The Effects of Milk Replacer Supplemented with Ascophyllum nodosum as a Novel Ingredient to Prevent Neonatal Diarrhea in Dairy Calves and Improve Their Health Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, Experimental Design and Treatment
2.2. Metabolic Profile, Antioxidant Barrier, and Immunoenzymatic Analysis in Serum Samples
2.2.1. Metabolic Profile
2.2.2. Antioxidant Barrier
2.2.3. Immunoenzymatic Tests for Serum Concentration of Trefoil Factor 3 and Diamine Oxidase
2.3. Microbiological and Molecular Analysis of Fecal Samples
2.3.1. Bacterial Count
- (i)
- Total bacteria: Plate Count Agar (PCA) (Liofilchem, Teramo, Italy). Incubation lasted three days at 30 °C (Merck, Taufkirchen, Germany);
- (ii)
- Lactic acid bacteria: de Man, Rogosa and Sharpe Agar (MRSA) (Liofilchem, Teramo, Italy). Incubation lasted three days at 30 °C under microaerophilic conditions (Merck, Taufkirchen, Germany);
- (iii)
- Coliform bacteria: Violet Red Bile Lactose Agar (VRBLA) (Liofilchem, Teramo, Italy). Incubation lasted 18–24 h at 35 °C under microaerophilic conditions (Merck, Taufkirchen, Germany).
2.3.2. Bacterial DNA Extraction and Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. Zootechnical Performances
3.2. Metabolic Profile, Antioxidant Barrier, and Immunoenzymatic Analysis of Serum Samples
3.2.1. Metabolic Profile
3.2.2. Oxidative Status of Blood Serum
3.2.3. Immunoenzymatic Test
3.3. Diarrhea Occurrence and Fecal Samples Analysis
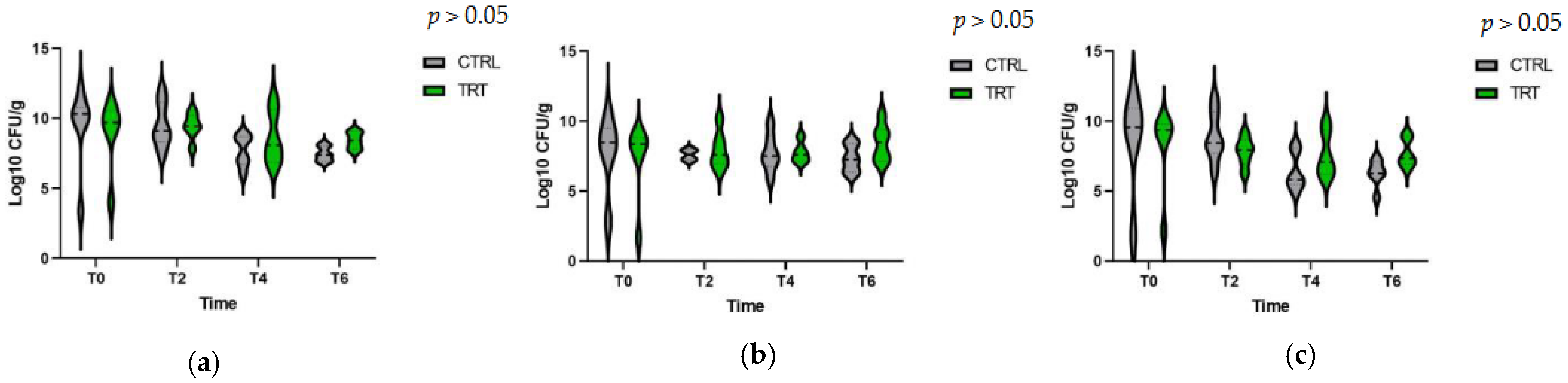
3.4. Bacterial Count in Fecal Samples
3.5. PCR, Molecular Microbiology
4. Discussion
4.1. Zootechnical Performances
4.2. Metabolic Profile, Antioxidant Barrier, and Immunoenzymatic Analysis of Serum Samples
4.3. Diarrhea Occurrence and Fecal Samples Analysis
4.4. Bacterial Count in Fecal Samples
4.5. PCR, Molecular Microbiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, B.L.; Guadagnin, A.R.; Fehlberg, L.K.; Sugimoto, Y.; Shinzato, I.; Drackley, J.K.; Cardoso, F.C. Feeding rumen-protected lysine to dairy cows prepartum improves performance and health of their calves. J. Dairy Sci. 2022, 105, 2256–2274. [Google Scholar] [CrossRef]
- Smith, D.R. Field Disease Diagnostic Investigation of Neonatal Calf Diarrhea. Vet. Clin. Food Anim. Pract. 2012, 28, 465–481. [Google Scholar] [CrossRef]
- Weber, L.P.; Dreyer, S.; Heppelmann, M.; Schaufler, K.; Homeier-Bachmann, T.; Bachmann, L. Prevalence and Risk Factors for ESBL/AmpC-E. coli in Pre-Weaned Dairy Calves on Dairy Farms in Germany. Microorganisms 2021, 9, 2135. [Google Scholar] [CrossRef] [PubMed]
- De Campos, J.L.; Kates, A.; Steinberger, A.; Sethi, A.; Suen, G.; Shutske, J.; Safdar, N.; Goldberg, T.; Ruegg, P.L. Quantification of antimicrobial usage in adult cows and preweaned calves on 40 large Wisconsin dairy farms using dose-based and mass-based metrics. J. Dairy Sci. 2021, 104, 4727–4745. [Google Scholar] [CrossRef] [PubMed]
- Hommels, N.M.C.; Ferreira, F.C.; van den Borne, B.H.P.; Hogeveen, H. Antibiotic use and potential economic impact of implementing selective dry cow therapy in large US dairies. J. Dairy Sci. 2021, 104, 8931–8946. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.U.; Breitenbuecher, J.; Gomez, J.P.; Samah, F.; Fausak, E.; Van Noord, M. Vaccination for the Prevention of Neonatal Calf Diarrhea in Cow-Calf Operations: A Scoping Review. Vet. Anim. Sci. 2022, 15, 100238. [Google Scholar] [CrossRef]
- Bokma, J.; Boone, R.; Deprez, P.; Pardon, B. Risk factors for antimicrobial use in veal calves and the association with mortality. J. Dairy Sci. 2019, 102, 607–618. [Google Scholar] [CrossRef]
- EUR-Lex. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, 276, 43–167. [Google Scholar]
- Heinrichs, A.J.; Heinrichs, B.S. A prospective study of calf factors affecting first-lactation and lifetime milk production and age of cows when removed from the herd1. J. Dairy Sci. 2011, 94, 336–341. [Google Scholar] [CrossRef]
- Palczynski, L.J.; Bleach, E.C.L.; Brennan, M.L.; Robinson, P.A. Appropriate Dairy Calf Feeding from Birth to Weaning: “It’s an Investment for the Future”. Animals 2020, 10, 116. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 268, 29–43. [Google Scholar]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Frazzini, S.; Scaglia, E.; Dell’Anno, M.; Reggi, S.; Panseri, S.; Giromini, C.; Lanzoni, D.; Sgoifo Rossi, C.A.; Rossi, L. Antioxidant and Antimicrobial Activity of Algal and Cyanobacterial Extracts: An In Vitro Study. Antioxidants 2022, 11, 992. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Narayan, B.; Kumar, C.S.; Sashima, T.; Maeda, H.; Hosokawa, M.; Miyashita, K. Composition, functionality and potential applications of seaweed lipids. In Biocatalysis and Bioenergy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 463–490. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.A.M.; Fremaut, D.; De Smet, S.; Dierick, N.A. In vitro characterisation of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. Anim. Feed. Sci. Technol. 2009, 151, 111–127. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Council Directive 2008/119/EC of 18 December 2008 Laying Down Minimum Standards for the Protection of Calves (Codified Version). 2019. Available online: http://data.europa.eu/eli/dir/2008/119/2019-12-14/eng (accessed on 10 July 2023).
- Samarasinghe, M.B.; Sehested, J.; Weisbjerg, M.R.; Van Der Heide, M.E.; Nørgaard, J.V.; Vestergaard, M.; Hernández-Castellano, L.E. Feeding milk supplemented with Ulva sp., Ascophyllum nodosum, or Saccharina latissima to preweaning dairy calves: Effects on growth, gut microbiota, gut histomorphology, and short-chain fatty acids in digesta. J. Dairy Sci. 2021, 104, 12117–12126. [Google Scholar] [CrossRef]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact Brown Seaweed (Ascophyllum Nodosum) in Diets of Weaned Piglets: Effects on Performance, Gut Bacteria and Morphology and Plasma Oxidative Status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef]
- Gahan, D.A.; Lynch, M.B.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. Performance of Weanling Piglets Offered Low-, Medium- or High-Lactose Diets Supplemented with a Seaweed Extract from Laminaria spp. Animal 2009, 3, 24–31. [Google Scholar] [CrossRef]
- Amaral-Phillips, D.M.; Scharko, P.B.; Johns, J.T.; Franklin, S. Feeding and Managing Baby Calves from Birth to 3 Months of Age. UK Cooperative Extension Service, University of Kentucky, ASC-161. 2006. Available online: http://www2.ca.uky.edu/agcomm/pubs/asc/asc161/ASC161.PDF (accessed on 10 July 2023).
- Dell’Anno, M.; Reggi, S.; Caprarulo, V.; Hejna, M.; Sgoifo Rossi, C.A.; Callegari, M.L.; Baldi, A.; Rossi, L. Evaluation of Tannin Extracts, Leonardite and Tributyrin Supplementation on Diarrhoea Incidence and Gut Microbiota of Weaned Piglets. Animals 2021, 11, 1693. [Google Scholar] [CrossRef]
- Rossi, L.; Dell’Orto, V.; Vagni, S.; Sala, V.; Reggi, S.; Baldi, A. Protective effect of oral administration of transgenic tobacco seeds against verocytotoxic Escherichia coli strain in piglets. Vet. Res. Commun. 2014, 38, 39–49. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Scaglia, E.; Reggi, S.; Grossi, S.; Sgoifo Rossi, C.A.; Frazzini, S.; Caprarulo, V.; Rossi, L. Evaluation of tributyrin supplementation in milk replacer on diarrhoea occurrence in preweaning Holstein calves. Animal 2023, 17, 100791. [Google Scholar] [CrossRef]
- Bellali, S.; Lagier, J.-C.; Raoult, D.; Bou Khalil, J. Among Live and Dead Bacteria, the Optimization of Sample Collection and Processing Remains Essential in Recovering Gut Microbiota Components. Front. Microbiol. 2019, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Imai, T.; Kondo, M.; Ban-Tokuda, T.; Yamada, Y. Effects of the supplementation of a calcium soap containing medium-chain fatty acids on the fecal microbiota of pigs, lactating cows, and calves. Anim. Sci. J. 2021, 92, e13636. [Google Scholar] [CrossRef] [PubMed]
- Dubernet, S.; Desmasures, N.; Guéguen, M. A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol. Lett. 2002, 214, 271–275. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, K.; Fukushima, M.; Kawaguchi, R. Detection and identification of Escherichia coli, Shigella, and Salmonella by microarrays using the gyrB gene. Biotechnol. Bioeng. 2003, 83, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Abrar, A.; Kondo, M.; Kitamura, T.; Ban-Tokuda, T.; Matsui, H. Effect of Supplementation of Rice Bran and Fumarate Alone or in Combination on in Vitro Rumen Fermentation, Methanogenesis and Methanogens. Anim. Sci. J. 2016, 87, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.; Meredith, H.; Vigors, S.; McDonnell, M.J.; Ryan, M.; Thornton, K.; O’Doherty, J.V. Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species Laminaria digitata improved growth rate and intestinal structure in young chicks, but does not influence Campylobacter jejuni colonisation. Anim. Feed Sci. Technol. 2017, 232, 71–79. [Google Scholar] [CrossRef]
- O’Doherty, J.V.; Venardou, B.; Rattigan, R.; Sweeney, T. Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs. Animals 2021, 11, 2644. [Google Scholar] [CrossRef]
- Silva, F.G.; Conceição, C.; Pereira, A.M.F.; Cerqueira, J.L.; Silva, S.R. Literature Review on Technological Applications to Monitor and Evaluate Calves’ Health and Welfare. Animals 2023, 13, 1148. [Google Scholar] [CrossRef]
- Gelsinger, S.L.; Heinrichs, A.J.; Jones, C.M. A meta-analysis of the effects of preweaned calf nutrition and growth on first-lactation performance. J. Dairy Sci. 2016, 99, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, M.B.; Sehested, J.; Weisbjerg, M.R.; Vestergaard, M.; Hernández-Castellano, L.E. Milk supplemented with dried seaweed affects the systemic innate immune response in preweaning dairy calves. J. Dairy Sci. 2021, 104, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Curtis, G.; Argo, C.M.; Jones, D.; Grove-White, D. The impact of early life nutrition and housing on growth and reproduction in dairy cattle. PLoS ONE 2018, 13, e0191687. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, G.; Cattaneo, L.; Trevisi, E.; Liotta, L.; Minuti, A.; Arfuso, F.; Lopreiato, V. Effects of Weaning Age on Plasma Biomarkers and Growth Performance in Simmental Calves. Animals 2022, 12, 1168. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Wu, C.-H.; Chen, W.-C.; Pan, C.-H.; Chen, Y.-W.; Tsao, S.-P.; Chen, C.-J.; Huang, H.-Y. Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats. Nutrients 2022, 14, 4665. [Google Scholar] [CrossRef]
- Evglevskiy, A.A.; Shvets, O.M.; Mikhaleva, T.I. Clinical and metabolic effects of the original iodine metabolic composition in the experiment on calves. E3S Web Conf. 2021, 285, 04003. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Li, F.-C.; Li, Y.-K.; Fan, Y.-C.; Wang, K. Plasma concentration of diamine oxidase (DAO) predicts 1-month mortality of acute-on-chronic hepatitis B liver failure. Clin. Chim. Acta 2018, 484, 164–170. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kang, J.-H.; Cho, H.-C.; Yu, D.-H.; Park, J. Changes in serum protein electrophoresis profiles and acute phase proteins in calves with diarrhea. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2021, 85, 45–50. [Google Scholar]
- Bozukluhan, K.; Merhan, O.; Gokce, H.I.; Deveci, H.A.; Gokce, G.; Ogun, M.; Marasli, S. Alterations in lipid profile in neonatal calves affected by diarrhea. Vet. World 2017, 10, 786–789. [Google Scholar] [CrossRef]
- Ok, M.; Yildiz, R.; Hatipoglu, F.; Baspinar, N.; Ider, M.; Üney, K.; Ertürk, A.; Durgut, M.K.; Terzi, F. Use of intestine-related biomarkers for detecting intestinal epithelial damage in neonatal calves with diarrhea. Am. J. Vet. Res. 2020, 81, 139–146. [Google Scholar] [CrossRef]
- Fukuda, T.; Tsukano, K.; Nakatsuji, H.; Suzuki, K. Plasma diamine oxidase activity decline with diarrhea severity in calves indicating systemic dysfunction related to intestinal mucosal damage. Res. Vet. Sci. 2019, 126, 127–130. [Google Scholar] [CrossRef]
- Santos, F.H.R.; Paula, M.R.D.; Lezier, D.; Silva, J.T.; Santos, G.; Bittar, C.M.M. Essential oils for dairy calves: Effects on performance, scours, rumen fermentation and intestinal fauna. Animal 2015, 9, 958–965. [Google Scholar] [CrossRef]
- Gomez, D.E.; Arroyo, L.G.; Costa, M.C.; Viel, L.; Weese, J.S. Characterization of the fecal bacterial microbiota of healthy and diarrheic dairy calves. J. Vet. Intern. Med. 2017, 31, 928–939. [Google Scholar] [CrossRef]
- Barry, J.; Bokkers, E.A.M.; Berry, D.P.; de Boer, I.J.M.; McClure, J.; Kennedy, E. Associations between colostrum management, passive immunity, calf-related hygiene practices, and rates of mortality in preweaning dairy calves. J. Dairy Sci. 2019, 102, 10266–10276. [Google Scholar] [CrossRef]
- Lora, I.; Gottardo, F.; Contiero, B.; Dall Ava, B.; Bonfanti, L.; Stefani, A.; Barberio, A. Association between passive immunity and health status of dairy calves under 30 days of age. Prev. Vet. Med. 2018, 152, 12–15. [Google Scholar] [CrossRef]
- Meale, S.J.; Li, S.; Azevedo, P.; Derakhshani, H.; Plaizier, J.C.; Khafipour, E.; Steele, M.A. Development of Ruminal and Fecal Microbiomes Are Affected by Weaning But Not Weaning Strategy in Dairy Calves. Front. Microbiol. 2016, 7, 582. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, F.; Patrone, V.; Prandini, A.; Sigolo, S.; Dell’Anno, M.; Rossi, L.; Senizza, A.; Morelli, L.; Callegari, M.L. Implications of Tributyrin on Gut Microbiota Shifts Related to Performances of Weaning Piglets. Microorganisms 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]






| Analyte | Composition (% as Fed) |
|---|---|
| Crude protein | 23.00 |
| Ether extract | 18.00 |
| Crude fiber | 0.10 |
| Ash | 7.50 |
| Lys | 2.10 |
| Ca | 1.00 |
| P | 0.70 |
| Na | 0.50 |
| Target | Forward/Reverse | Nucleotide Sequence | Amplicon (bp) | Reference |
|---|---|---|---|---|
| Lactobacillus spp. | Fw | 5′–CTTGTACACACCGCCCGTCA–3′ | 250 | [29] |
| Rv | 5′–CTCAAAACTAAACAAAGTTTC–3′ | |||
| Bifidobacterium spp. | Fw | 5′–CTCCTGGAAACGGGTGG–3′ | 549–563 | [30] |
| Rv | 5′–GGTGTTCTTCCCGATATCTACA–3′ | |||
| E. coli | Fw | 5′–ATGCTTAGTGCTGGTTTAGGG–3′ | 248 | [31] |
| Rv | 5′–GCCTTCATCATTTCGCTTTC–3′ | |||
| Total bacteria | Fw | 5′–CGGCAACGAGCGCAACCC–3′ | 130 | [32] |
| Rv | 5′–CCATTGTAGCACGTGTGTAGCC–3′ |
| Analyte | LSMeans | SE | p-Value | |
|---|---|---|---|---|
| CTRL | TRT | |||
| Albumin (g/L) | 41.96 * | 48.43 * | 1.374 | 0.014 * |
| Albumin/globulin (A/G) | 1.22 | 1.25 | 0.090 | 0.869 |
| Beta-hydroxyb-utyrate (mmol/L) | 0.07 | 0.09 | 0.010 | 0.165 |
| Calcium (mmol/L) | 3.75 * | 4.48 * | 0.161 | 0.018 * |
| Gamma-glutamyl transferase (IU/L) | 39.42 | 45.75 | 5.212 | 0.414 |
| Globulin (g/L) | 35.62 | 39.73 | 3.079 | 0.436 |
| Glucose (mmol/L) | 8.36 | 9.66 | 0.491 | 0.118 |
| Magnesium (mmol/L) | 1.17 | 1.26 | 0.052 | 0.296 |
| Non-esterified fatty acid (mmol/L) | 0.67 | 0.66 | 0.073 | 0.207 |
| Phosphorus (mmol/L) | 4.01 * | 4.84 * | 0.170 | 0.012 * |
| Total bilirubin (µmol/L) | 5.56 | 6.44 | 4.770 | 0.267 |
| Total cholesterol (mmol/L) | 4.47 * | 6.61 * | 0.392 | 0.006 * |
| Total protein (g/L) | 77.51 | 88.27 | 4.019 | 0.133 |
| Triglycerides (mmol/L) | 0.81 | 0.98 | 0.100 | 0.260 |
| Urea (mmol/L) | 2.72 | 2.78 | 0.233 | 0.842 |
| Mean ± SD | CTRL T6 | TRT T6 |
|---|---|---|
| DAO | 21.11 ± 1.972 | 18.56 ± 3.504 |
| TFF-3 | 1.61 ± 0.523 | 1.70 ± 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaglia, E.; Reggi, S.; Canala, B.; Frazzini, S.; Dell’Anno, M.; Hejna, M.; Rossi, L. The Effects of Milk Replacer Supplemented with Ascophyllum nodosum as a Novel Ingredient to Prevent Neonatal Diarrhea in Dairy Calves and Improve Their Health Status. Vet. Sci. 2023, 10, 618. https://doi.org/10.3390/vetsci10100618
Scaglia E, Reggi S, Canala B, Frazzini S, Dell’Anno M, Hejna M, Rossi L. The Effects of Milk Replacer Supplemented with Ascophyllum nodosum as a Novel Ingredient to Prevent Neonatal Diarrhea in Dairy Calves and Improve Their Health Status. Veterinary Sciences. 2023; 10(10):618. https://doi.org/10.3390/vetsci10100618
Chicago/Turabian StyleScaglia, Elena, Serena Reggi, Benedetta Canala, Sara Frazzini, Matteo Dell’Anno, Monika Hejna, and Luciana Rossi. 2023. "The Effects of Milk Replacer Supplemented with Ascophyllum nodosum as a Novel Ingredient to Prevent Neonatal Diarrhea in Dairy Calves and Improve Their Health Status" Veterinary Sciences 10, no. 10: 618. https://doi.org/10.3390/vetsci10100618
APA StyleScaglia, E., Reggi, S., Canala, B., Frazzini, S., Dell’Anno, M., Hejna, M., & Rossi, L. (2023). The Effects of Milk Replacer Supplemented with Ascophyllum nodosum as a Novel Ingredient to Prevent Neonatal Diarrhea in Dairy Calves and Improve Their Health Status. Veterinary Sciences, 10(10), 618. https://doi.org/10.3390/vetsci10100618









