Antimicrobial Residue Accumulation Contributes to Higher Levels of Rhodococcus equi Carrying Resistance Genes in the Environment of Horse-Breeding Farms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection
2.2. Questionnaire Collection
2.3. Sample Collection
2.4. Sample Processing
2.4.1. R. equi Quantification
2.4.2. Antimicrobial Residue Quantification
2.5. Data Analysis
3. Results
3.1. Descriptive Data from Questionnaires
3.2. Prevalence of R. equi Carrying Antimicrobial Resistance Genes (AMRGs)
3.3. Prevalence of Multidrug-Resistant R. equi
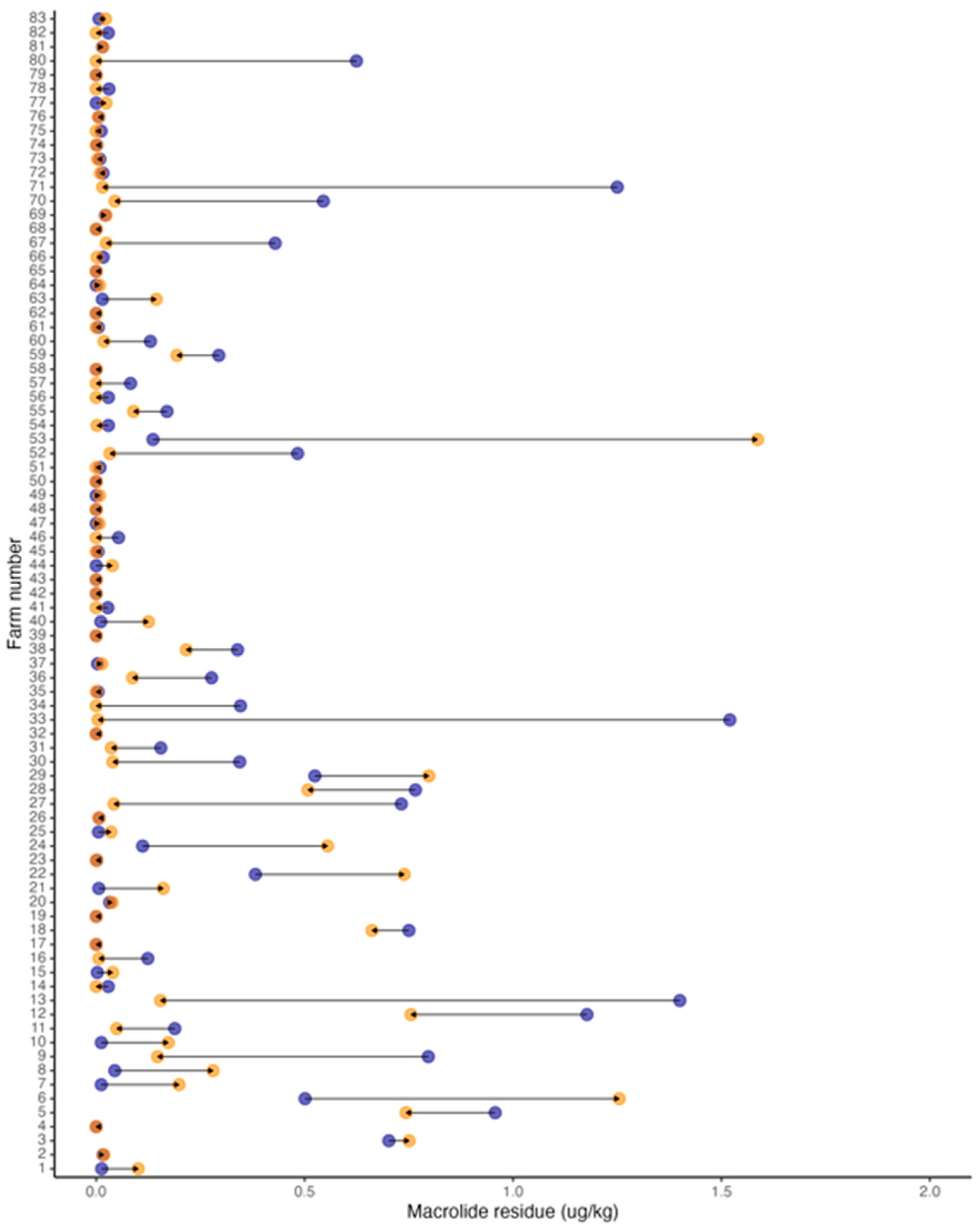
3.4. Prevalence of Antimicrobial Residues
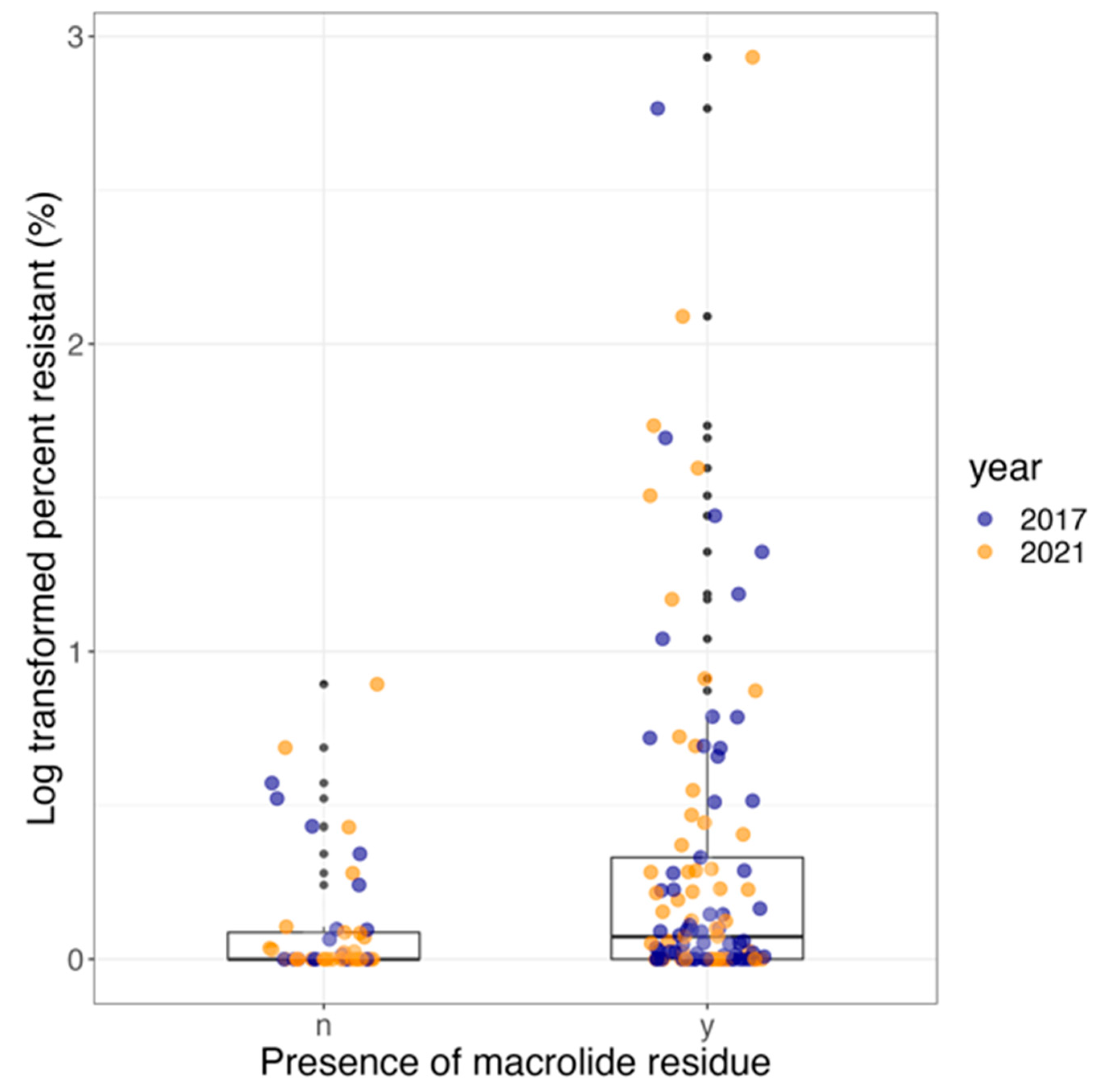
3.5. Effect of Antimicrobial Use and Year on R. equi Carrying AMRGs
3.6. Effect of Using Thoracic Ultrasound Screening (TUS) and Year on R. equi Carrying AMRGs
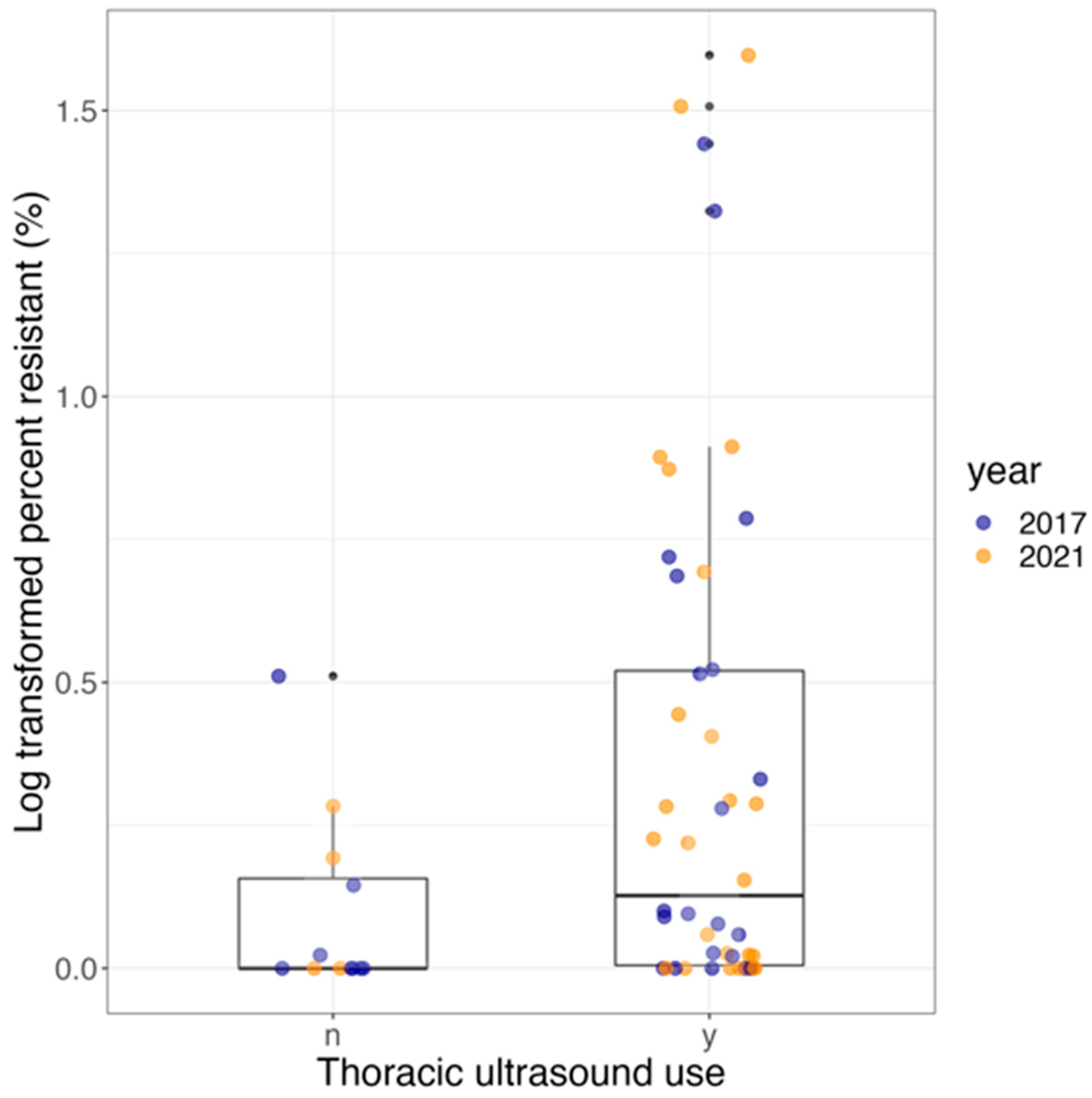
3.7. Effect of Using Thoracic Ultrasound Screening (TUS) and Year on Antimicrobial Residues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 September 2023).
- Centers for Disease Control. Get Smart: Know When Antibiotics Work. 2010. Available online: www.cdc.gov/Features/GetSmart (accessed on 1 September 2023).
- Food and Drug Administration. FDA Task Force on Antimicrobial Resistance: Key Recommendations and Report. 2000. Available online: http://fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM143458.pdf (accessed on 1 September 2023).
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Blount, K.F.; Breaker, R.R. Riboswitches as antibacterial drug targets. Nat. Biotechnol. 2006, 24, 1558–1564. [Google Scholar] [CrossRef]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef]
- Goh, E.-B.; Yim, G.; Tsui, W.; McClure, J.; Surette, M.G.; Davies, J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Giguère, S.; Cohen, N.; Chaffin, M.K.; Slovis, N.; Hondalus, M.; Hines, S.; Prescott, J. Diagnosis, treatment, control, and prevention of infections caused by Rhodococcus equi in foals. J. Vet. Intern. Med. 2011, 25, 1209–1220. [Google Scholar] [CrossRef]
- Verville, T.D.; Huycke, M.M.; Greenfield, R.A.; Fine, D.P.; Kuhls, T.L.; Slater, L.N. Rhodococcus equi Infections of Humans: 12 Cases and a Review of the Literature. Medicine 1994, 73, 119–132. [Google Scholar] [CrossRef]
- Stranahan, L.W.; Plumlee, Q.D.; Lawhon, S.D.; Cohen, N.D.; Bryan, L.K. Rhodococcus equi Infections in Goats: Characterization of Virulence Plasmids. Vet. Pathol. 2018, 55, 273–276. [Google Scholar] [CrossRef]
- Giguère, S.; Hondalus, M.K.; Yager, J.A.; Darrah, P.; Mosser, D.M.; Prescott, J.F. Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein a in intracellular survival and virulence of Rhodococcus equi. Infect. Immun. 1999, 67, 3548–3557. [Google Scholar] [CrossRef]
- Slovis, N.M.; McCracken, J.; Mundy, D. How to use thoracic ultrasound to screen foals for Rhodococcus equi at affected farms. Proc. Am. Assoc. Equine Pract. 2005, 51, 274–278. [Google Scholar]
- Giguère, S.; Lee, E.A.; Guldbech, K.M.; Berghaus, L.J. In vitro synergy, pharmacodynamics, and postantibiotic effect of 11 antimicrobial agents against Rhodococcus equi. Vet. Microbiol. 2012, 160, 207–213. [Google Scholar] [CrossRef]
- Huber, L.; Giguère, S.; Slovis, N.M.; Carter, C.N.; Barr, B.S.; Cohen, N.D.; Elam, J.; Erol, E.; Locke, S.J.; Phillips, E.D.; et al. Emergence of resistance to macrolides and rifampin in clinical isolates of rhodococcus equi from foals in central kentucky, 1995 to 2017. Antimicrob. Agents Chemother. 2018, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Cohen, N.D.; Slovis, N.M.; Hanafi, A.; Schuckert, A.; Berghaus, L.; Greiter, M.; Hart, K.A. Prevalence and risk factors associated with emergence of Rhodococcus equi resistance to macrolides and rifampicin in horse-breeding farms in Kentucky, USA. Vet. Microbiol. 2019, 235, 243–247. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.M.; Halling-Sørensen, B.; Ingerslev, F.; Hansen, S.H. Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2004, 1038, 157–170. [Google Scholar] [CrossRef]
- Grimm, M.B.; Cohen, N.D.; Slovis, N.M.; Mundy, G.D.; Harrington, J.R.; Libal, M.C.; Takai, S.; Martens, R.J. Evaluation of fecal samples from mares as a source of Rhodococcus equi for their foals by use of quantitative bacteriologic culture and colony immunoblot analyses. Am. J. Vet. Res. 2007, 68, 63–71. [Google Scholar] [CrossRef]
- Ladrón, N.; Fernández, M.; Agüero, J.; Zörn, B.G.; Vázquez-Boland, J.A.; Navas, J. Rapid identification of Rhodococcus equi by a PCR assay targeting the choE gene. J. Clin. Microbiol. 2003, 41, 3241–3245. [Google Scholar] [CrossRef]
- Halbert, N.D.; Reitzel, R.A.; Martens, R.J.; Cohen, N.D. Evaluation of a multiplex polymerase chain reaction assay for simultaneous detection of Rhodococcus equi and the vapA gene. Am. J. Vet. Res. 2005, 66, 1380–1385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anastasi, E.; Giguère, S.; Berghaus, L.J.; Hondalus, M.K.; Willingham-Lane, J.M.; MacArthur, I.; Cohen, N.D.; Roberts, M.C.; Vazquez-Boland, J.A. Novel transferable erm(46) determinant responsible for emerging macrolide resistance in Rhodococcus equi. J. Antimicrob. Chemother. 2015, 70, 3184–3190. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Slovis, N.M.; Álvarez-Narváez, S.; Hart, K.A.; Greiter, M.; Morris, E.R.A.; Cohen, N.D. The novel and transferable erm(51) gene confers macrolides, lincosamides and streptogramins B (MLSB) resistance to clonal Rhodococcus equi in the environment. Environ. Microbiol. 2020, 22, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. vol. 29th edition, Jan. 2019. Available online: https://clsi.org/media/1930/m100ed28_sample.pdf (accessed on 1 September 2023).
- Berghaus, L.J.; Giguère, S.; Guldbech, K.; Warner, E.; Ugorji, U.; Berghaus, R.D. Comparison of etest, disk diffusion, and broth macrodilution for in vitro susceptibility testing of Rhodococcus equi. J. Clin. Microbiol. 2015, 53, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chhonker, Y.S.; Bisen, A.C.; Prasad, Y.D.; Tulsankar, S.L.; Chandasana, H.; Dey, T.; Verma, S.K.; Bala, V.; Kanojiya, S.; et al. Rapid and simultaneous analysis of multiple classes of antimicrobial drugs by liquid chromatography-tandem mass spectrometry and its application to routine biomedical, food, and soil analyses. ACS Omega 2020, 5, 31584–31597. [Google Scholar] [CrossRef]
- Huber, L.; Giguère, S.; Hart, K.A.; Slovis, N.M.; Greiter, M.E.; Dailey, C.A.; Cohen, N.D. Association between antimicrobial treatment of subclinical pneumonia in foals and selection of macrolide- and rifampicin-resistant Rhodococcus equi strains at horse-breeding farms in central Kentucky. J. Am. Vet. Med. Assoc. 2021, 258, 648–653. [Google Scholar] [CrossRef]
- Tu, Z.; Shui, J.; Liu, J.; Tuo, H.; Zhang, H.; Lin, C.; Feng, J.; Feng, Y.; Su, W.; Zhang, A. Exploring the abundance and influencing factors of antimicrobial resistance genes in manure plasmidome from swine farms. J. Environ. Sci. 2023, 124, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and Antibiotic Resistance in Agroecosystems: State of the Science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Bester, K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ. Pollut. 2006, 143, 565–571. [Google Scholar] [CrossRef]
- Berglund, F.; Böhm, M.-E.; Martinsson, A.; Ebmeyer, S.; Österlund, T.; Johnning, A.; Larsson, D.G.J.; Kristiansson, E. Comprehensive screening of genomic and metagenomic data reveals a large diversity of tetracycline resistance genes. Microb. Genom. 2020, 6, e000455. [Google Scholar] [CrossRef]
- Prescott, J.F. Rhodococcus equi: An animal and human pathogen. Clin. Microbiol. Rev. 1991, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes following Land Application of Manure Waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [PubMed]
- Val-Calvo, J.; Darcy, J.; Gibbons, J.; Creighton, A.; Egan, C.; Buckley, T.; Schmalenberger, A.; Fogarty, U.; Scortti, M.; Vázquez-Boland, J.A. International Spread of Multidrug-Resistant Rhodococcus equi. Emerg. Infect. Dis. 2022, 28, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Vogwill, T.; Comfort, A.C.; Furió, V.; MacLean, R.C. Persistence and resistance as complementary bacterial adaptations to antibiotics. J. Evol. Biol. 2016, 29, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, M.; Shah, D.H.; Besser, T.E.; Ullman, J.L.; Call, D.R. Urine from Treated Cattle Drives Selection for Cephalosporin Resistant Escherichia coli in Soil. PLoS ONE 2012, 7, e48919. [Google Scholar] [CrossRef]
- Reichel, R.; Rosendahl, I.; Peeters, E.T.; Focks, A.; Groeneweg, J.; Bierl, R.; Schlichting, A.; Amelung, W.; Thiele-Bruhn, S. Effects of slurry from sulfadiazine- (SDZ) and difloxacin- (DIF) medicated pigs on the structural diversity of microorganisms in bulk and rhizosphere soil. Soil Biol. Biochem. 2013, 62, 82–91. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.-P.; Fu, J.; Zhou, Z.-Q.; Zhang, N.; Guo, L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fertil. Soils 2014, 50, 939–947. [Google Scholar] [CrossRef]
- Manzetti, S.; Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef]
- Duan, M.; Li, H.; Gu, J.; Tuo, X.; Sun, W.; Qian, X.; Wang, X. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce. Environ. Pollut. 2017, 224, 787–795. [Google Scholar] [CrossRef]
- Carlson, J.C.; Mabury, S.A. Dissipation kinetics and mobility of chlortetracycline, tylosin, and monensin in an agricultural soil in Northumberland County, Ontario, Canada. Environ. Toxicol. Chem. 2006, 25, 1–10. [Google Scholar] [CrossRef]
- Davis, J.G.; Truman, C.C.; Kim, S.C.; Ascough, J.C.; Carlson, K.; Ii, J.C.A. Antibiotic Transport via Runoff and Soil Loss. J. Environ. Qual. 2006, 35, 2250–2260. [Google Scholar] [CrossRef]
- McGuire, J.M.; Bunch, R.L.; Anderson, R.C.; Boaz, H.E.; Flynn, E.H.; Powell, H.M.; Smith, J.W. Ilotycin, a new antibiotic. Antibiot. Chemother. 1952, 2, 281–283. [Google Scholar]
- Fernandes, P.; Martens, E.; Pereira, D. Nature nurtures the design of new semi-synthetic macrolide antibiotics. J. Antibiot. 2017, 70, 527–533. [Google Scholar] [CrossRef]
- Burton, A.J.; Giguère, S.; Sturgill, T.L.; Berghaus, L.J.; Slovis, N.M.; Whitman, J.L.; Levering, C.; Kuskie, K.R.; Cohen, N.D. Macrolide- and rifampin-resistant Rhodococcus equi on a horse breeding farm, Kentucky, USA. Emerg. Infect. Dis. 2013, 19, 282–285. [Google Scholar] [CrossRef]
- Shi, H.; Hu, X.; Li, W.; Zhang, J.; Hu, B.; Lou, L. Soil Component: A Potential Factor Affecting the Occurrence and Spread of Antibiotic Resistance Genes. Antibiotics 2023, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.; Hernandez-Leal, L.; van der Maas, P.; Heederik, D.; Mevius, D.; Schmitt, H. The impact of manure and soil texture on antimicrobial resistance gene levels in farmlands and adjacent ditches. Sci. Total Environ. 2020, 737, 139563. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Gu, X.; Zheng, Y.; Delgado-Moreno, L.; Jia, W.; Ye, Q.; Wang, W. The fate of erythromycin in soils and its effect on soil microbial community structure. Sci. Total Environ. 2022, 820, 153373. [Google Scholar] [CrossRef]
- Huijbers, P.M.; Flach, C.-F.; Larsson, D.J. A conceptual framework for the environmental surveillance of antibiotics and antibiotic resistance. Environ. Int. 2019, 130, 104880. [Google Scholar] [CrossRef]
- Arnold-Lehna, D.; Venner, M.; Berghaus, L.J.; Berghaus, R.; Giguère, S. Changing policy to treat foals with Rhodococcus equi pneumonia in the later course of disease decreases antimicrobial usage without increasing mortality rate. Equine Vet. J. 2020, 52, 531–537. [Google Scholar] [CrossRef] [PubMed]

| Data from Farms with Questionnaire (29 Farms) | 2017 | 2021 |
|---|---|---|
| Percentage of foals treated with any antimicrobial (median, IQR) | 10% (0–20%) | 12% (3–27%) |
| Percentage of foals treated with macrolides (median, IQR) | 0% (0–10%) | 2% (0–7%) |
| Proportion of foals treated with rifampicin (median, IQR) | 7% (0–19%) | 7% (0–18%) |
| Number of farms that treated foals with any antimicrobials (%) | 19/29 (66%) | 23/29 (79%) |
| Number of farms that treated foals with macrolides (%) | 11/29 (38%) | 20/29 (69%) |
| Number of farms that treated foals with rifampicin (%) | 18/29 (62%) | 22/29 (76%) |
| Density of animals/acre (median, IQR) | 0.85 (0.61–1.15) | 0.62 (0.44–1.06) |
| Foal mortality (median, IQR) | 0.00 (0–0.00) | 0.00 (0–0.002) |
| TUS in Farms Over Time (56 farms) | N (%) | |
| Number of farms that used TUS in 2017 Number of farms that used TUS in 2021 Number of farms that used TUS in the last decade Number of farms that did not use TUS in the last decade Number of farms that did use TUS from 2014 to 2017 but discontinued use of TUS after 2017 Number of farms that did not use TUS from 2014 to 2017 but began use of TUS after 2017 | 43/56 (77%) | |
| 37/56 (66%) | ||
| 32/56 (57%) | ||
| 8/56 (14%) | ||
| 15/56 (27%) | ||
| 1/56 (2%) | ||
| MDR-R. equi in Farms | N (%) | Percentage MDR R. equi (Median, Range) |
|---|---|---|
| Farms with MDR R. equi in 2017 | 52/83 (63%) | 0.05% (0.00–15%) |
| Farms with MDR R. equi in 2021 | 46/83 (55%) | 0.03% (0.00–18%) |
| Farms with increased MDR R. equi | 32/83 (39%) | 0.30% (0.00–2%) |
| Farms with decreased MDR R. equi | 30/83 (36%) | 0.40% (0.00–15%) |
| Farms that remained MDR R. equi-free | 21/83 (25%) | 0.00% (0.00–0.00%) |
| Farms that increased from zero MDR R. equi | 9/83 (11%) | 0.20% (0.07–0.90%) |
| Farms that decreased to zero MDR R. equi | 15/83 (18%) | 0.20% (0.05–0.30%) |
| Antimicrobial | Class | MIC (Median, Range) | % Susceptible | % Non-Susceptible |
|---|---|---|---|---|
| Azithromycin | Macrolides | >256 (NA) | 0% | 100% |
| Clarithromycin | Macrolides | >256 (NA) | 0% | 100% |
| Erythromycin | Macrolides | >256 (24–>256) | 0% | 100% |
| Tetracycline | Tetracyclines | 8 (4–12) | 0% | 100% |
| Doxycycline | Tetracyclines | 1 (0.023–>256) | 94% | 6% |
| Rifampicin | Ansamycins | >256 (0.032–>256) | 2% | 98% |
| Quinupristin-Dalfopristin | Streptogramins | 24 (3–>256) | 0% | 100% |
| Trimethoprim-sulfamethoxazole | Aminopyrimidines/Sulfonamides | 0.75 (0.19–>256) | 66% | 34% |
| Vancomycin | Glycopeptides | 0.19 (0.025–0.75) | 100% | 0% |
| Clindamycin | Lincosamides | >256 (3–>256) | 0% | 100% |
| Antimicrobial Residue in Farms | N (%) | Macrolide Residue (Median, Range; μg/Kg) |
|---|---|---|
| Farms with macrolide residue in 2017 | 64/83 (77%) | 0.016 (0.00–1.52) |
| Farms with macrolide residue in 2021 | 53/83 (64%) | 0.013 (0.00–1.58) |
| Farms with increased macrolide residue | 26/83 (31%) | 0.070 (0.020–0.26) |
| Farms with decreased macrolide residue | 43/83 (52%) | 0.030 (0.010–0.090) |
| Farms remained macrolide residue free | 14/83 (17%) | 0.00 (0.00–0.00) |
| Farms that increased from zero macrolide residue | 5/83 (6%) | 0.010 (0.010–0.020) |
| Farms that decreased to zero macrolide residue | 16/83 (19%) | 0.030 (0.00–0.040) |
| Negative Binomial Model | Estimate | SE | p-Value |
|---|---|---|---|
| Year 2021 (reference: 2017) | 0.22 | 0.30 | 0.48 |
| Presence of residue (reference: absence) | 1.12 | 0.51 | 0.02 * |
| Logistic Model | OR | 95% CI | p-value |
| Year 2021 (reference: 2017) | 0.75 | 0.33–1.65 | 0.45 |
| Presence of residue (reference: absence) | 3.55 | 1.19–10.52 | 0.03 * |
| Negative Binomial Model | Estimate | SE | p-Value |
|---|---|---|---|
| Year 2021 (reference: 2017) | 0.003 | 0.34 | 0.99 |
| Have used TUS at least in the last 4 years (reference: never used TUS or stopped this practice for at least 4 years) | 0.73 | 0.43 | 0.08 |
| Logistic Model | OR | 95% CI | p-value |
| Year 2021 (reference: 2017) | 0.79 | 0.31–2.05 | 0.63 |
| Have used TUS at least in the last 4 years (reference: never used TUS or stopped this practice for at least 4 years) | 5.43 | 1.24–23.89 | 0.03 * |
| Logistic Model | OR | 95% CI | p-Value |
|---|---|---|---|
| Year 2021 (reference: 2017) | 0.44 | 0.14–1.46 | 0.18 |
| Have used TUS at least in the last 4 years (reference: never used TUS or stopped this practice for at least 4 years) | 6.36 | 0.76–52.52 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, C.; Cohen, N.D.; Slovis, N.; Boersma, M.; Gaonkar, P.P.; Golden, D.R.; Huber, L. Antimicrobial Residue Accumulation Contributes to Higher Levels of Rhodococcus equi Carrying Resistance Genes in the Environment of Horse-Breeding Farms. Vet. Sci. 2024, 11, 92. https://doi.org/10.3390/vetsci11020092
Higgins C, Cohen ND, Slovis N, Boersma M, Gaonkar PP, Golden DR, Huber L. Antimicrobial Residue Accumulation Contributes to Higher Levels of Rhodococcus equi Carrying Resistance Genes in the Environment of Horse-Breeding Farms. Veterinary Sciences. 2024; 11(2):92. https://doi.org/10.3390/vetsci11020092
Chicago/Turabian StyleHiggins, Courtney, Noah D. Cohen, Nathan Slovis, Melissa Boersma, Pankaj P. Gaonkar, Daniel R. Golden, and Laura Huber. 2024. "Antimicrobial Residue Accumulation Contributes to Higher Levels of Rhodococcus equi Carrying Resistance Genes in the Environment of Horse-Breeding Farms" Veterinary Sciences 11, no. 2: 92. https://doi.org/10.3390/vetsci11020092
APA StyleHiggins, C., Cohen, N. D., Slovis, N., Boersma, M., Gaonkar, P. P., Golden, D. R., & Huber, L. (2024). Antimicrobial Residue Accumulation Contributes to Higher Levels of Rhodococcus equi Carrying Resistance Genes in the Environment of Horse-Breeding Farms. Veterinary Sciences, 11(2), 92. https://doi.org/10.3390/vetsci11020092






