Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Beta-Adrenergic Agonists
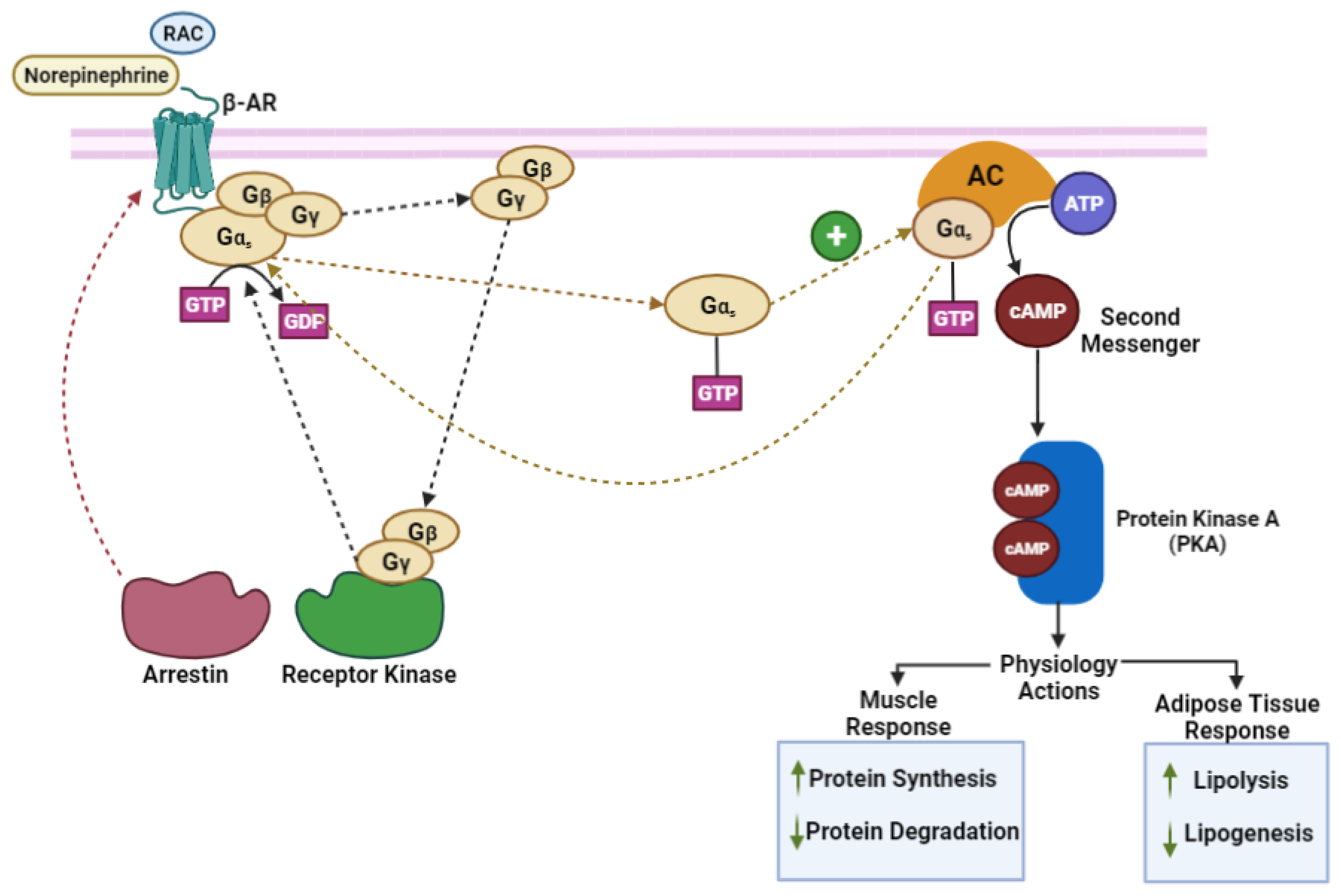
3. Mechanism of Action
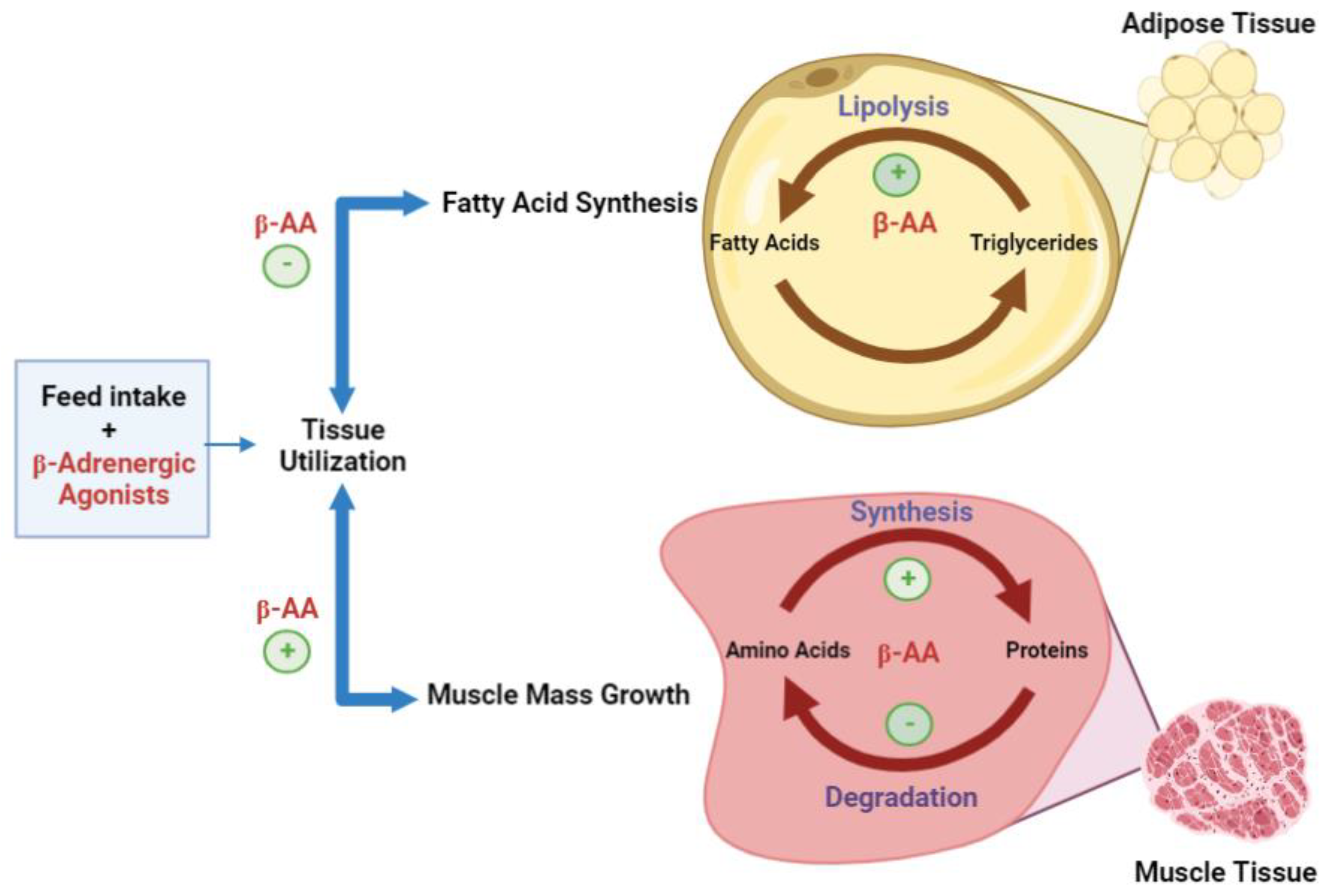
3.1. Effects on Skeletal Muscle Deposition
3.2. Effects on Protein Accretion
4. Effects of Catecholamines and Beta-Adrenergic Agonists on the Microbiome
4.1. Rumen Bacteria
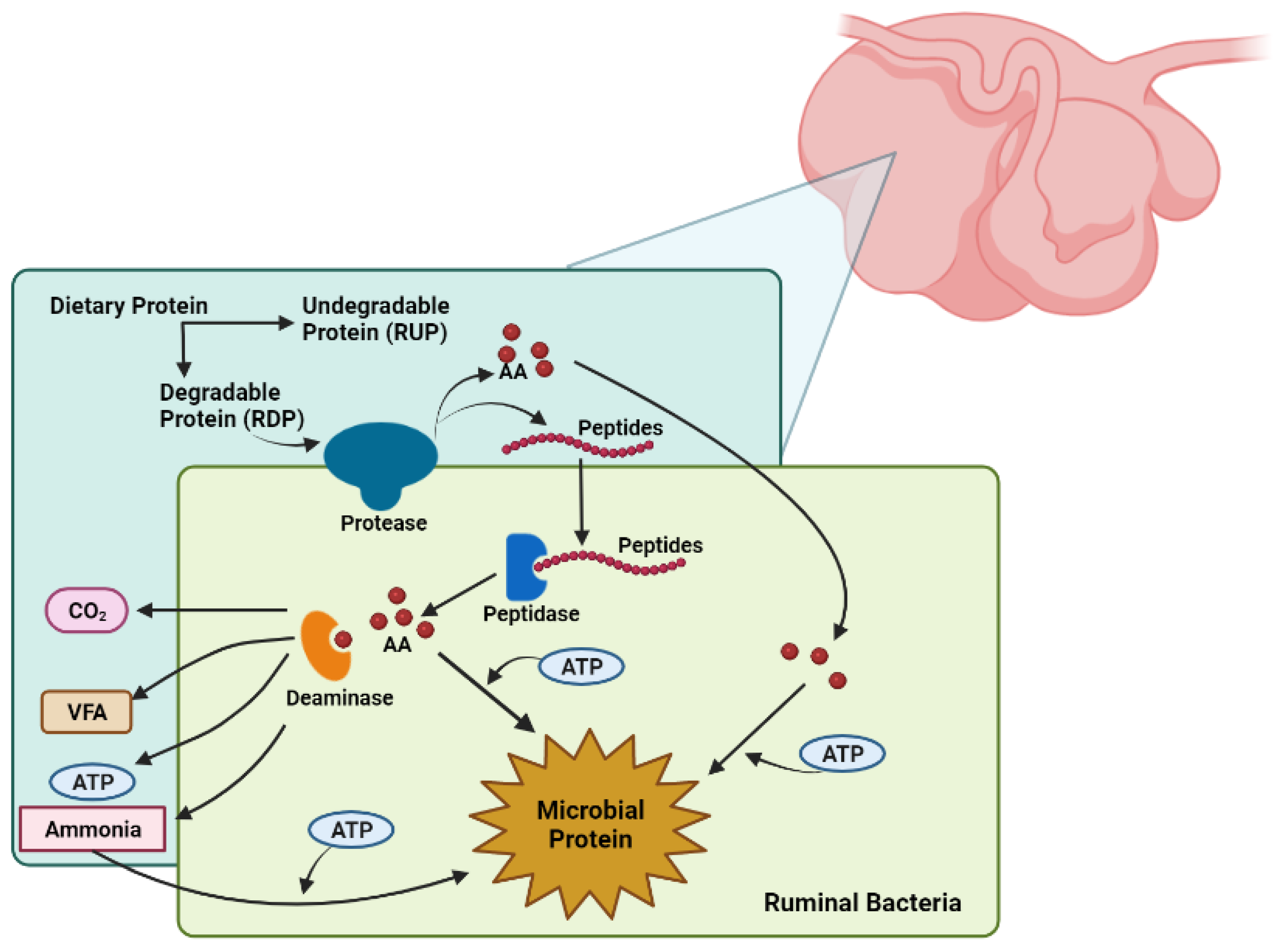
4.2. Protein Degradation in the Rumen
4.3. Microbial Protein Synthesis
4.4. Effects of Beta-Adrenergic Agonists on Protein, Supplemented Protein, and Nitrogen Utilization
4.5. Effect of Beta-Adrenergic Agonist on the Bacteria Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffy, E.M. Impact of Beta-Adrenergic Agonist Supplementation and Heat Stress on the Microbiome and Gastrointestinal Transcriptome of Sheep. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2019. [Google Scholar]
- Bradford, G. Contributions of animal agriculture to meeting global human food demand. Livest. Prod. Sci. 1999, 59, 95–112. [Google Scholar] [CrossRef]
- Samuelson, K.L.; Hubbert, M.E.; Oosthuysen, E.R.; Bester, Z.; Löest, C.A. Effects of Protein Concentration and Degradability on Performance and Carcass Characteristics of Finishing Heifers Receiving 0 or 400 mg Ractopamine Hydrochloride; American Society of Animal Science: Champaign, IL, USA, 2016. [Google Scholar]
- Galyean, M.L. Protein levels in beef cattle finishing diets: Industry application, university research, and systems results. J. Anim. Sci. 1996, 74, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Gleghorn, J.F.; Elam, N.A.; Galyean, M.L.; Duff, G.C.; Cole, N.A.; Rivera, J.D. Effects of crude protein concentration and degradability on performance, carcass characteristics, and serum urea nitrogen concentrations in finishing beef steers. J. Anim. Sci. 2004, 82, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Lobley, G.E.; Connell, A.; Lomax, M.A.; Brown, D.S.; Milne, E.; Calder, A.G.; Farningham, D.A.H. Hepatic detoxification of ammonia in the ovine liver: Possible consequences for amino acid catabolism. Br. J. Nutr. 1995, 73, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.; Brister, D.; Burdette, B.; Hutcheson, J.; Nordstrom, S.; Reinhardt, C.; Shelton, T.; Newcomb, H. Revalor Implant Strategies. Revalor-S Tech. Bull. 2005, 12. [Google Scholar]
- Harsh, B. Effects of Ractopamine Hydrochloride on Nutrient Digestibility, Environmental N Excretion, Regulation of Skeletal Muscle Growth, and Beta-Receptor Subtypes in Finishing Beef Steers; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2018. [Google Scholar]
- Sanchez, P.H.; Tracey, L.N.; Browne-Silva, J.; Lodge-Ivey, S.L. Propionibacterium acidipropionici P169 and glucogenic precursors improve rumen fermentation of low-quality forage in beef cattle. J. Anim. Sci. 2014, 92, 1738–1746. [Google Scholar] [CrossRef]
- Avendaño-Reyes, L.; Torres-Rodríguez, V.; Meraz-Murillo, F.; Pérez-Linares, C.; Figueroa-Saavedra, F.; Robinson, P. Effects of two β-adrenergic agonists on finishing performance, carcass characteristics, and meat quality of feedlot steers. J. Anim. Sci. 2006, 84, 3259–3265. [Google Scholar] [CrossRef]
- Arp, T.; Howard, S.; Woerner, D.; Scanga, J.; McKenna, D.; Kolath, W.; Chapman, P.L.; Tatum, J.D.; Belk, K.E. Effects of dietary ractopamine hydrochloride and zilpaterol hydrochloride supplementation on performance, carcass traits, and carcass cutability in beef steers. J. Anim. Sci. 2014, 92, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Tatum, J.; Engle, T.; Mitchell, M.; Laudert, S.; Schroeder, A.; Platter, W.J. Effects of ractopamine supplementation on growth performance and carcass characteristics of feedlot steers differing in biological type. J. Anim. Sci. 2007, 85, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Bryant, T.; Engle, T.; Galyean, M.; Wagner, J.; Tatum, J.; Anthony, R.; Laudert, S.B. Effects of ractopamine and trenbolone acetate implants with or without estradiol on growth performance, carcass characteristics, adipogenic enzyme activity, and blood metabolites in feedlot steers and heifers. J. Anim. Sci. 2010, 88, 4102–4119. [Google Scholar] [CrossRef]
- Abney, C.; Vasconcelos, J.; McMeniman, J.; Keyser, S.; Wilson, K.; Vogel, G.; Galyean, M.L. Effects of ractopamine hydrochloride on performance, rate and variation in feed intake, and acid-base balance in feedlot cattle. J. Anim. Sci. 2007, 85, 3090–3098. [Google Scholar] [CrossRef]
- Bohrer, B.; Edenburn, B.; Boler, D.; Dilger, A.; Felix, T. Effect of feeding ractopamine hydrochloride (Optaflexx) with or without supplemental zinc and chromium propionate on growth performance, carcass characteristics, and meat quality of finishing steers. J. Anim. Sci. 2014, 92, 3988–3996. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Titgemeyer, E.; Drouillard, J.; Loe, E.; Depenbusch, B.; Webb, A. Effects of ractopamine and protein source on growth performance and carcass characteristics of feedlot heifers. J. Anim. Sci. 2006, 84, 2795–2800. [Google Scholar] [CrossRef]
- Beermann, D.; Hogue, D.; Fishell, V.; Dalrymple, R.; Ricks, C.A. Effects of cimaterol and fishmeal on performance, carcass characteristics and skeletal muscle growth in lambs. J. Anim. Sci. 1986, 62, 370–380. [Google Scholar] [CrossRef]
- Bell, A.W.; Bauman, D.E.; Beermann, D.H.; Harrell, R.J. Nutrition, development and efficacy of growth modifiers in livestock species. J. Nutr. 1998, 128, 360S–363S. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.T.; Rathmann, R.J.; Reuter, R.R.; Leibovich, J.; McMeniman, J.P.; Hales, K.E.; Covey, T.L.; Miller, M.F.; Nichols, W.T.; Galyean, M.L. Effects of duration of zilpaterol hydrochloride feeding and days on the finishing diet on feedlot cattle performance and carcass traits. J. Anim. Sci. 2008, 86, 2005–2015. [Google Scholar] [CrossRef]
- Elam, N.A.; Vasconcelos, J.T.; Hilton, G.; VanOverbeke, D.L.; Lawrence, T.E.; Montgomery, T.H.; Nichols, W.T.; Streeter, M.N.; Hutcheson, J.P.; Yates, D.A.; et al. Effect of zilpaterol hydrochloride duration of feeding on performance and carcass characteristics of feedlot cattle. J. Anim. Sci. 2009, 87, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.L.; Krehbiel, C.R.; Cranston, J.J.; Yates, D.A.; Hutcheson, J.P.; Nichols, W.T.; Streeter, M.N.; Bechtol, D.T.; Johnson, E.; TerHune, T.; et al. Dietary zilpaterol hydrochloride. I. Feedlot performance and carcass traits of steers and heifers. J. Anim. Sci. 2009, 87, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. The pharmacokinetics, metabolism, and tissue residues of β-adrenergic agonists in livestock. J. Anim. Sci. 1998, 76, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Smith, S.B.; Chung, K.Y. Historical Overview of the Effect of β-Adrenergic Agonists on Beef Cattle Production. Asian-Australas. J. Anim. Sci. 2014, 27, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Mersmann, H.; Smith, S.; Smith, D. Beta-adrenergic agonists, their receptors, and growth: Special reference to the peculiarities in pigs. Biol. Fat Meat Anim. Curr. Adv. 1995, 1–34. [Google Scholar]
- Mersmann, H.J. Overview of the effects of β-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 1998, 76, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen*. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Bylund, D.B. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar] [PubMed]
- Minneman, K.P.; Hegstrand, L.R.; Molinoff, P.B. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol. Pharmacol. 1979, 16, 34–46. [Google Scholar] [PubMed]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1-and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Krief, S.; Lönnqvist, F.; Raimbault, S.; Baude, B.; Van Spronsen, A.; Arner, P.; Strosberg, A.D.; Ricquier, D.; Emorine, L.J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J. Clin. Investig. 1993, 91, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Mills, S. Quantitative analysis of beta-adrenergic receptor subtypes in pig tissues. J. Anim. Sci. 2002, 80, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Sillence, M.; Matthews, M. Classical and atypical binding sites for β-adrenoceptor ligands and activation of adenylyl cyclase in bovine skeletal muscle and adipose tissue membranes. Br. J. Pharmacol. 1994, 111, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Laudert, S.; Vogel, G.; Schroeder, A.; Platter, W. (Eds.) Effect of ractopamine HCl on growth and carcass traits of finishing heifers fed to slaughter. In Journal of Dairy Science; American Dairy Science Association: Savoy, IL, USA, 2007. [Google Scholar]
- Ungemach, F.R. Ractopamine (addendum). WHO Food Addit. Ser. 2004, 53. Available online: https://inchem.org/documents/jecfa/jecmono/v53je08.htm (accessed on 20 April 2023).
- NRC. Metabolic Modifiers: Effects on the Nutrient Requirements of Food-Producing Animals; National Academies: Washington, DC, USA, 1994.
- Kim, Y.; Sainz, R.; Summers, R.; Molenaar, P. Cimaterol reduces β-adrenergic receptor density in rat skeletal muscles. J. Anim. Sci. 1992, 70, 115–122. [Google Scholar] [CrossRef]
- Johnson, B.J. Mechanism of Action of Beta Adrenergic Agonists and Potential Residue Issues; American Meat Association Reference Paper; American Meat Science Association: Champaign, IL, USA, 2014; pp. 1–6. [Google Scholar]
- Williams, N.; Cline, T.; Schinckel, A.; Jones, D. The impact of ractopamine, energy intake, and dietary fat on finisher pig growth performance and carcass merit. J. Anim. Sci. 1994, 72, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Mills, S. Implications of feedback regulation of beta-adrenergic signaling. J. Anim. Sci. 2002, 80 (Suppl. S1), E30–E35. [Google Scholar]
- Rathmann, R.J.; Mehaffey, J.M.; Baxa, T.J.; Nichols, W.T.; Yates, D.A.; Hutcheson, J.P.; Brooks, J.C.; Johnson, B.J.; Miller, M.F. Effects of duration of zilpaterol hydrochloride and days on the finishing diet on carcass cutability, composition, tenderness, and skeletal muscle gene expression in feedlot steers. J. Anim. Sci. 2009, 87, 3686–3701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez, J.; Carter, J.; Johnson, D.; Ouellette, S.; Johnson, S. Effect of ractopamine-hydrochloride and trenbolone acetate on longissimus muscle fiber area, diameter, and satellite cell numbers in cull beef cows. J. Anim. Sci. 2007, 85, 1893–1901. [Google Scholar] [CrossRef]
- Baxa, T.; Hutcheson, J.; Miller, M.; Brooks, J.; Nichols, W.; Streeter, M.; Yates, D.A.; Johnson, B.J. Additive effects of a steroidal implant and zilpaterol hydrochloride on feedlot performance, carcass characteristics, and skeletal muscle messenger ribonucleic acid abundance in finishing steers. J. Anim. Sci. 2010, 88, 330–337. [Google Scholar] [CrossRef]
- Beermann, D.H.; Butler, W.R.; Hogue, D.; Fishell, V.; Dalrymple, R.; Ricks, C.; Scanes, C.G. Cimaterol-induced muscle hypertrophy and altered endocrine status in lambs. J. Anim. Sci. 1987, 65, 1514–1524. [Google Scholar] [CrossRef]
- Bergen, W.G.; Johnson, S.E.; Skjaerlund, D.M.; Babiker, A.S.; Ames, N.K.; Merkel, R.A.; Anderson, D.B. Muscle protein metabolism in finishing pigs fed ractopamine. J. Anim. Sci. 1989, 67, 2255–2262. [Google Scholar] [CrossRef]
- Reeds, P.; Fuller, M.; Nicholson, B. Metabolic basis of energy expenditure with particular reference to protein. In Substrate and Energy Metabolism in Man; CRC Press: Boca Raton, FL, USA, 1985; pp. 46–57. [Google Scholar]
- Cong, M.; Thompson, V.F.; Goll, D.E.; Antin, P.B. The bovine calpastatin gene promoter and a new N-terminal region of the protein are targets for cAMP-dependent protein kinase activity. J. Biol. Chem. 1998, 273, 660–666. [Google Scholar] [CrossRef]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Berdeaux, R.; Stewart, R. cAMP signaling in skeletal muscle adaptation: Hypertrophy, metabolism, and regeneration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1–E17. [Google Scholar] [CrossRef] [PubMed]
- Pringle, T.; Calkins, C.R.; Koohmaraie, M.; Jones, S.J. Effects over time of feeding a β-adrenergic agonist to wether lambs on animal performance, muscle growth, endogenous muscle proteinase activities, and meat tenderness. J. Anim. Sci. 1993, 71, 636–644. [Google Scholar] [CrossRef][Green Version]
- Strydom, P.; Frylinck, L.; Montgomery, J.; Smith, M. The comparison of three β-agonists for growth performance, carcass characteristics and meat quality of feedlot cattle. Meat Sci. 2009, 81, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Killefer, J.; Koohmaraie, M. Bovine skeletal muscle calpastatin: Cloning, sequence analysis, and steady-state mRNA expression. J. Anim. Sci. 1994, 72, 606–614. [Google Scholar] [CrossRef]
- Grant, A.; Helferich, W.; Merkel, R.; Bergen, W. Effects of phenethanolamines and propranolol on the proliferation of cultured chick breast muscle satellite cells. J. Anim. Sci. 1990, 68, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545–546, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Baldwin, R.; Allison, M. Rumen metabolism. J. Anim. Sci. 1983, 57 (Suppl. S2), 461–477. [Google Scholar] [PubMed]
- Yokoyama, M.; Johnson, K. Microbiología del Rumen e Intestine; El Rumiante Fisiología Digestiva y Nutrición Acribia: Zaragoza, Spain, 1988; pp. 137–157. [Google Scholar]
- Firkins, J.L.; Yu, Z. Characterization and quantification of the microbial populations of the rumen. In Ruminant Physiology. Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Sejrsen, K., Hvelplund, T., Nielson, M.O., Eds.; Wageningen Academic: Wageningen, The Netherlands, 2006; pp. 19–54. [Google Scholar]
- Edwards, J.E.; Huws, S.A.; Kim, E.J.; Lee, M.R.F.; Kingston-Smith, A.H.; Scollan, N.D. Advances in microbial ecosystem concepts and their consequences for ruminant agriculture. Animal 2008, 2, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.E.; Suárez, M.C. Principales Factores que Afectan la Actividad Celulolítica Bacteriana en Ruminates; Fondo Editorial Biogénesis, 2005; pp. 15–43. [Google Scholar]
- Wang, Z.; Elekwachi, C.; Jiao, J.; Wang, M.; Tang, S.; Zhou, C.; Tan, Z.; Forster, R.J. Changes in metabolically active bacterial community during rumen development, and their alteration by rhubarb root powder revealed by 16S rRNA amplicon sequencing. Front. Microbiol. 2017, 8, 159. [Google Scholar] [CrossRef]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef] [PubMed]
- Cobarrubias, L.; Anzola Vásquez, H.; Cuesta Peralta, A.; Abril, C.; Rincón, T. Manipulación de los Microorganismos Ruminales con Diferentes Sustratos, 1. Pruebas In Vitro; Revista ACOVEZ: Bogotá, Colombia, 1996; v 21 (1); pp. 4+7–11. ISSN 0120-1530. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Galindo, J.; Stuart, R.; Fundora, O.; Regalado, E.; Piedra, R.; Delgado, D.; Pérez, M. Efecto de la suplementación en la población microbiana ruminal de toros que consumen residuos de centros de limpieza de caña. Rev. Cubana Cinc. Agric. 1993, 27, 171. [Google Scholar]
- Carulla, J.E.; Pardo, O.; Hess, D.; Gonzalez, S.V. El uso de la Urea Sanguinea y/o Urea en Leche Como Herramienta Para Determinar el Balance Energia Proteina a Nivel Ruminal; Publicaci6n Universidad Nacional de Colombia: Bogotá, Colombia, 1998. [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001.
- Walker, E.; Drouillard, S. Effects of catecholamines on gut microflora and potential for beta-adrenergic agonists to impact ruminal fermentation. Open Agric. J. 2012, 6, 57–66. [Google Scholar] [CrossRef]
- Brock, F.; Forsberg, C.; Buchanan-Smith, J. Proteolytic activity of rumen microorganisms and effects of proteinase inhibitors. Appl. Environ. Microbiol. 1982, 44, 561–569. [Google Scholar] [CrossRef]
- Duff, G.C. Integrating lifetime nutrition: From cow/calf to stocker to feedlot. Vet. Clin. North Am. Food Anim. Pract. 2007, 23, 177–191. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Galyean, M. Nutritional recommendations of feedlot consulting nutritionists: The 2007 Texas Tech University survey. J. Anim. Sci. 2007, 85, 2772–2781. [Google Scholar] [CrossRef]
- León, S.; Chicco, C. Degradación ruminal in situ de diferentes fuentes de proteína [In siter ruminal degradation of protein sources]. Zootec. Trop. Venez. 1991, 9, 3–24. [Google Scholar]
- Russell, J.B. Rumen Microbiology and Its Role in Ruminant Nutrition; Department of Microbiology, Cornell University: Ithaca, NY, USA, 2002. [Google Scholar]
- Church, D. The Ruminant Animal Digestive Physiology and Nutrition Waveland Press; Ine: Prospect Height, IL, USA, 1993. [Google Scholar]
- Firkins, J.L. Maximizing Microbial Protein Synthesis in the Rumen. J. Nutr. 1996, 126 (Suppl. S4), 1347S–1354S. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Beef Cattle; National Academy of Sciences-NRC: Washington, DC, USA, 1996.
- Hackmann, T.J.; Firkins, J.L. Maximizing efficiency of rumen microbial protein production. Front. Microbiol. 2015, 6, 465. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; McDonald, I. The inter-relationships of individual proteins and carbohydrates during fermentation in the rumen of the sheep. I. The fermentation of casein in the presence of starch or other carbohydrate materials. J. Agric. Sci. 1958, 51, 108–118. [Google Scholar] [CrossRef]
- Stern, M.; Hoover, H.; Sniffen, C.; Crooker, B.; Knowlton, P. Effects of nonstructural carbohydrate, urea and soluble protein levels on microbial protein synthesis in continuous culture of rumen contents. J. Anim. Sci. 1978, 47, 944–956. [Google Scholar] [CrossRef]
- Russell, J.B.; O’connor, J.; Fox, D.; Van Soest, P.; Sniffen, C. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A. Various factors affecting microbial protein synthesis in the rumen. Vet. World 2008, 1, 186. [Google Scholar]
- Chanjula, P.; Wanapat, M.; Wachirapakorn, C.; Rowlinson, P. Effect of synchronizing starch sources and protein (NPN) in the rumen on feed intake, rumen microbial fermentation, nutrient utilization and performance of lactating dairy cows. Asian-Australas. J. Anim. Sci. 2004, 17, 1400–1410. [Google Scholar] [CrossRef]
- Reynolds, C.; Kristensen, N.B. Nitrogen recycling through the gut and the nitrogen economy of ruminants: An asynchronous symbiosis. J. Anim. Sci. 2008, 86 (Suppl. S14), E293–E305. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Shen, W.; Zhao, S.; Wang, J. Synchrony degree of dietary energy and nitrogen release influences microbial community, fermentation, and protein synthesis in a rumen simulation system. Microorganisms 2020, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Satter, L.; Slyter, L. Effect of ammonia concentration on rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef]
- Giraldo, L.M.; Medina, G.E.; Osorio, F. Utilización del Nitrógeno por los Ruminates; Fondo Editorial Biogénesis, 2005; pp. 45–66. [Google Scholar]
- Beermann, D. β-adrenergic agonists and growth. In The Endocrinology of Growth, Development and Metabolism in Vertebrates; Schriebman, M.P., Scanes, C.G., Pang, P.K.T., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 345–366. [Google Scholar]
- Boyd, R.; Bauman, D.; Fox, D.; Scanes, C. Impact of metabolism modifiers on protein accretion and protein and energy requirements of livestock. J. Anim. Sci. 1991, 69 (Suppl. S2), 56–75. [Google Scholar] [CrossRef]
- Brake, D.; Titgemeyer, E.; Jones, M. Effect of nitrogen supplementation and zilpaterol–HCl on urea kinetics in steers consuming corn-based diets. J. Anim. Physiol. Anim. Nutr. 2011, 95, 409–416. [Google Scholar] [CrossRef]
- Walker, C.; Drouillard, J. Effects of ractopamine hydrochloride are not confined to mammalian tissue: Evidence for direct effects of ractopamine hydrochloride supplementation on fermentation by ruminal microorganisms. J. Anim. Sci. 2010, 88, 697–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canfield, P.; Paraskeva, P. Beta-adrenoceptor agonist stimulation of acid secretion by rat stomach in vitro is mediated by ‘atypical’ beta-adrenoceptors. Br. J. Pharmacol. 1992, 106, 583. [Google Scholar] [CrossRef]
- Lyte, M.; Bailey, M.T. Neuroendocrine–bacterial interactions in a neurotoxin-induced model of trauma. J. Surg. Res. 1997, 70, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sainz, R.D. Beta-adrenergic agonists and hypertrophy of skeletal muscles. Life Sci. 1992, 50, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Matthews, J.; Socransky, S.; Freestone, P.; Williams, P.; Chapple, I. Stress and the periodontal diseases: Effects of catecholamines on the growth of periodontal bacteria in vitro. Oral Microbiol. Immunol. 2002, 17, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ruckebusch, Y. Pharmacology of reticulo-ruminal motor function. J. Vet. Pharmacol. Ther. 1983, 6, 245–272. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Thompson, D. Adrenergic control of motor and secretory function in the gastrointestinal tract. Aliment. Pharmacol. Ther. 1992, 6, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Brikas, P.; Bueno, L.; Fioramonti, J. Central and peripheral β-adrenergic influences on reticulo-rumen and upper-gut myoelectrical activity in sheep. J. Vet. Pharmacol. Ther. 1989, 12, 430–437. [Google Scholar] [CrossRef]
- Leek, B. Pharmacology: Review: Reticuloruminal motility—A pharmacological target with a difference? Vet. Q. 2001, 23, 26–31. [Google Scholar] [CrossRef]
- Costanzo, L.S. Physiology, E-Book: Elsevier Health Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Aschenbach, J.R.; Borau, T.; Gäbel, G. Glucose uptake via SGLT-1 is stimulated by β2-adrenoceptors in the ruminal epithelium of sheep. J. Nutr. 2002, 132, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Edrington, T.; Callaway, T.; Ives, S.; Engler, M.; Welsh, T.; Hallford, D.; Genovese, K.J.; Anderson, R.C.; Nisbet, D.J. Effect of ractopamine HCl supplementation on fecal shedding of Escherichia coli O157: H7 and Salmonella in feedlot cattle. Curr. Microbiol. 2006, 53, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kinney, K.S.; Austin, C.E.; Morton, D.S.; Sonnenfeld, G. Norepinephrine as a growth stimulating factor in bacteria—Mechanistic studies. Life Sci. 2000, 67, 3075–3085. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfau, A.P.; Shepherd, E.A.; Martin, M.G.; Ascolese, S.; Mason, K.M.; Egert-McLean, A.M.; Voy, B.H.; Myer, P.R. Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review. Vet. Sci. 2023, 10, 579. https://doi.org/10.3390/vetsci10090579
Pfau AP, Shepherd EA, Martin MG, Ascolese S, Mason KM, Egert-McLean AM, Voy BH, Myer PR. Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review. Veterinary Sciences. 2023; 10(9):579. https://doi.org/10.3390/vetsci10090579
Chicago/Turabian StylePfau, Alison P., Elizabeth A. Shepherd, M. Gabbi Martin, Sophia Ascolese, Katie M. Mason, Amanda M. Egert-McLean, Brynn H. Voy, and Phillip R. Myer. 2023. "Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review" Veterinary Sciences 10, no. 9: 579. https://doi.org/10.3390/vetsci10090579
APA StylePfau, A. P., Shepherd, E. A., Martin, M. G., Ascolese, S., Mason, K. M., Egert-McLean, A. M., Voy, B. H., & Myer, P. R. (2023). Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review. Veterinary Sciences, 10(9), 579. https://doi.org/10.3390/vetsci10090579





