Improving Cryopreservation Efficiency and Pregnancy Rate through Superovulation with Follicle-Stimulating Hormone in Korean Hanwoo Cows via Ovum Pick Up
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
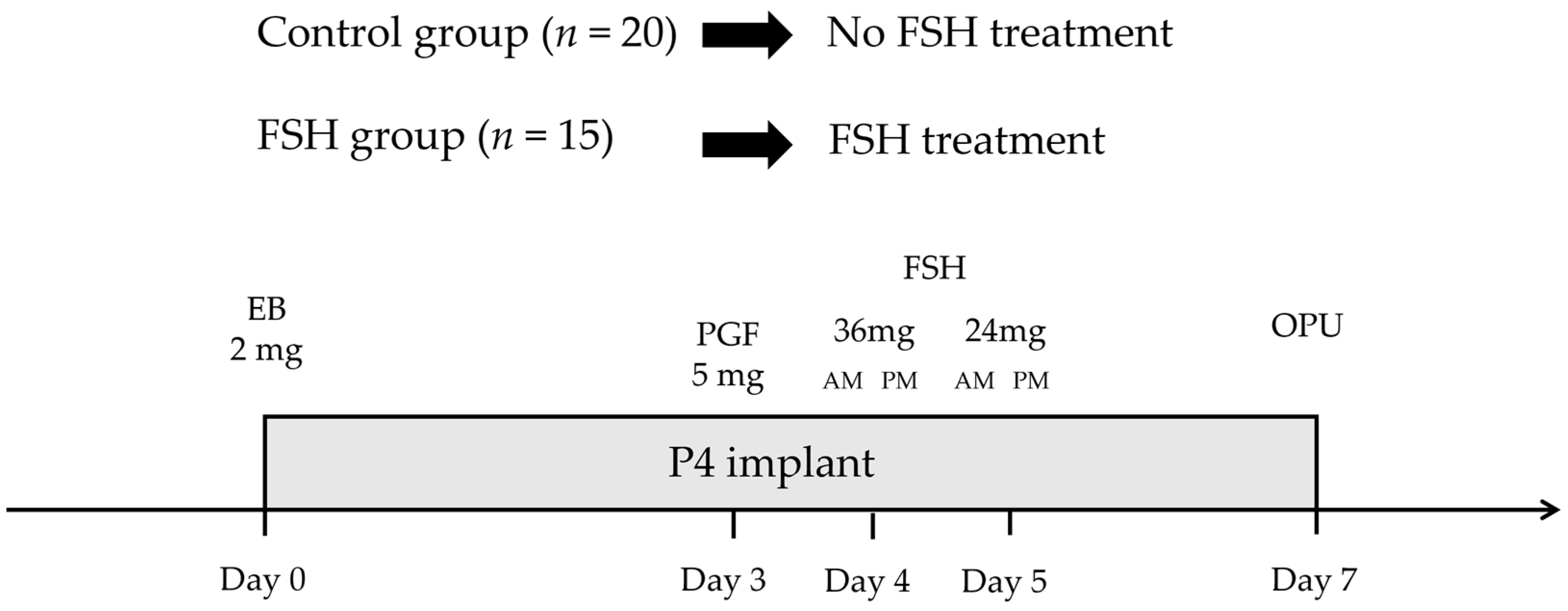
2.1. Animal and Management
2.2. Experimental Methodology
2.3. Ultrasonographic Scans
2.4. Procedures for Obtaining Oocytes under Ultrasound Guidance
2.5. Oocyte Characteristics: Grade and Germinal Vesicle Chromatin Status
2.6. Embryo Vitrification and Warming
2.7. Cell Counts before Vitrification and after Warming
2.8. Embryo Transfer and Pregnancy Assessment
2.9. Statistical Analysis
3. Results
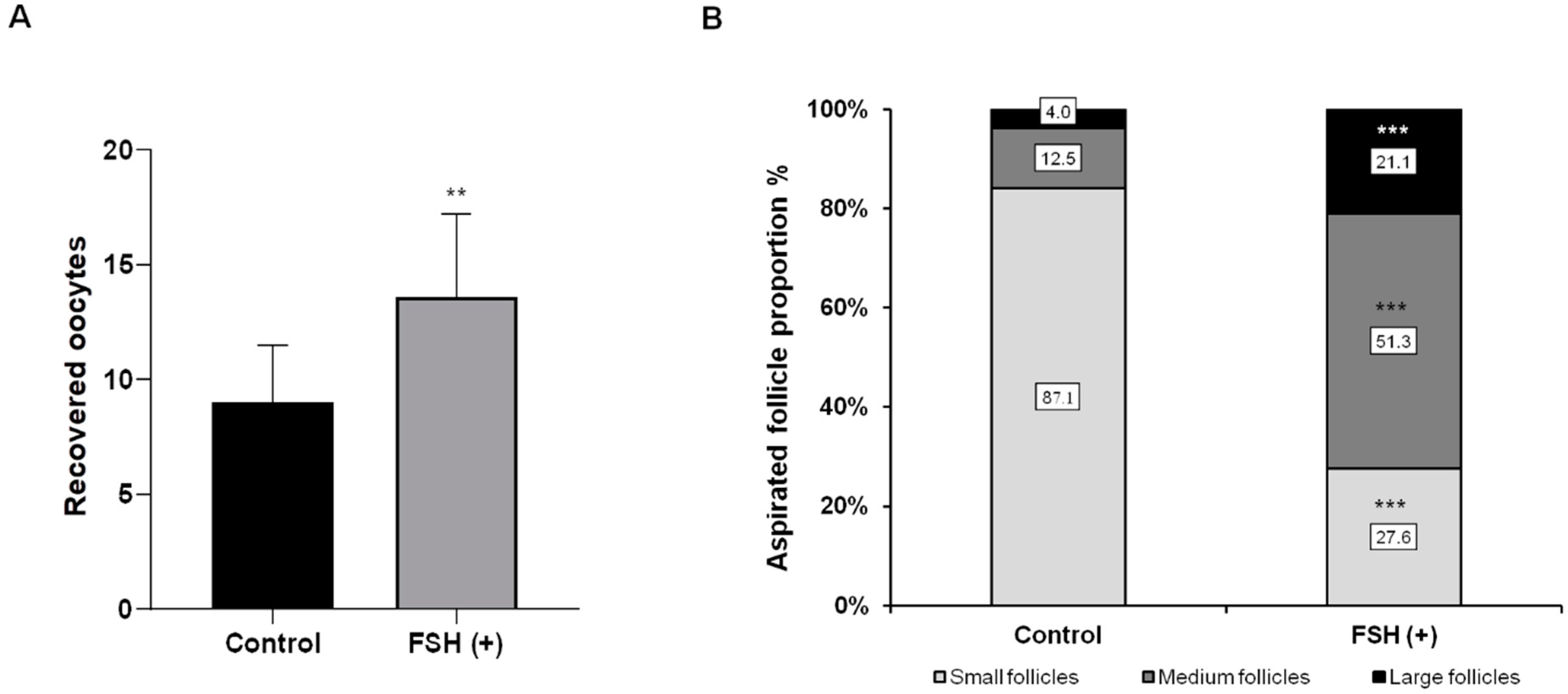
3.1. Ovarian Response to Hormonal Treatment
3.2. Collecting Oocytes and Producing Embryos In Vitro
3.3. Assessment of the Survival Rates of Blastocysts after Vitrification and Warming and Analysis of Staining
3.4. Comparison of Pregnancy Rates after Embryo Transfer to Hanwoo Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Wagtendonk-de Leeuw, A.M. Ovum pick up and in vitro production in the bovine after use in several generations: A 2005 status. Theriogenology 2006, 65, 914–925. [Google Scholar] [CrossRef]
- Pontes, J.H.; Nonato-Junior, I.; Sanches, B.V.; Ereno-Junior, J.C.; Uvo, S.; Barreiros, T.R.; Oliveira, J.A.; Hasler, J.F.; Seneda, M.M. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 2009, 71, 690–697. [Google Scholar] [CrossRef]
- Blondin, P. Logistics of large scale commercial IVF embryo production. Reprod. Fertil. Dev. 2016, 29, 32–36. [Google Scholar] [CrossRef]
- Joao Viana, M.D.L.R. Luis Nasser, Reuben Mapletoft 2021 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2022, 40, 1–18. [Google Scholar]
- Viana, J. 2020 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2021, 39, 1–14. [Google Scholar]
- Sirard, M.A. 40 years of bovine IVF in the new genomic selection context. Reproduction 2018, 156, R1–R7. [Google Scholar] [CrossRef]
- Mikkola, M.; Hasler, J.F.; Taponen, J. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 2019, 32, 104–124. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Ginther, O.J. The theory of follicle selection in cattle. Domest. Anim. Endocrinol. 2016, 57, 85–99. [Google Scholar] [CrossRef]
- Bo, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef]
- Hasler, J.F. Forty years of embryo transfer in cattle: A review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology 2014, 81, 152–169. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. The ART of studying early embryo development: Progress and challenges in ruminant embryo culture. Theriogenology 2014, 81, 49–55. [Google Scholar] [CrossRef]
- Farin, P.W.; Crosier, A.E.; Farin, C.E. Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology 2001, 55, 151–170. [Google Scholar] [CrossRef]
- Ongaratto, F.L.; Cedeno, A.V.; Rodriguez-Villamil, P.; Tribulo, A.; Bo, G.A. Effect of FSH treatment on cumulus oocyte complex recovery by ovum pick up and in vitro embryo production in beef donor cows. Anim. Reprod. Sci. 2020, 214, 106274. [Google Scholar] [CrossRef]
- de Carvalho, J.G.S.; de Carvalho, N.A.T.; Bayeux, B.M.; Watanabe, Y.F.; Watanabe, O.Y.; Mingoti, R.D.; Baruselli, P.S. Superstimulation prior to the ovum pick-up improves the in vitro embryo production in nulliparous, primiparous and multiparous buffalo (Bubalus bubalis) donors. Theriogenology 2019, 138, 164–168. [Google Scholar] [CrossRef]
- da Silva, J.C.B.; Ferreira, R.M.; Maturana Filho, M.; Naves, J.R.; Santin, T.; Pugliesi, G.; Madureira, E.H. Use of FSH in two different regimens for ovarian superstimulation prior to ovum pick up and in vitro embryo production in Holstein cows. Theriogenology 2017, 90, 65–73. [Google Scholar] [CrossRef]
- De Roover, R.; Feugang, J.M.; Bols, P.E.; Genicot, G.; Hanzen, C. Effects of ovum pick-up frequency and FSH stimulation: A retrospective study on seven years of beef cattle in vitro embryo production. Reprod. Domest. Anim. 2008, 43, 239–245. [Google Scholar] [CrossRef]
- Machado, G.M.; Carvalho, J.O.; Filho, E.S.; Caixeta, E.S.; Franco, M.M.; Rumpf, R.; Dode, M.A. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology 2009, 71, 1289–1297. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Sakoda, J.N.; Gama, I.L.; Bayeux, B.M.; Lodde, V.; Luciano, A.M.; Buratini, J. Characterization and control of oocyte large-scale chromatin configuration in different cattle breeds. Theriogenology 2020, 141, 146–152. [Google Scholar] [CrossRef]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte Selection for In Vitro Embryo Production in Bovine Species: Noninvasive Approaches for New Challenges of Oocyte Competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef]
- Lodde, V.; Modina, S.; Galbusera, C.; Franciosi, F.; Luciano, A.M. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol. Reprod. Dev. 2007, 74, 740–749. [Google Scholar] [CrossRef]
- Vajta, G.; Holm, P.; Kuwayama, M.; Booth, P.J.; Jacobsen, H.; Greve, T.; Callesen, H. Open Pulled Straw (OPS) vitrification: A new way to reduce cryoinjuries of bovine ova and embryos. Mol. Reprod. Dev. 1998, 51, 53–58. [Google Scholar] [CrossRef]
- Spricigo, J.F.; Diogenes, M.N.; Leme, L.O.; Guimaraes, A.L.; Muterlle, C.V.; Silva, B.D.; Sola-Oriol, D.; Pivato, I.; Silva, L.P.; Dode, M.A. Effects of Different Maturation Systems on Bovine Oocyte Quality, Plasma Membrane Phospholipid Composition and Resistance to Vitrification and Warming. PLoS ONE 2015, 10, e0130164. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Evaluation and classification of bovine embryos. Anim. Reprod. 2018, 10, 344–348. [Google Scholar]
- Baruselli, P.S.; Ferreira, R.M.; Sales, J.N.; Gimenes, L.U.; Sa Filho, M.F.; Martins, C.M.; Rodrigues, C.A.; Bo, G.A. Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology 2011, 76, 1583–1593. [Google Scholar] [CrossRef]
- Sirard, M.A.; Desrosier, S.; Assidi, M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology 2007, 68 (Suppl. 1), S71–S76. [Google Scholar] [CrossRef]
- Morotti, F.; Sanches, B.V.; Pontes, J.H.; Basso, A.C.; Siqueira, E.R.; Lisboa, L.A.; Seneda, M.M. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology 2014, 81, 696–701. [Google Scholar] [CrossRef]
- Morotti, F.; Santos, G.M.G.; Junior, C.K.; Silva-Santos, K.C.; Roso, V.M.; Seneda, M.M. Correlation between phenotype, genotype and antral follicle population in beef heifers. Theriogenology 2017, 91, 21–26. [Google Scholar] [CrossRef]
- Silva-Santos, K.C.; Santos, G.M.; Koetz Junior, C.; Morotti, F.; Siloto, L.S.; Marcantonio, T.N.; Urbano, M.R.; Oliveira, R.L.; Lima, D.C.; Seneda, M.M. Antral follicle populations and embryo production—In vitro and in vivo—Of Bos indicus-taurus donors from weaning to yearling ages. Reprod. Domest. Anim. 2014, 49, 228–232. [Google Scholar] [CrossRef]
- Ghanem, N.; Jin, J.I.; Kim, S.S.; Choi, B.H.; Lee, K.L.; Ha, A.N.; Song, S.H.; Kong, I.K. The Anti-Mullerian Hormone Profile is Linked with the In Vitro Embryo Production Capacity and Embryo Viability after Transfer but Cannot Predict Pregnancy Outcome. Reprod. Domest. Anim. 2016, 51, 301–310. [Google Scholar] [CrossRef]
- Machatkova, M.; Krausova, K.; Jokesova, E.; Tomanek, M. Developmental competence of bovine oocytes: Effects of follicle size and the phase of follicular wave on in vitro embryo production. Theriogenology 2004, 61, 329–335. [Google Scholar] [CrossRef]
- Seneda, M.M.; Esper, C.R.; Garcia, J.M.; Oliveira, J.A.; Vantini, R. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim. Reprod. Sci. 2001, 67, 37–43. [Google Scholar] [CrossRef]
- Bo, G.A.; Rogan, D.R.; Mapletoft, R.J. Pursuit of a method for single administration of pFSH for superstimulation in cattle: What we have learned. Theriogenology 2018, 112, 26–33. [Google Scholar] [CrossRef]
- Chaubal, S.A.; Ferre, L.B.; Molina, J.A.; Faber, D.C.; Bols, P.E.; Rezamand, P.; Tian, X.; Yang, X. Hormonal treatments for increasing the oocyte and embryo production in an OPU-IVP system. Theriogenology 2007, 67, 719–728. [Google Scholar] [CrossRef]
- Castilho, C.; Garcia, J.M.; Renesto, A.; Nogueira, G.P.; Brito, L.F. Follicular dynamics and plasma FSH and progesterone concentrations during follicular deviation in the first post-ovulatory wave in Nelore (Bos indicus) heifers. Anim. Reprod. Sci. 2007, 98, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Rodrigues, C.A.; Castro Netto, A.; Guerreiro, B.M.; Silveira, C.R.A.; Freitas, B.G.; Braganca, L.G.M.; Marques, K.N.G.; Sa Filho, M.F.; Bo, G.A.; et al. Efficacy of a single intramuscular injection of porcine FSH in hyaluronan prior to ovum pick-up in Holstein cattle. Theriogenology 2016, 85, 877–886. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Sanches, C.P.; Seddon, A.S.; Veras, M.B.; Lima, F.A.; Monteiro, P.L.J., Jr.; Wiltbank, M.C.; Sartori, R. Short communication: Follicle superstimulation before ovum pick-up for in vitro embryo production in Holstein cows. J. Dairy Sci. 2016, 99, 9307–9312. [Google Scholar] [CrossRef]
- Pontes, J.H.; Melo Sterza, F.A.; Basso, A.C.; Ferreira, C.R.; Sanches, B.V.; Rubin, K.C.; Seneda, M.M. Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nelore cattle (Bos indicus) donors. Theriogenology 2011, 75, 1640–1646. [Google Scholar] [CrossRef]
- Sarwar, Z.; Sagheer, M.; Sosa, F.; Saad, M.; Hassan, M.; Husnain, A.; Arshad, U. Meta-analysis to determine effects of treatment with FSH when there is progestin-priming on in-vitro embryo production using ovum pick-up in Bos taurus cows. Anim. Reprod. Sci. 2020, 221, 106590. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, J.; Dufort, I.; Robert, C.; Dias, F.C.F.; Anzar, M. Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage. PLoS ONE 2017, 12, e0187268. [Google Scholar] [CrossRef]
- Pitangui-Molina, C.P.; Vireque, A.A.; Tata, A.; Belaz, K.R.; Santos, V.G.; Ferreira, C.R.; Eberlin, M.N.; Silva-de-Sa, M.F.; Ferriani, R.A.; Rosa, E.S.A.C. Effect of soybean phosphatidylcholine on lipid profile of bovine oocytes matured in vitro. Chem. Phys. Lipids 2017, 204, 76–84. [Google Scholar] [CrossRef]
- Gomez, E.; Munoz, M.; Rodriguez, A.; Caamano, J.N.; Facal, N.; Diez, C. Vitrification of bovine blastocysts produced in vitro inflicts selective damage to the inner cell mass. Reprod. Domest. Anim. 2009, 44, 194–199. [Google Scholar] [CrossRef]
- Do, V.H.; Catt, S.; Kinder, J.E.; Walton, S.; Taylor-Robinson, A.W. Vitrification of in vitro-derived bovine embryos: Targeting enhancement of quality by refining technology and standardising procedures. Reprod. Fertil. Dev. 2019, 31, 837–846. [Google Scholar] [CrossRef]
- Kocyigit, A.; Cevik, M. Correlation between the cryosurvival, cell number and diameter in bovine in vitro produced embryos. Cryobiology 2016, 73, 203–208. [Google Scholar] [CrossRef]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.A. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol. Reprod. 2002, 66, 38–43. [Google Scholar] [CrossRef]
- Kang, S.-S.; Kim, U.-H.; Lee, S.-D.; Lee, M.-S.; Han, M.-H.; Cho, S.-R. Recovery Efficiency of Cumulus Oocyte Complexes (COCs) according to Collection Frequency for Ovum Pick-up (OPU) Method in Hanwoo Cow. J. Anim. Reprod. Biotechnol. 2019, 34, 300–304. [Google Scholar] [CrossRef]

| No. of Oocytes (Session) | Grade I | Grade II | Oocyte Grade (I + II)% | Grade III | Grade IV | Oocyte Grade (III + IV)% | |
|---|---|---|---|---|---|---|---|
| Control | 1022 (120) | 321 (31.4) | 193 (18.8) | 514 (50.2) | 330 (32.3) | 178 (17.5) | 508 (49.8) |
| FSH (+) | 1125 (90) | 702 (62.4) | 342 (30.4) | 1044 (88.2) | 72 (6.4) | 9 (0.8) | 40 (7.2) |
| Group | Number of Oocytes Generated by IVM (Session) | Number of Developed | ||
|---|---|---|---|---|
| Cleaved Embryos (%) | 8-Cell Stage (%) | Blastocysts (%) | ||
| Control | 884 (120) | 621 (70.2) | 464 (52.4) | 275 (31.1) ‡ |
| FSH (+) | 1116 (90) | 917 (82.2) | 828 (74.2) | 499 (44.7) † |
| Quality Grade | Group | Number of Cultured Embryos | Number (%) of Developing Embryos | ||
|---|---|---|---|---|---|
| in Culture | Re-Expansion | to Hatched Blastocyst | |||
| 1 | Control | 90 | 82 (91.1) | 68 (75.6) | 53 (58.9) ‡ |
| FSH (+) | 84 | 82 (97.6) | 73 (86.9) | 64 (76.2) † | |
| 2–3 | Control | 90 | 68 (75.6) | 35 (38.9) | 24 (26.7) † |
| FSH (+) | 52 | 43 (82.7) | 33 (57.9) | 28 (53.8) † | |
| Group | Blastocysts Diameter µm (±SD) | Time | No. of Blastocysts | Total Cell Number Mean (±SD) |
|---|---|---|---|---|
| Control | 186 (±34.8) † | Before vitrification | 54 | 160.1 (±28.2) ‡ |
| After warming | 46 § | 131.4 (±26.7) † | ||
| FSH (+) | 194 (±32.2) † | Before vitrification | 69 | 193.2 (±43.6) † |
| After warming | 63 § | 167.1 (±39.1) † |
| Group | No. of Farms | Blastocysts | Number of Recipients Transferred | Number of Recipients Pregnant (%) |
|---|---|---|---|---|
| Control | 45 | Fresh blastocysts | 412 | 186 (45.1) |
| Post-thaw blastocysts | 102 | 37 (36.2) | ||
| FSH (+) | 31 | Fresh blastocysts | 155 | 94 (60.6) |
| Post-thaw blastocysts | 155 | 79 (50.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Yi, J. Improving Cryopreservation Efficiency and Pregnancy Rate through Superovulation with Follicle-Stimulating Hormone in Korean Hanwoo Cows via Ovum Pick Up. Vet. Sci. 2023, 10, 578. https://doi.org/10.3390/vetsci10090578
Kim D, Yi J. Improving Cryopreservation Efficiency and Pregnancy Rate through Superovulation with Follicle-Stimulating Hormone in Korean Hanwoo Cows via Ovum Pick Up. Veterinary Sciences. 2023; 10(9):578. https://doi.org/10.3390/vetsci10090578
Chicago/Turabian StyleKim, Daehyun, and Junkoo Yi. 2023. "Improving Cryopreservation Efficiency and Pregnancy Rate through Superovulation with Follicle-Stimulating Hormone in Korean Hanwoo Cows via Ovum Pick Up" Veterinary Sciences 10, no. 9: 578. https://doi.org/10.3390/vetsci10090578
APA StyleKim, D., & Yi, J. (2023). Improving Cryopreservation Efficiency and Pregnancy Rate through Superovulation with Follicle-Stimulating Hormone in Korean Hanwoo Cows via Ovum Pick Up. Veterinary Sciences, 10(9), 578. https://doi.org/10.3390/vetsci10090578







