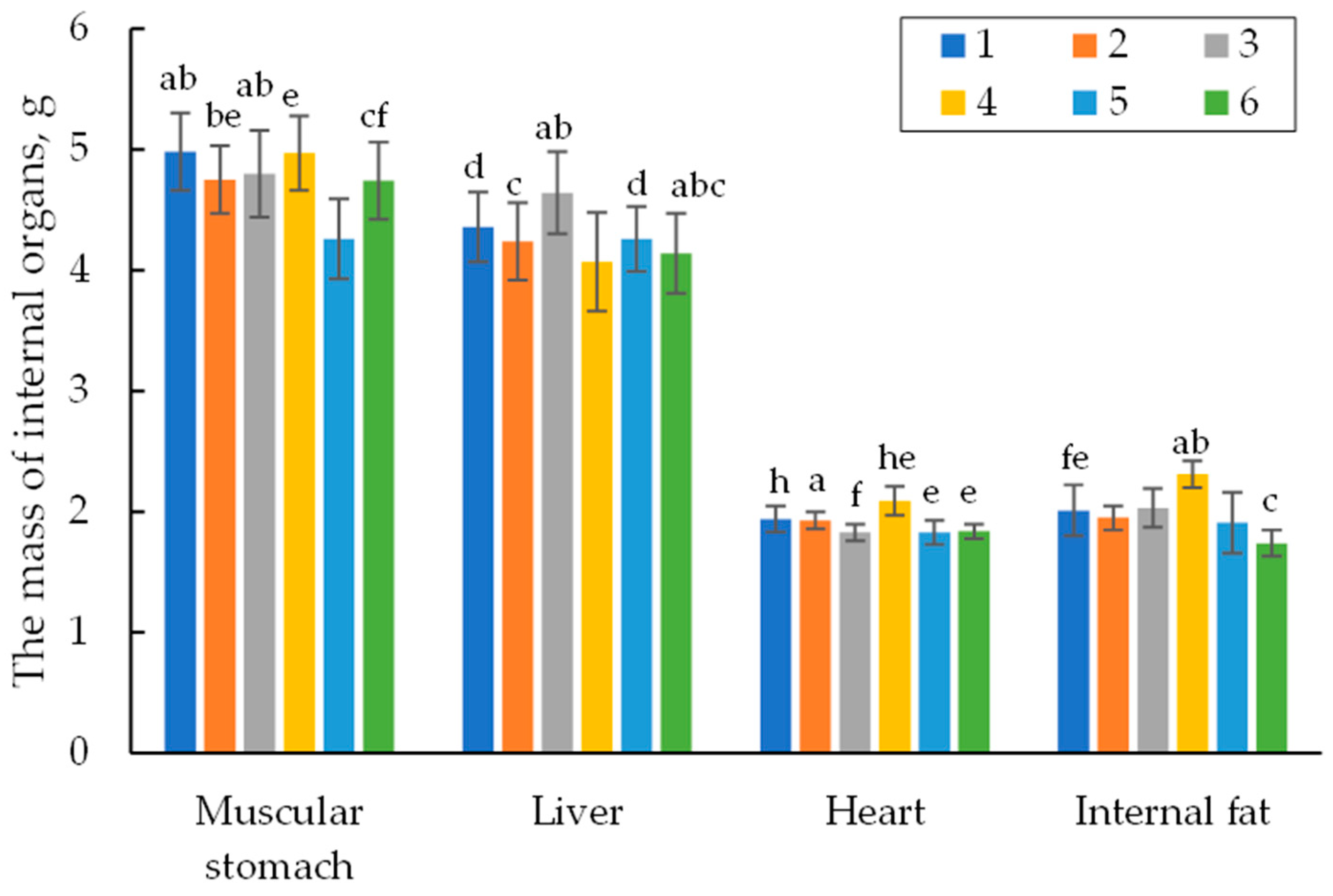
3.1.2. Carcass Criteria and Organs on the 42nd Day of Life
3.1.3. Chemical Composition of Quails’ Bones
3.2. Stage 2
3.2.1. Growth Performance of the Quails at the Age of 60 Days
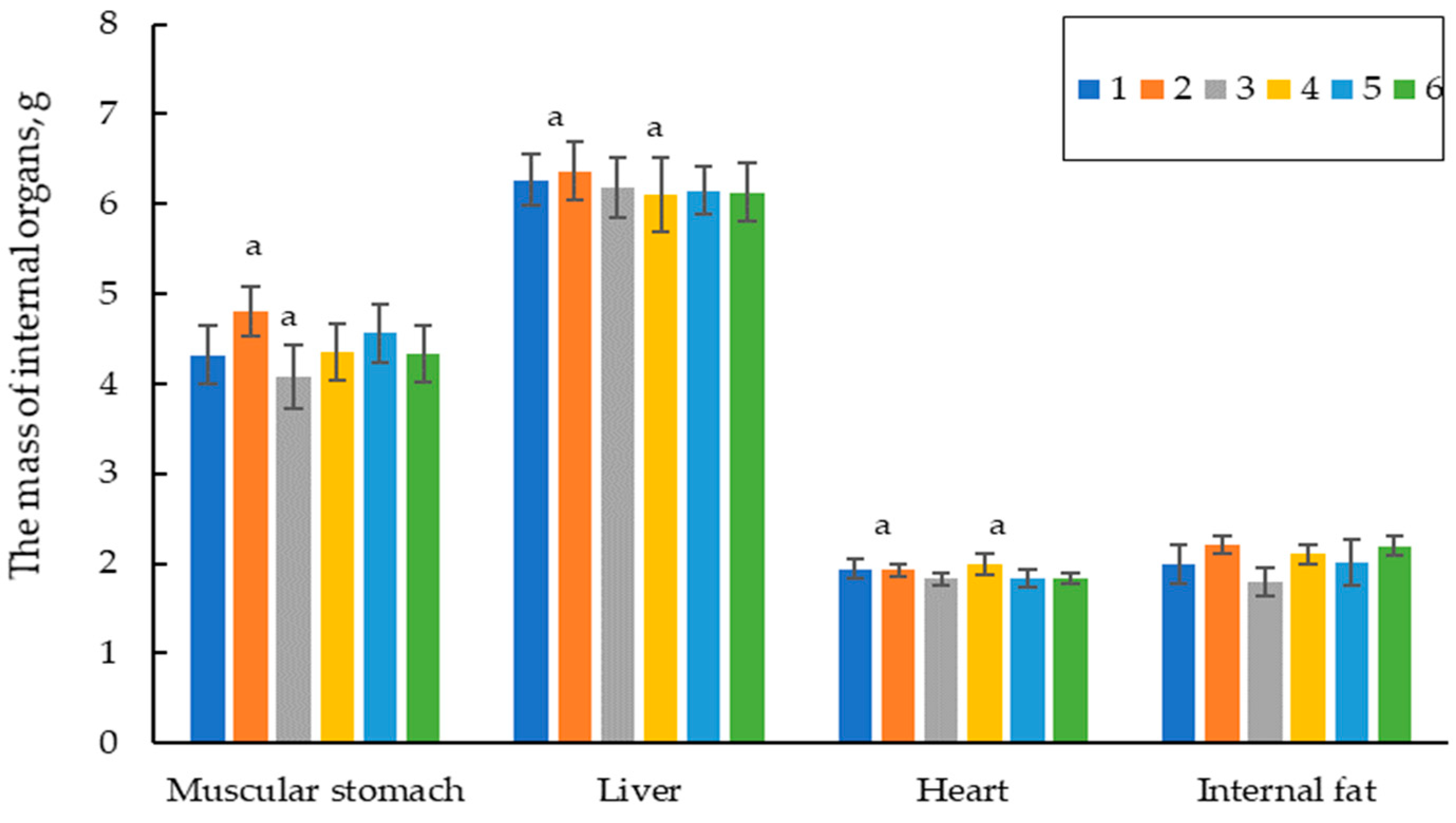
3.2.2. Carcass Criteria, Organs, and Egg Laying Productivity on the 60th Day of Life
3.2.3. The Effect of the Phytases on Residual Phosphorus and Macro- and Microelements in the Quails’ Excreta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lisunova, L.I. Perepelovodstvo—It’s profitable. Our Agric. 2021, 252, 79–81. [Google Scholar]
- El Sabry, M.I.; Hassan, S.S.A.; Zaki, M.M.; Stino, F.K.R. Stocking density: A clue for improving social behavior, welfare, health indices along with productivity performances of quail (Coturnix coturnix)—A review. Trop. Anim. Health Prod. 2022, 54, 83. [Google Scholar] [CrossRef] [PubMed]
- Osmanyan, A.K.; Slashcheva, Y.V.; Komarchev, A.S. Planting density when growing quail depending on the age at slaughter. Poult. Poult. Prod. 2022, 2, 28–32. [Google Scholar]
- Osmanyan, A.K.; Slashcheva, Y.V.; Komarchev, A.S. Efficiency of quail meat production under various light conditions. Poult. Farming 2022, 6, 37–41. [Google Scholar]
- Reuter, Y.S.; Degtyareva, T.N.; Degtyareva, O.N. Selection features and acquisition of a breeding herd of quails. Bull. Agrar. Sci. 2022, 1, 60–64. [Google Scholar] [CrossRef]
- Akupiyan, O.S.; Sheveleva, N.A. Breeding quails as a promising direction in agricultural business. Problems and solutions of modern agrarian economy. XXI Inter. Scient. Indust. Conf. 2017, 2, 170–171. [Google Scholar]
- Maga, J.A. Phytate: Its chemistry, occurrence, food interactions, nutritional significance and methods of analysis. J. Agric. Food Chem. 1982, 30, 1–9. [Google Scholar] [CrossRef]
- Raboy, V. Myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003, 64, 1033–1043. [Google Scholar] [CrossRef]
- Lenkova, T.N. Intestinal microbiota and productive qualities broilers using phytase to increase the digestibility of phosphorus and nutrients from compound feeds. Agric. Biol. 2020, 55, 406–416. [Google Scholar] [CrossRef]
- Bhavsar, K.; Khire, J.M. Current research and future perspectives of phytase bioprocessing. RSC Adv. 2014, 4, 26677–26691. [Google Scholar] [CrossRef]
- Gessler, N.N.; Serdyuk, E.G.; Isakova, E.P.; Deryabina, Y.I. Phytases and the Prospects for Their Application. Appl. Biochem. Microb. 2018, 54, 352–360. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Isakova, E.P.; Gessler, N.N.; Deryabina, Y.I. Advances in immobilization of phytases and their application. Bioresour. Technol. 2023, 379, 129030. [Google Scholar] [CrossRef]
- Miller, K.K.; Alper, H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262. [Google Scholar] [CrossRef]
- Lu, R.; Cao, L.; Wang, K.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica to produce advanced biofuels: Current status and perspectives. Bioresour. Technol. 2021, 341, 125877. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef]
- Serdyuk, E.G.; Isakova, E.P.; Gessler, N.N.; Trubnikova, E.V.; Antipov, A.N.; Deryabina, Y.I. Activity of neutral phytase from Obesumbacterium proteus in recombinant strains of Yarrowia lipolytica under cultivation on low-grade vegetable substrate. Prikl. Biochem. Microb. 2019, 55, 498–505. [Google Scholar] [CrossRef]
- Isakova, E.P.; Gessler, N.N.; Deryabina, Y.I. Comparative Assay of Phytase Activity in Yarrowia lipolytica Strains Transformed with the Neutrophilic Phytase Genome from Obesumbacterium proteus in Batch Cultivation. Appl. Biochem. Microb. 2022, 58, S126–S131. [Google Scholar] [CrossRef]
- Danilova, M.A.; Epova, E.Y.; Trubnikova, E.V.; Badrutdinov, N.V.; Kokoreva, A.S.; Pusev, M.S.; Deryabina, Y.I.; Isakova, E.P. Encapsulated Phytase Produced by Recombinant Yarrowia lipolytica Exhibits High Efficiency on Broiler Chickens in Low Dosage. Appl. Sci. 2022, 12, 11999. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Butt, A. Replacement of Fish Meal with Poultry By–Product Meal (Chicken Intestine) as a Protein Source in Grass Carp Fry Diet. Pak. J. Zool. 2012, 44, 1373–1381. [Google Scholar]
- Ziarat, M.M.; Kermanshahi, H.; Mogaddam, H.N.; Heravi, R.M. Performance of an Escherichia coli phytase expressed in Lactococcus lactis on nutrient retention, bone traits and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2020, 104, 909–917. [Google Scholar] [CrossRef]
- Shastak, Y.; Witzig, M.; Hartung, K.; Bessei, W.; Rodehutscord, M. Comparison and evaluation of bone measurements for the assessment of mineral phosphorus sources in broilers. Poult. Sci. 2012, 91, 2210–2220. [Google Scholar] [CrossRef]
- Norton, J.D.; Yang, S.P.; Diffley, P. Influence of source and quantity of protein on the development of immunity and resistance to African trypanosomiasis. Infect. Immun. 1986, 51, 455–460. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Abd El-Hack, M.; Alagawany, M.; Arif, M.; Emam, M.; Saeed, V.; Arain, M.A.; Siyal, F.A.; Patra, A.; Elnesr, S.S.; Ullah Khan, R. The uses of microbial phytase as a feed additive in poultry nutrition-a review. Ann. Anim. Sci. 2018, 18, 639–658. [Google Scholar] [CrossRef]
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef]
- Kim, T.W.; Lei, X.G. An improved method for a rapid determination of phytase activity in animal feed. J. Anim. Sci. 2005, 83, 1062–1067. [Google Scholar] [CrossRef]
- Alam, S.; Masood, S.; Zaneb, H.; Rabbani, I.; Khan, R.U.; Shah, M.; Ashraf, S.; Alhidary, I.A. Effect of Bacillus cereus and Phytase on the Expression of Musculoskeletal Strength and Gut Health in Japanese Quail (Coturnix japonica). J. Poult. Sci. 2020, 57, 200–204. [Google Scholar] [CrossRef]
- Oduguwa, O.; Pirgozliev, V.; Acamovic, T. Energy metabolizability and digestibility of amino acids by broilers fed malted sorghum sprouts supplemented with polyethylene glycol, charcoal phytase and xylanase. Br. Poult. Sci. 2007, 48, 55–63. [Google Scholar] [CrossRef]
- Mitchell, D.B.; Vogel, K.; Weimann, B.J.; Pasamontes, L.; van Loon, A.P.G.M. The phytase subfamily of histidine acid phosphatases: Isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 1997, 143, 245–252. [Google Scholar] [CrossRef]
- Troesch, B.; Jing, H.; Laillou, A.; Fowler, A. Absorption Studies Show that Phytase from Aspergillus niger Significantly Increases Iron and Zinc Bioavailability from Phytate-Rich Foods. Food Nutr. Bull. 2013, 34, 90–101. [Google Scholar] [CrossRef]
- Prasad, C.; Mandal, A.; Gowda, N.; Sharma, K.; Pattanaik, A.; Tyagi, P. Enhancing phosphorus utilization for better animal production and environment sustainability. Curr. Sci. 2015, 108, 1315–1319. [Google Scholar]
- Kumar, A.; Chanderman, A.; Makolomakwe, M.; Perumal, K.; Singh, S. Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Critical Rev. Environ. Sci. Technol. 2016, 46, 556–591. [Google Scholar] [CrossRef]
- Balaban, N.P.; Suleimanova, A.D.; Valeeva, L.R.; Chastukhina, I.B.; Rudakova, N.L.; Sharipova, M.R. Microbial Phytases and Phytate: Exploring Opportunities for Sustainable Phosphorus Management in Agriculture. Amer. J. Mol. Biol. 2017, 7, 11–29. [Google Scholar] [CrossRef]
- Ptak, A.; Bedford, M.R.; Świątkiewicz, S.; Żyła, K.; Józefiak, D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS ONE 2015, 10, e0119770. [Google Scholar] [CrossRef]
- Mikityuk, A.; Epifanov, V.; Simonov, G.; Zoteev, V.; Kerzhner, A.; Moseev, P. An enzyme supplement in the diet of quails. Compound Feed 2019, 7–8, 49–51. [Google Scholar] [CrossRef]
- Rezaeipour, V.; Barsalani, A.; Abdullahpour, R. Effects of phytase supplementation on growth performance, jejunum morphology, liver health, and serum metabolites of Japanese quails fed sesame (Sesamum indicum) meal-based diets containing graded levels of protein. Trop. Anim. Health Prod. 2016, 48, 1141–1146. [Google Scholar] [CrossRef]
- Mehraei Hamzekolaei, M.H.; Zamani Moghaddam, A.K.; Tohidifar, S.S.; Dehghani Samani, A.; Heydari, A. The effects of transportation stress on Japanese quail (Coturnix Coturnix japonica) fed corn-based diet in comparison with wheat-based diet supplemented with xylanase and phytase. J. Anim. Physiol. Anim. Nutr. 2016, 100, 618–622. [Google Scholar] [CrossRef]
- Vieira, B.S.; Caramori Junior, J.G.; Correa, G.S.S.; Colvara, I.G.; Brusamarelo, E.; Pereira, T.V.S.; Barbosa, S.A.P.V.; Oliveira, C.F.S. Combination of phytase and citric acid, but not phytase alone, ensures regular rates of growth and bone mineralization in quails under severe phosphorus restriction. J. Anim. Physiol. Anim. Nutr. 2019, 103, 555–563. [Google Scholar] [CrossRef]
- Ravindran, V.; Bryden, W.L.; Kornegay, E.T. Phytate: Occurrences, bioavailability and implications in poultry nutrition. Avian Poult. Biol. Rev. 1995, 6, 125–143. [Google Scholar]
- Lim, H.S.; Namkung, H.; Paik, I.K. Effects of phytase supplementation on the performance, egg quality, and phosphorous excretion of laying hens fed different levels of dietary calcium and nonphytate phosphorous. Poult. Sci. 2003, 82, 92–99. [Google Scholar] [CrossRef]
- Casartelli, E.M.; Junqueira, O.M.; Laurentiz, A.C.; Filardi, R.S.; Lucas Júnior, J.; Araujo, L.F. Effect of phytase in laying hen diets with different phosphorus sources. Braz. J. Poult. Sci. 2005, 7, 93–98. [Google Scholar] [CrossRef]


| 1 kg of the Feed Contains (%) | Groups | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 (9 g/kg) | Group 4 (9 g/kg) | Group 5 (3 g/10 kg) | Group 6 (9 g/10 kg) | |
| Soybean meal | 42.54 | 42.42 | 42.91 | 43.06 | 42.40 | 42.46 |
| Wheat | 28.60 | 29.14 | 27.23 | 26.68 | 29.19 | 28.95 |
| Corn | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Sunflower oil | 4.77 | 4.62 | 5.14 | 5.29 | 4.61 | 4.67 |
| OPP | - | - | 0.90 | 0.90 | - | - |
| Ladozym proxy | - | - | - | - | 0.03 | 0.09 |
| Monocalcium phosphate | 1.50 | 0.99 | 0.99 | 1.51 | 0.89 | 0.99 |
| Limestone Ca | 1.19 | 1.44 | 1.44 | 1.18 | 1.49 | 1.44 |
| Premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 |
| Methionine | 0.27 | 0.26 | 0.27 | 0.27 | 0.26 | 0.27 |
| Lysine | 0.09 | 0.09 | 0.08 | 0.08 | 0.09 | 0.09 |
| Threonine | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Choline chloride | 0.08 | 0.08 | 0.08 | 0.07 | 0.08 | 0.08 |
| Soda | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| FEKORD enzyme | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| 100 g of the feed contains (%) | ||||||
| Metabolizable energy (kcal) | 300 | 300 | 300 | 300 | 300 | 300 |
| Crude fiber | 24.85 | 24.85 | 24.85 | 24.85 | 24.85 | 24.85 |
| Raw fiber | 4.28 | 4.28 | 4.27 | 4.26 | 4.28 | 4.28 |
| Calcium | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total phosphorus | 0.77 | 0.65 | 0.65 | 0.77 | 0.63 | 0.65 |
| Phosphorus assimilated | 0.45 | 0.35 | 0.35 | 0.45 | 0.45 | 0.35 |
| Sodium | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chlorine | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Lysine assimilated | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| Methionine assimilated | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 |
| Threonine assimilated | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| 1 kg of the Feed Contains (%) | Groups | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 (5 g/kg) | Group 4 (5 g/kg) | Group 5 (3 g/10 kg) | Group 6 (9 g/10 kg) | |
| Soybean meal | 11.11 | 11.03 | 10.10 | 10.18 | 11.12 | 11.06 |
| Wheat | 54.79 | 55.14 | 54.64 | 54.30 | 54.75 | 55.02 |
| Corn | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Sunflower seed cake | 13.39 | 13.37 | 13.82 | 13.84 | 13.39 | 13.37 |
| Sunflower oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| OPP | - | - | 0.50 | 0.50 | - | - |
| Ladozym proxy | - | - | - | - | 0.03 | 0.09 |
| Monocalcium phosphate | 1.56 | 1.05 | 1.06 | 1.57 | 1.56 | 1.05 |
| Limestone Ca | 1.87 | 2.13 | 2.13 | 1.87 | 1.87 | 2.13 |
| Premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| Methionine | 0.03 | 0.03 | 0.04 | 0.04 | 0.03 | 0.03 |
| Lysine | 0.30 | 0.30 | 0.37 | 0.36 | 0.30 | 0.30 |
| Threonine | - | - | 0.39 | 0.39 | - | - |
| Choline chloride | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| The FEKORD enzyme | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| 100 g of the feed contains (%) | ||||||
| Metabolizable energy (kcal) | 2750 | 2750 | 2750 | 2750 | 2750 | 2750 |
| Crude fiber | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 |
| Raw fiber | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Calcium | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Total phosphorus | 0.80 | 0.68 | 0.68 | 0.80 | 0.80 | 0.68 |
| Phosphorus assimilated | 0.45 | 0.35 | 0.35 | 0.45 | 0.45 | 0.35 |
| Sodium | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chlorine | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 |
| Lysine assimilated | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| Methionine assimilated | 0.29 | 0.29 | 0.30 | 0.30 | 0.29 | 0.29 |
| 1 kg of the Feed Contains (%) | Groups | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 (5 g/kg) | Group 4 (5 g/kg) | Group 5 (3 g/10 kg) | Group 6 (9 g/10 kg) | |
| Soybean meal | 19.95 | 19.84 | 20.05 | 20.16 | 19.96 | 19.87 |
| Wheat | 31.92 | 32.49 | 31.39 | 30.84 | 31.86 | 32.29 |
| Corn | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Sunflower seed cake | 8.57 | 8.53 | 8.60 | 8.64 | 8.57 | 8.54 |
| Full-fat soy | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Sunflower oil | 3.78 | 3.63 | 3.93 | 4.08 | 3.80 | 3.69 |
| OPP | - | - | 0.50 | 0.50 | - | - |
| Ladozym proxy | - | - | - | - | 0.03 | 0.09 |
| Monocalcium phosphate | 1.48 | 0.96 | 0.97 | 1.48 | 1.48 | 0.97 |
| Limestone Са | 6.27 | 6.53 | 6.53 | 6.27 | 6.27 | 6.53 |
| Premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Methionine | 0.09 | 0.08 | 0.09 | 0.09 | 0.09 | 0.08 |
| Choline chloride | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| FEKORD enzyme | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| 100 g of the feed contains (%) | ||||||
| Metabolizable energy (кcal) | 2850 | 2850 | 2850 | 2850 | 2850 | 2850 |
| Crude fiber | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 |
| Raw fiber | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Calcium | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 |
| Phosphorus total | 0.79 | 0.67 | 0.67 | 0.79 | 0.79 | 0.67 |
| Phosphorus assimilated | 0.45 | 0.35 | 0.35 | 0.45 | 0.45 | 0.35 |
| Sodium | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chloride | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Methionine assimilated | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| Day of the Quail’s Life | Average Body Weight (g) | |||||
|---|---|---|---|---|---|---|
| 1 (Positive Control) | 2 (Negative Control) | 3 | 4 | 5 | 6 | |
| BW 7d | 39.2 ± 0.52 | 37.5 ± 0.68 | 38.2 ± 0.50 | 39.5 ± 0.55 | 38.7 ± 0.51 | 37.88 ± 0.68 |
| Ratio with the positive control | 0.00 | −1.70 | −1.00 | +0.30 | −0.50 | −1.32 |
| Ratio with the positive control (%) | 0.00% | −4.34% | −2.55% | +0.77% | −1.28% | 3.37% |
| BW 14d | 80.4 ± 1.08 | 78.7 ± 1.08 | 78.8 ± 1.06 | 81.9 ± 0.89 | 80.9 ± 1.14 | 79.6 ± 1.38 |
| Ratio with the positive control | 0.00 | −1.70 | −1.60 | +1.50 | +0.50 | −0.80 |
| Ratio with the positive control (%) | 0.00% | −2.10% | −1.99% | +1.87% | +0.66% | −1.00% |
| BW 21d | 140.9 ± 1.81 | 138.9 ± 1.87 | 139.7 ± 1.47 | 142.6 ± 1.43 | 142 ± 1.80 | 136.3 ± 1.94 |
| Ratio with the positive control | 0.0 | −2.00 | −1.20 | +1.70 | +1.10 | −3.70 |
| Ratio with the positive control (%) | 0.0% | −1.42% | −0.85% | +1.21% | +0.78% | −3.26% |
| BW 28d | 169 ± 1.88 a | 168.2 ± 2.24 | 167.9 ± 1.69 b | 171.4 ± 1.80 a,b | 170.65 ± 2.19 a | 164.4 ± 2.18 |
| Ratio with the positive control | 0.0 | −0.8 | −1.10 | +2.40 | +1.65 | −4.6 |
| Ratio with the positive control (%) | 0.0% | −0.47% | −0.65% | +1.42% | +0.06% | −2.74% |
| BW 35d | 196.9 ± 3.52 a | 195.9 ± 4.90 | 196.5 ± 2.73 a,b | 201.1 ± 3.28 a,b | 196.2 ± 2.92 a | 192.1 ± 3.61 |
| Ratio with the positive control | 0.0 | −1.00 | −0.40 | +4.20 | −0.70 | −4.80 |
| Ratio with the positive control (%) | 0.0% | −0.51% | −0.20% | +2.13% | −0.36% | −2.44% |
| BW 42d | hens | |||||
| 218.5 ± 4.04 a | 216 ± 5.39 | 218.1 ± 3.60 a,b | 240.4 ± 4.15 a,b | 233.9 ± 6.96 a | 215.25 ± 6.09 | |
| Ratio with the positive control | 0.0 | −2.5 | −0.40 | +21.9 | +15.40 | −3.25 |
| Ratio with the positive control (%) | 0.0% | −1.14% | −1.18% | +10.02% | +7.05% | −1.49% |
| BW 42d | roosters | |||||
| 194.7 ± 4.73 a | 190.6 ± 4.89 | 191.7 ± 4.14 a,b | 196.9 ± 3.94 a,b | 189.0 ± 4.23 a | 180.3 ± 3.18 | |
| Ratio with the positive control | 0.0 | −4.10 | −3.00 | +2.20 | −5.7 | −14.40 |
| Ratio with the positive control (%) | 0.0% | −2.11% | −1.54% | +1.13% | −2.93% | −7.40% |
| Group Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Average daily gain (g) | 4.99 ± 0.13 a | 4.94 ± 0.15 a | 4.91 ± 0.09 b | 5.00 ± 0.1 b | 4.84 ± 0.11 b | 4.73 ± 0.13 d |
| Feed consumption per head per day (g) | 19.66 ± 0.8 a | 19.71 ± 0.7 b | 19.43 ± 0.8 a | 19.59 ± 0.9 c | 19.66 ± 0.8 a | 19.33 ± 0.9 d |
| Feed consumption per kg of live weight (kg) | 3.75 ± 0.3 a | 3.80 ± 0.2 c | 3.77 ± 0.4 b | 3.73 ± 0.3 a | 3.87 ± 0.2 d | 3.89 ± 0.1 d |
| FCR * | 3.99 | 3.99 | 3.95 | 3.91 | 4.06 | 4.08 |
| Group Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Yield of carcass parts: breast thigh + drumstick | 39.3 ± 2.4 a 24.8 ± 3.0 b | 38.4 ± 1.9 c 23.8 ± 1.7 c | 37.1 ± 1.8 b 23.5 ± 2.0 c | 40.5 ± 3.1 a 25.1 ± 2.3 c | 36.6 ± 3.1 b 22.4 ± 1.9 d | 35.7 ± 3.2 a 21.6 ± 1.9 c |
| Yield of muscle parts: breast (fillet) thigh + drumstick | 27.3 ± 1.9 a 17.0 ± 1.2 b | 26.5 ± 1.7 b 16.9 ± 1.2 c | 25.3 ± 1.7 a 16.2 ± 1.3 b | 28.4 ± 1.8 18.1 ± 1.3 a | 24.2 ± 1.9 a 15.5 ± 1.4 | 23.8 ± 1.8 b 15.1 ± 1.4 |
| % | Groups | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Calcium | 20.25 | 22.19 | 21.76 | 21.87 | 21.90 | 21.84 |
| Phosphorus | 10.48 | 10.99 | 10.85 | 11.18 | 11.10 | 11.04 |
| Group Number | Hens | Roosters | Average Live Weight |
|---|---|---|---|
| 1 | 273.3 ± 5.78 a | 211.5 ± 5.50 a | 260.9 ± 9.48 a |
| 2 | 251.3 ± 6.15 a | 208.5 ± 2.50 a | 242.7 ± 10.29 |
| 3 | 268.2 ± 6.80 | 206.5 ± 2.80 | 255.7 ± 9.92 |
| 4 | 276.3 ± 8.86 a | 218.4 ± 3.50 a | 264.7 ± 10.40 a |
| 5 | 257.6 ± 6.34 a | 196.5 ± 6.50 a | 245.4 ± 9.48 a |
| 6 | 249.1 ± 4.50 a | 200.5 ± 4.50 a | 239.4 ± 6.15 |
| Group Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Average daily gain (g) | 2.4 ± 0.2 a | 1.51 ± 0.1 b | 2.31 ± 0.1 c | 2.59 ± 0.2 a | 1.90 ± 0.2 a | 1.81 ± 0.2 c |
| Feed consumption per 1 head per day (g) | 33.49 ± 2.3 | 33.02 ± 2.4 b | 31.95 ± 2.4 c | 32.82 ± 2.3 | 33.15 ± 2.4 b | 32.75 ± 2.1 c |
| Feed expenses per average body weight (kg) | 2.31 ± 0.1 a | 2.45 ± 0.1 b | 2.25 ± 0.1 c | 2.23 ± 0.2 b | 2.43 ± 0.1 c | 2.46 ± 0.2 a |
| Egg productivity (%) | 77.1 ± 4.8 | 73.6 ± 4.6 | 77.8 ± 4.7 | 86.8 ± 4.9 | 75.0 ± 4.5 | 77.8 ± 4.6 |
| FCR * | 2.31 | 2.45 | 2.25 | 2.23 | 2.43 | 2.46 |
| Groups | Macro-Element Amount (% of Dry Weight) | Trace Element Amount (µg/g of Dry Weight) | ||||
|---|---|---|---|---|---|---|
| P | Mg | K | Ca | Zn | Cu | |
| 14 days of the experiment | ||||||
| 1 | 1.27 | 0.38 | 2.06 | 1.37 | 1148 | 92 |
| 3 | 0.96 | 0.36 | 2.05 | 1.01 | 1158 | 113 |
| 4 | 1.30 | 0.46 | 2.35 | 1.21 | 1071 | 89 |
| 5 | 1.24 | 0.39 | 2.19 | 1.04 | 1205 | 91 |
| 28 days of the experiment | ||||||
| 1 | 1.47 | 0.44 | 1.91 | 1.93 | 681 | 55 |
| 3 | 1.32 | 0.45 | 2.00 | 1.66 | 784 | 65 |
| 4 | 1.38 | 0.44 | 1.90 | 1.63 | 723 | 62 |
| 5 | 1.61 | 0.47 | 1.89 | 1.85 | 642 | 48 |
| 42 days of the experiment | ||||||
| 1 | 1.42 | 0.41 | 1.85 | 1.82 | 539 | 51 |
| 3 | 1.47 | 0.43 | 1.79 | 1.79 | 565 | 49 |
| 4 | 1.41 | 0.46 | 1.83 | 1.81 | 551 | 48 |
| 5 | 1.53 | 0.42 | 1.81 | 1.93 | 558 | 50 |
| Diets for the Groups | Macro-Element Amount (% of Dry Weight) | Trace Element Amount (µg/g of Dry Weight) | ||||
|---|---|---|---|---|---|---|
| P | Mg | K | Ca | Zn | Cu | |
| 1 | 0.71 | 0.23 | 1.31 | 1.05 | 373 | 41 |
| 2 | 0.61 | 0.28 | 1.27 | 1.37 | 366 | 64 |
| 3 | 0.73 | 0.24 | 1.40 | 1.42 | 448 | 49 |
| 4 | 0.69 | 0.21 | 1.30 | 0.93 | 246 | 30 |
| 5 | 0.73 | 0.22 | 1.34 | 1.16 | 340 | 42 |
| 6 | 0.58 | 0.22 | 1.39 | 0.90 | 320 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovseychik, E.A.; Klein, O.I.; Gessler, N.N.; Deryabina, Y.I.; Lukashenko, V.S.; Isakova, E.P. The Efficacy of Encapsulated Phytase Based on Recombinant Yarrowia lipolytica on Quails’ Zootechnic Features and Phosphorus Assimilation. Vet. Sci. 2024, 11, 91. https://doi.org/10.3390/vetsci11020091
Ovseychik EA, Klein OI, Gessler NN, Deryabina YI, Lukashenko VS, Isakova EP. The Efficacy of Encapsulated Phytase Based on Recombinant Yarrowia lipolytica on Quails’ Zootechnic Features and Phosphorus Assimilation. Veterinary Sciences. 2024; 11(2):91. https://doi.org/10.3390/vetsci11020091
Chicago/Turabian StyleOvseychik, Ekanerina A., Olga I. Klein, Natalia N. Gessler, Yulia I. Deryabina, Valery S. Lukashenko, and Elena P. Isakova. 2024. "The Efficacy of Encapsulated Phytase Based on Recombinant Yarrowia lipolytica on Quails’ Zootechnic Features and Phosphorus Assimilation" Veterinary Sciences 11, no. 2: 91. https://doi.org/10.3390/vetsci11020091
APA StyleOvseychik, E. A., Klein, O. I., Gessler, N. N., Deryabina, Y. I., Lukashenko, V. S., & Isakova, E. P. (2024). The Efficacy of Encapsulated Phytase Based on Recombinant Yarrowia lipolytica on Quails’ Zootechnic Features and Phosphorus Assimilation. Veterinary Sciences, 11(2), 91. https://doi.org/10.3390/vetsci11020091





