Comparison of Absorbable and Nonabsorbable Sutures for Intradermal Skin Closure in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals
2.3. Inclusion Criteria
2.4. Experimental Design
2.5. Postsurgery Care
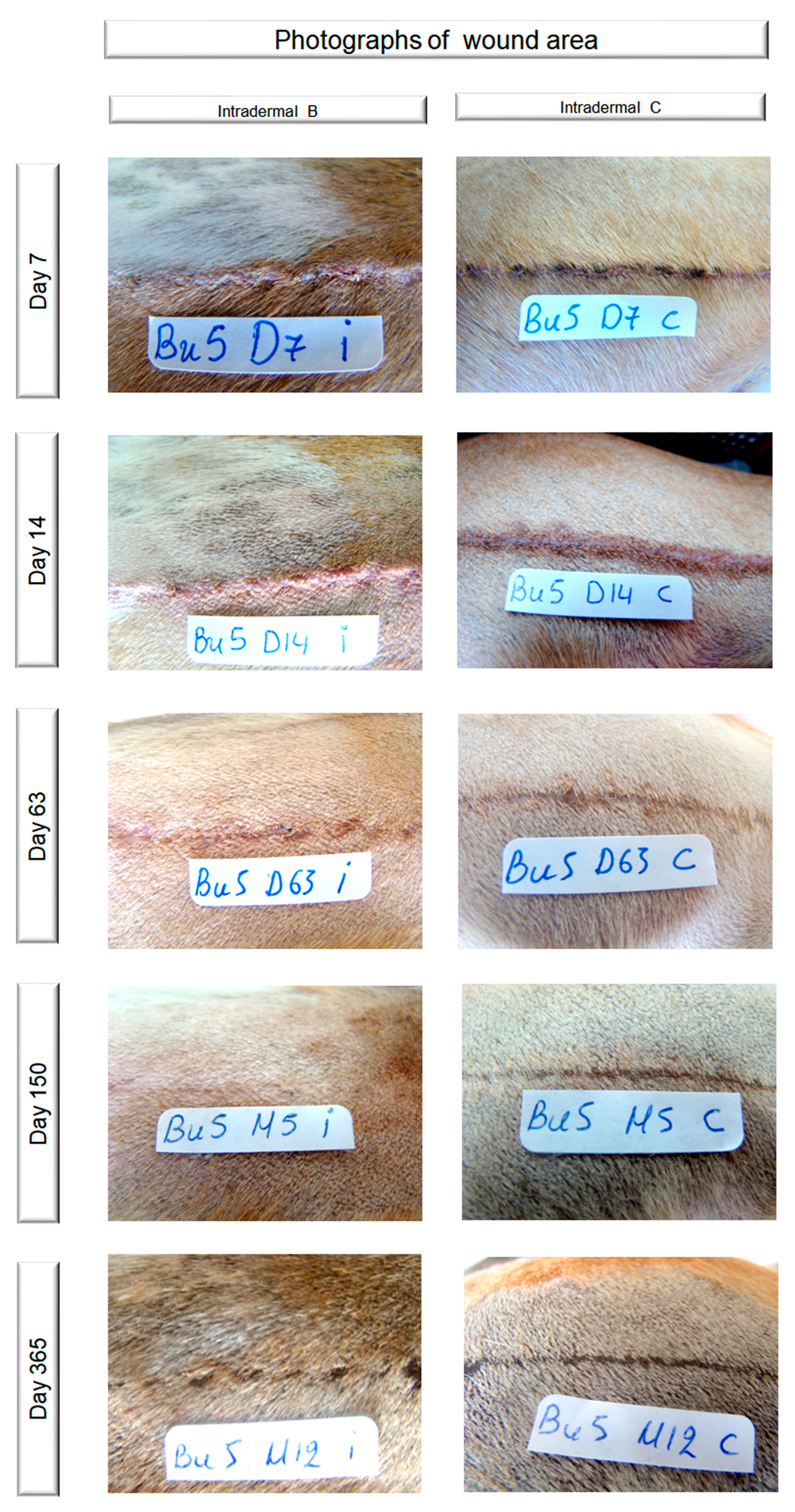
2.6. Cosmetic Evaluation
2.7. Clinical Evaluation
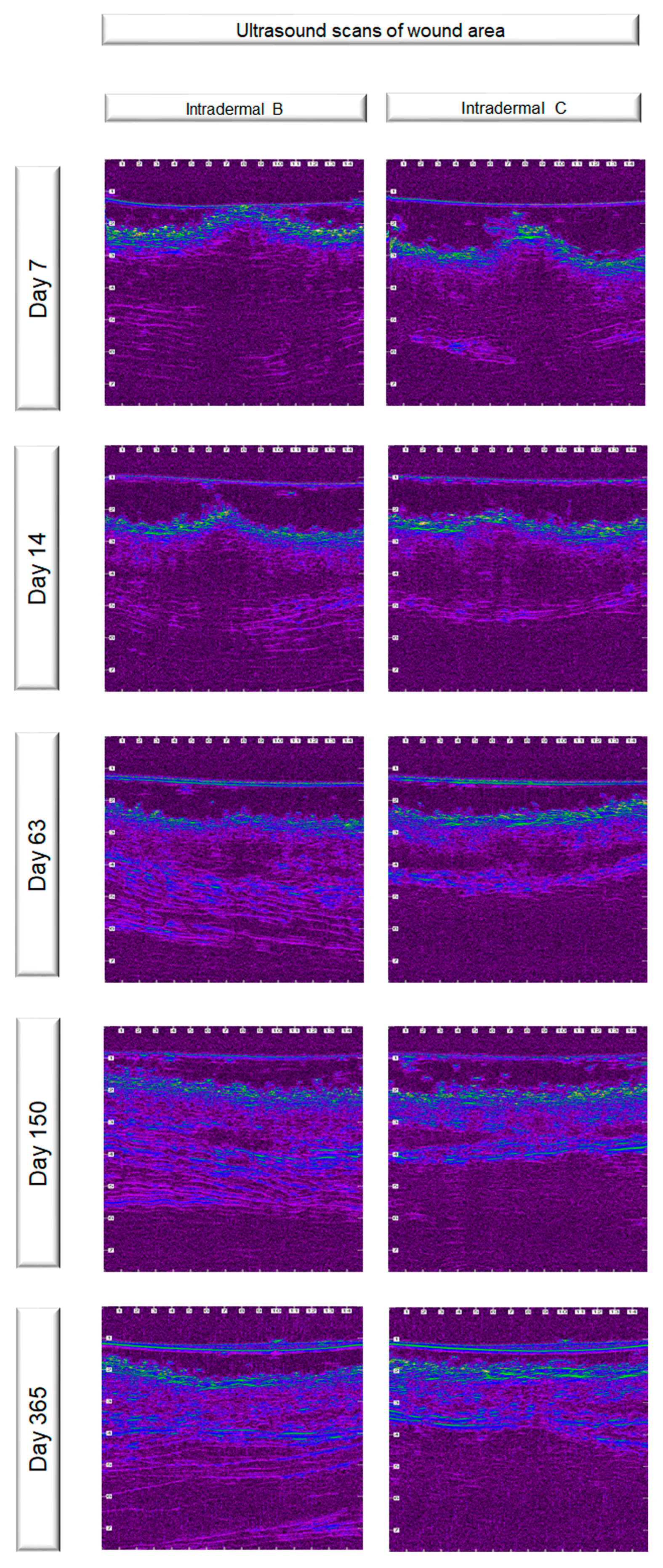
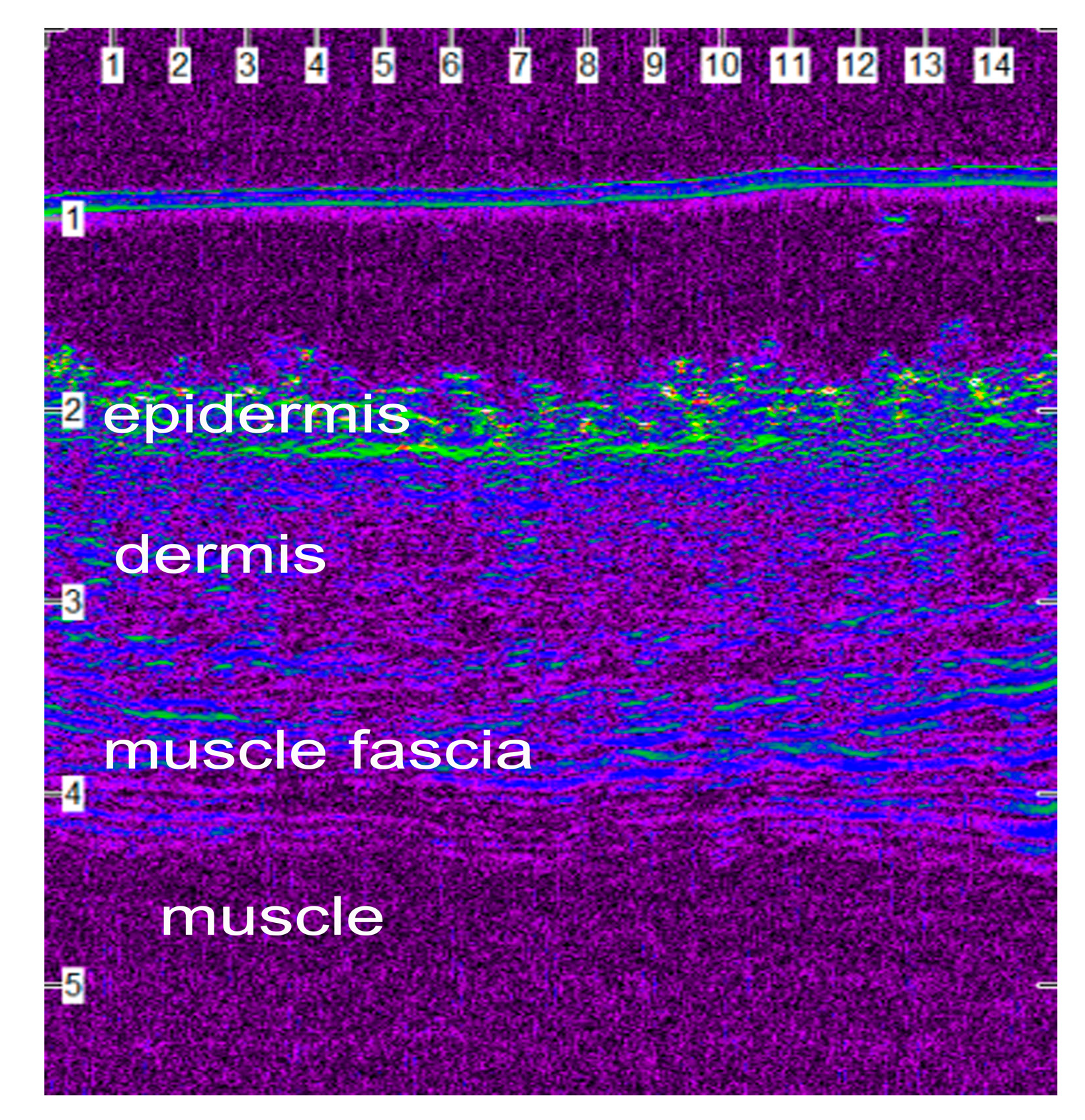
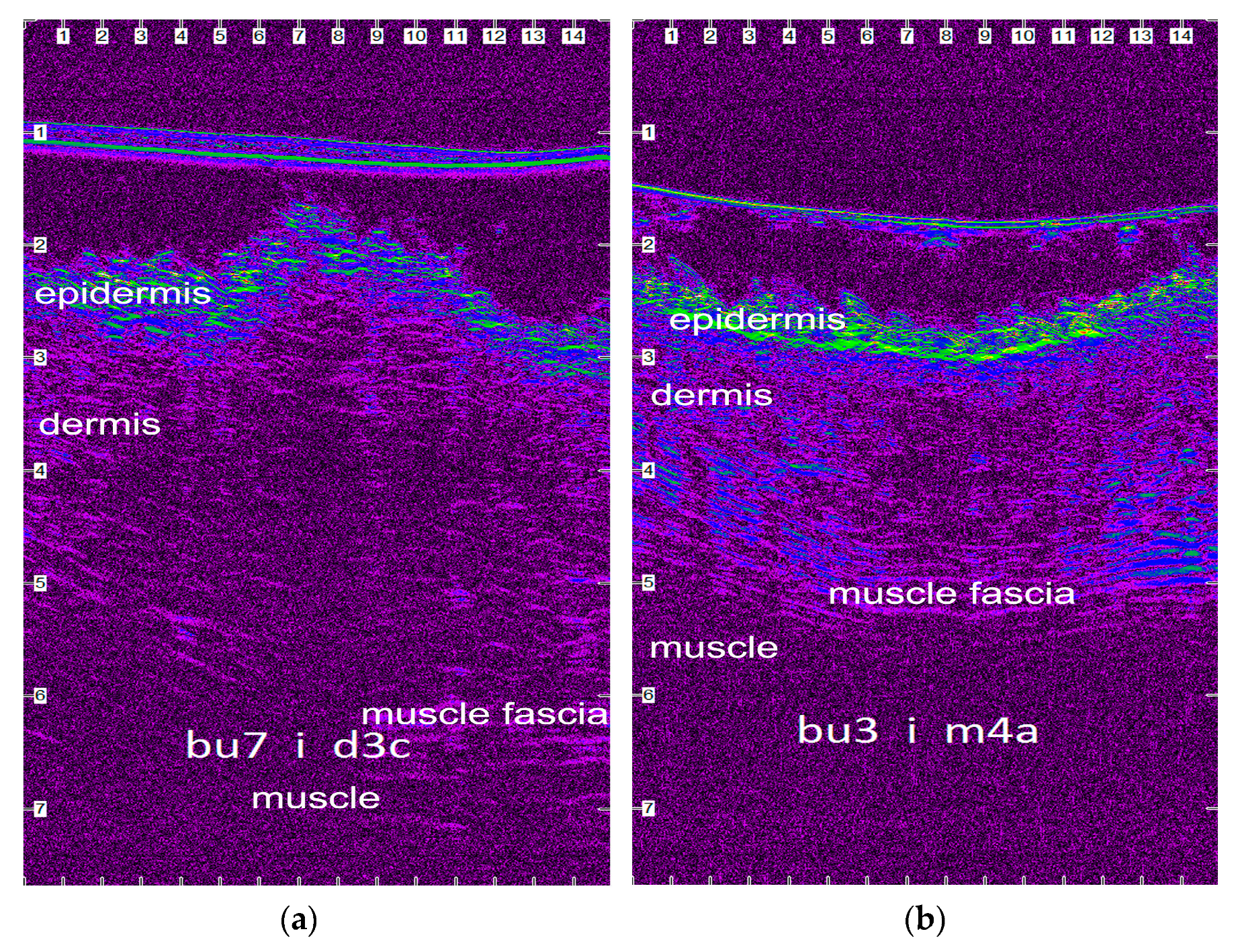
2.8. Ultrasonographic Evaluation
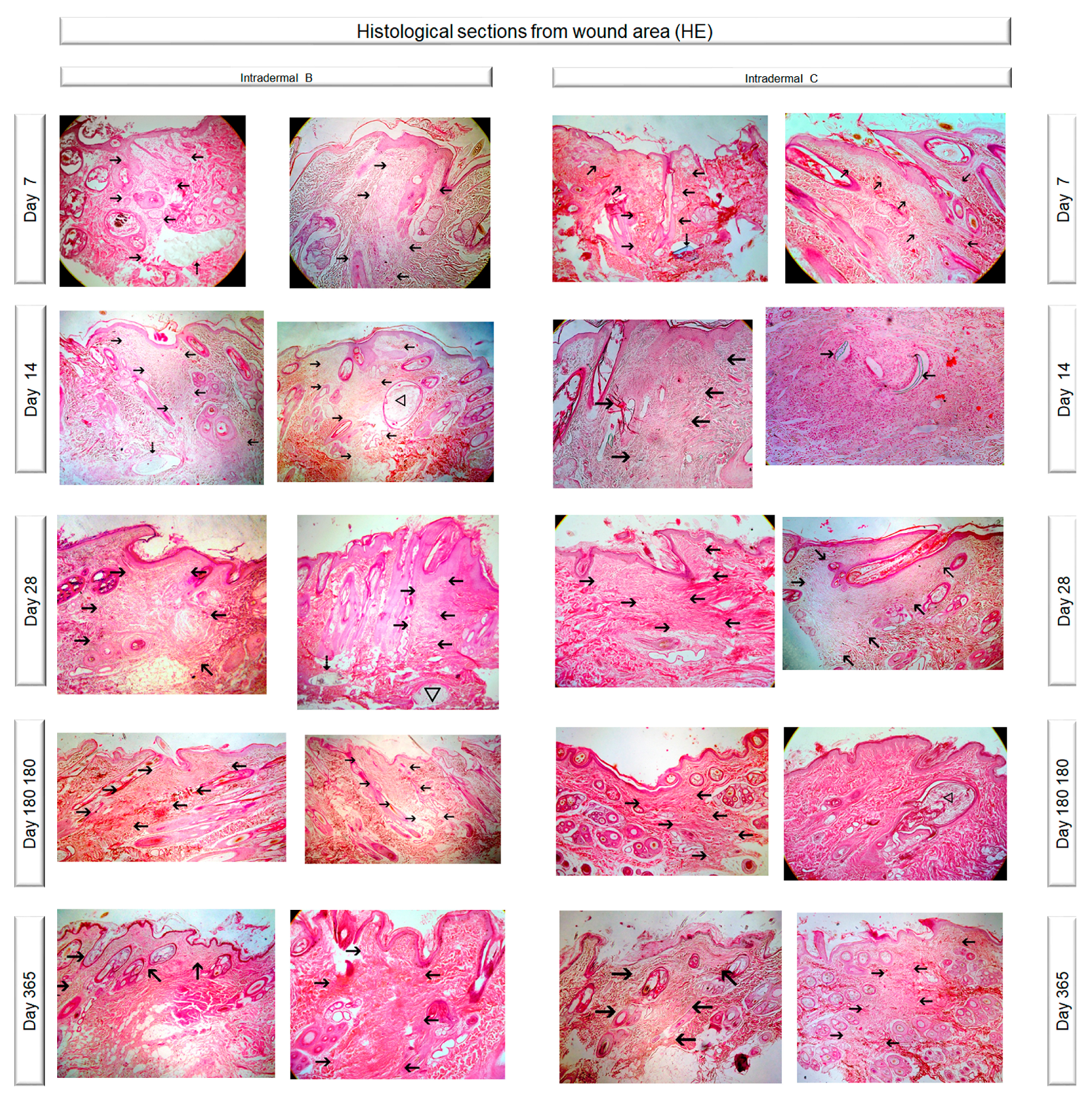
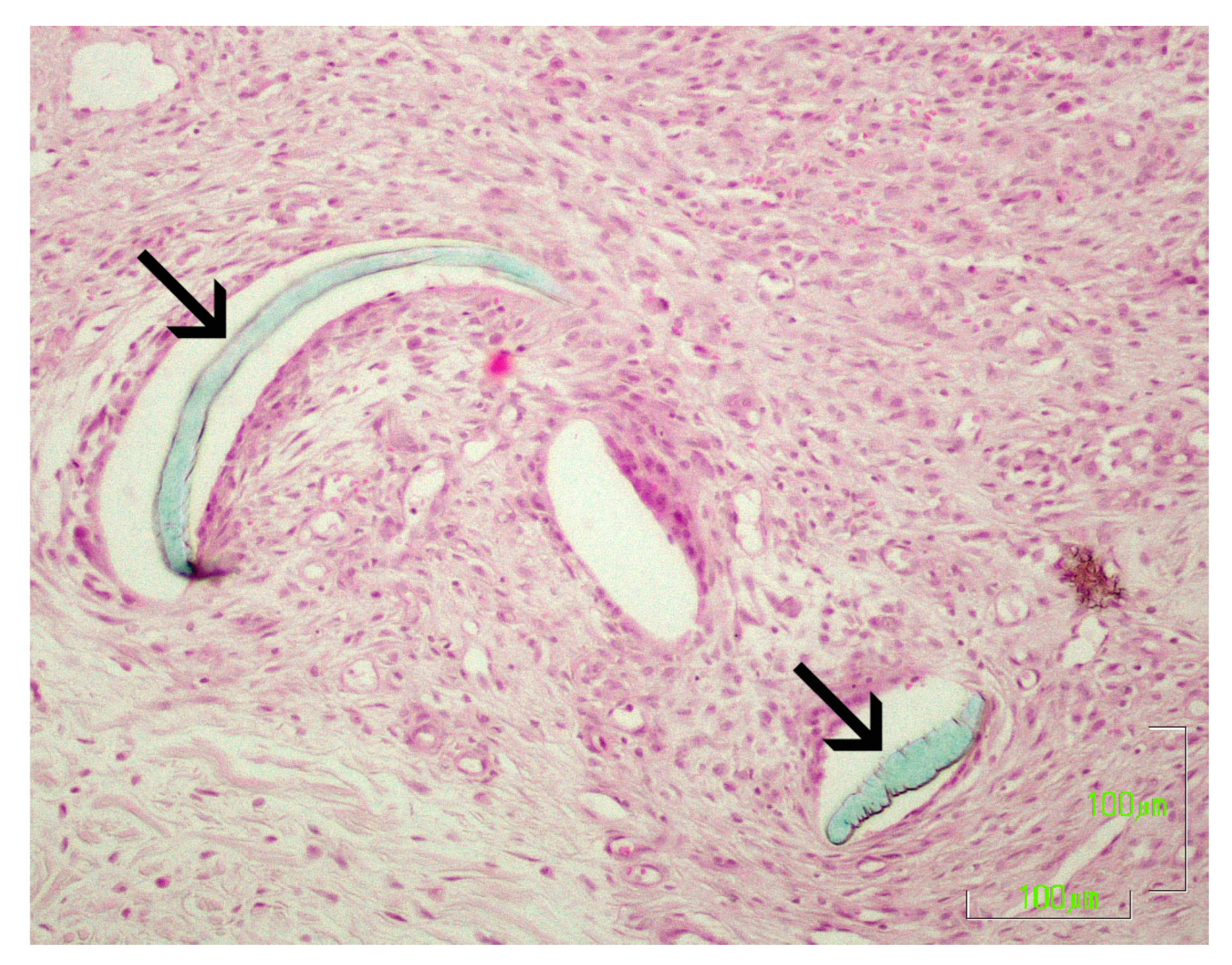
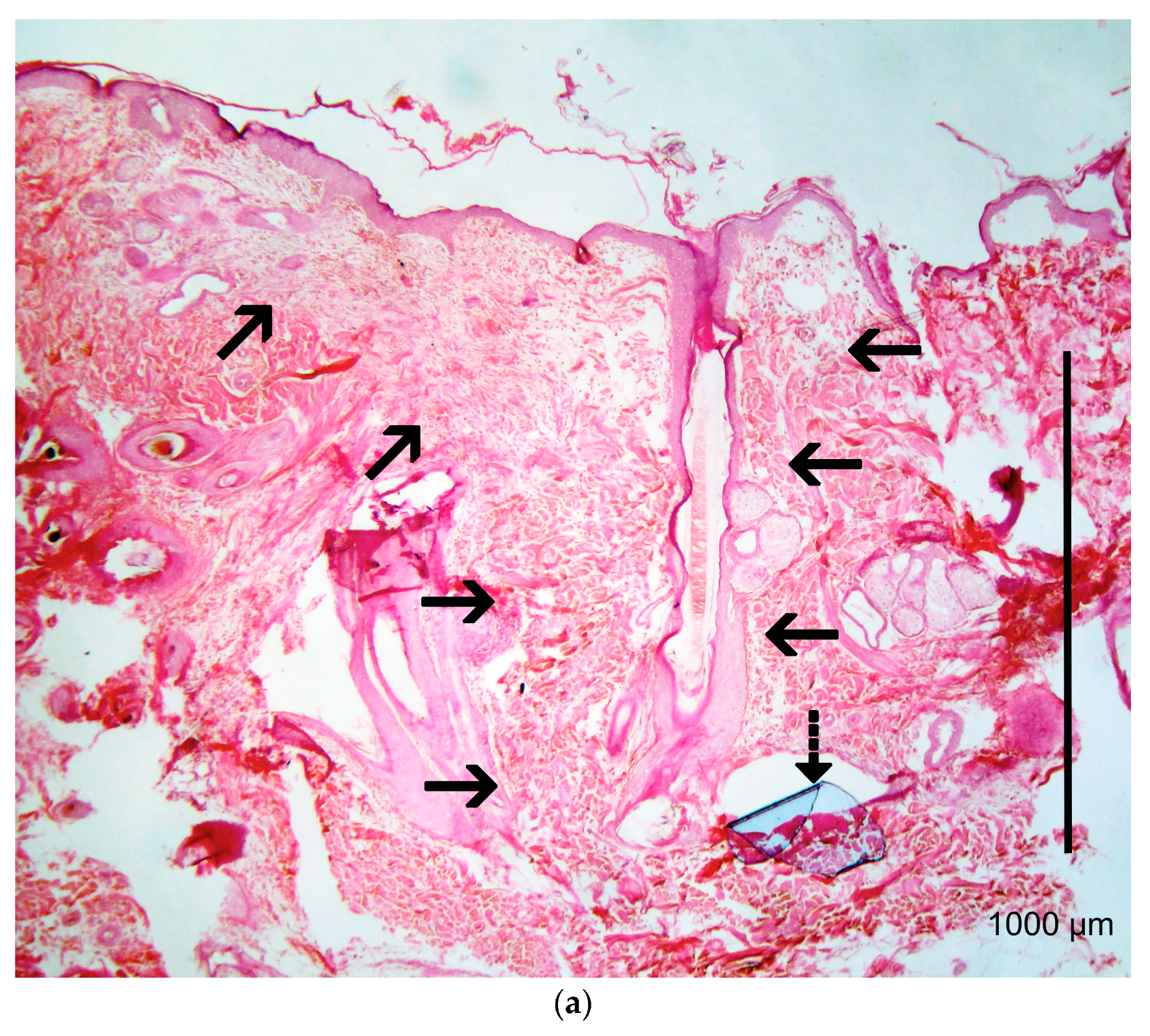
2.9. Histological Evaluation
2.10. Statistical Analysis
- Time period A (1st–8th po.d.), when inflammation, debridement and proliferation take place;
- Time period Β (9th–21st po.d.), when proliferation takes place;
- Time period C (22nd–63rd po.d.), when the early stage of maturation takes place;
- Time period D (64th–180th po.d.), when the median stage of maturation takes place;
- Time period E (181st–365th po.d.), when the late stage of maturation takes place.
3. Results
3.1. Technique Duration
3.2. Cosmetic Evaluation
3.3. Clinical Evaluation
3.3.1. Skin Thickness
3.3.2. Erythema
3.3.3. Scar Width
3.3.4. Inflammation and Abscessation
3.3.5. Exudate
3.3.6. Comedones
3.3.7. Hyperpigmentation
3.3.8. Suture Loss and Wound Dehiscence
3.3.9. Total Clinical Evaluation
3.4. Ultrasonographic Evaluation
3.5. Histological Evaluation
3.5.1. Necrosis
3.5.2. Epithelial Gap
3.5.3. Oedema
3.5.4. Inflammation
3.5.5. Presence of Suture Material and Tissue Reaction
3.5.6. Epithelial Thickness
3.5.7. Scar Width
3.5.8. Collagen Deposition, Fibroblast Presence and Angiogenesis
3.5.9. Total Histological Evaluation
3.5.10. Total Evaluation
3.6. Correlations
3.6.1. Correlation between u/s Estimated Wound Area and Clinically Evaluated Skin Thickening
3.6.2. Correlation between u/s Estimated Wound Area and Histologically Estimated Scar Width
4. Discussion
4.1. Cosmetic Evaluation
4.2. Clinical Evaluation
4.3. Ultrasonographic Evaluation
4.3.1. Ultrasonographic Findings
4.3.2. Correlations between Ultrasonographic Findings and Clinical and Histological Findings
4.4. Histological Evaluation
4.5. Total Scores for Each Technique
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smeak, D.D. Buried continuous intradermal suture closure. Compend. Contin. Educ. Pract. Vet. 1992, 14, 907–919. [Google Scholar]
- Kirpensteijn, J.; Maarschalkerweerd, R.J.; Koeman, J.P.; Kooistra, H.S.; van Sluijs, F.J. Comparison of two suture materials for intradermal skin closure in dogs. Vet. Q. 1997, 19, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, A.; Wilson, J.; Hare, J. A comparison of 2 different suture patterns for skin closure of canine ovariohysterectomy. Can. Vet. J. 2002, 43, 699–702. [Google Scholar] [PubMed]
- Papazoglou, L.G.; Tsioli, V.; Papaioannou, N.; Georgiadis, M.; Savvas, I.; Prassinos, N.; Kouti, V.; Bikiaris, D.; Hadzigiannakis, C.; Zavros, N. Comparison of absorbable and nonabsorbable sutures for intradermal skin closure in cats. Can. Vet. J. 2010, 51, 770–772. [Google Scholar] [PubMed]
- Gouletsou, P.G.; Prassinos, N.N.; Papazoglou, L.G.; Kostoulas, P.; Galatos, A.D. Comparison of continuous intradermal with simple interrupted suture pattern: An experimental study in dogs. Top. Companion Anim. Med. 2020, 41, 100454. [Google Scholar] [CrossRef]
- Gouletsou, P.; Prassinos, N.; Papazoglou, L.; Kostoulas, P.; Galatos, A. A Controlled Trial of Polyglytone 6211 versus Poliglecaprone 25 for Use in Intradermal Suturing in Dogs. Animals 2021, 11, 3094. [Google Scholar] [CrossRef]
- Vipond, M.N.; Ralph, D.J.; Stotter, A.T. Surgery in HIV-positive and AIDS patients: Indications and outcome. J. R. Coll. Surg. Edinb. 1991, 36, 254–258. [Google Scholar]
- Alam, M.; Posten, W.; Martini, M.C.; Wrone, D.A.; Rademaker, A.W. Aesthetic and functional efficacy of subcuticular running epidermal closures of the trunk and extremity: A rater-blinded randomized control trial. Arch. Dermatol. 2006, 142, 1272–1278. [Google Scholar] [CrossRef]
- Rosenzweig, L.B.; Abdelmalek, M.; Ho, J.; Hruza, G.J. Equal cosmetic outcomes with 5-0 poliglecaprone-25 versus 6-0 polypropylene for superficial closures. Dermatol. Surg. 2010, 36, 1126–1129. [Google Scholar] [CrossRef]
- Hopkinson, G.B.; Bullen, B.R. Removable subcuticular skin suture in acute appendicitis: A prospective comparative clinical trial. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 869. [Google Scholar] [CrossRef]
- Molea, G.; Schonauer, F.; Bifulco, G.; D’Angelo, D. Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (Polydioxanone, Poliglecaprone 25, Glycomer 631). Br. J. Plast. Surg. 2000, 53, 137–141. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Silva Junior, V.A.; Silva Neto, J.C.; Vasconcelos, B.C. Clinical and histopathological study of tissue reactivity to monofilament suture materials: Nylon and poliglecaprone 25 in rats. Acta Cir. Bras. 2005, 20, 284–291. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Schmidt, K.; Laske, M.; Ziegler, U.E. Comparison of various methods and materials for treatment of skin laceration by a 3-dimensional measuring technique in a pig experiment. Ann. Plast. Surg. 2007, 58, 566–572. [Google Scholar] [CrossRef]
- Court, M.H.; Bellenger, C.R. Comparison of adhesive polyurethane membrane and polypropylene sutures for closure of skin incisions in cats. Vet. Surg. 1989, 18, 211–215. [Google Scholar] [CrossRef]
- Hunt, T.K.; Hopf, H.; Hussain, Z. Physiology of wound healing. Adv. Ski. Wound Care 2000, 13, 6–11. [Google Scholar] [CrossRef]
- Scardino, M.S.; Swaim, S.F.; Sartin, E.A.; Steiss, J.E.; Spano, J.S.; Hoffman, C.E.; Coolman, S.L.; Peppin, B.L. Evaluation of treatment with a pulsed electromagnetic field on wound healing, clinicopathologic variables, and central nervous system activity of dogs. Am. J. Vet. Res. 1998, 59, 1177–1181. [Google Scholar]
- Gillette, R.L.; Swaim, S.F.; Sartin, E.A.; Bradley, D.M.; Coolman, S.L. Effects of a bioactive glass on healing of closed skin wounds in dogs. Am. J. Vet. Res. 2001, 62, 1149–1153. [Google Scholar] [CrossRef]
- Winkler, J.T.; Swaim, S.F.; Sartin, E.A.; Henderson, R.A.; Welch, J.A. The effect of a porcine-derived small intestinal submucosa product on wounds with exposed bone in dogs. Vet. Surg. 2002, 31, 541–551. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Swanson, N.A. Basic techniques. In Atlas of Cutaneous Surgery; Little, Brown, & Co: Boston, MA, USA, 1987; pp. 1–68. [Google Scholar]
- Stasko, T. Advanced suturing techniques and layered closures. In Cutaneous Surgery; Wheeland, R.G., Ed.; Saunders: Philadelphia, PA, USA, 1994; pp. 304–317. [Google Scholar]
- Swaim, S.F.; Henderson, R.A.J. Wound healing. In Small Animal Wound Management, 2nd ed.; Williams & Wilkins: Baltimore, MD, USA, 1997; pp. 1–12. [Google Scholar]
- Vipond, M.N.; Higgins, A.F. Subcuticular Prolene or PDS for skin closure? J. R. Coll. Surg. Edinb. 1991, 36, 97–99. [Google Scholar]
- Webster, R.C.; McCollough, E.G.; Giandello, P.R.; Smith, R.C. Skin wound approximation with new absorbable suture material. Arch Otolaryngol. 1985, 111, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, G. Stages of wound healing and their clinical relevance. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.C.; Campell, K. Structure and function of the skin. In Muller & Kirk’s Small Animal Dermatology, 7th ed.; Miller, W.G.C., Campell, K., Eds.; Elsevier: St. Louis, MO, USA, 2013; pp. 1–56. [Google Scholar]
- Miller, W.G.C.; Campell, K. Pigmentary abnormalities. In Muller & Kirk’s Small Animal Dermatology, 7th ed.; Miller, W.G.C., Campell, K., Eds.; Elsevier: St. Louis, MO, USA, 2013; pp. 618–629. [Google Scholar]
- Rudolph, R.; Klein, L. Healing processes in skin grafts. Surg. Gynecol. Obstet. 1973, 136, 641–654. [Google Scholar] [PubMed]
- Matsumoto, K.; Robb, E.; Warden, G.; Nordlund, J. Hyperpigmentation of human skin grafted on to athymic nude mice: Immunohistochemical study. Br. J. Dermatol. 1996, 135, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Mantis, P.; Lloyd, D.H.; Pfeiffer, D.; Stevens, K.; Auxilia, S.; Noli, C.; Auxilia, S.; Noli, C.; Abramo, F.; Miolo, A. Assessment of the Effect of an Aliamide-containing Topical Gel by Evaluation of the Reduction of Wound Volume Measured by High R. Wounds 2007, 19, 113–119. [Google Scholar]
- Diana, A.; Guglielmini, C.; Fracassi, F.; Pietra, M.; Balletti, E.; Cipone, M. Use of high-frequency ultrasonography for evaluation of skin thickness in relation to hydration status and fluid distribution at various cutaneous sites in dogs. Am. J. Vet. Res. 2008, 69, 1148–1152. [Google Scholar] [CrossRef]
- Mantis, P.; Tontis, D.; Church, D.; Lloyd, D.; Stevens, K.; Balomenos, D.; Gouletsou, P.G.; Gianoulopoulos, G.; Doukas, D.; Galatos, A.D.; et al. High-frequency ultrasound biomicroscopy of the normal canine haired skin. Vet. Dermatol. 2014, 25, 176-e145. [Google Scholar] [CrossRef]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Pope, E.R. Skin healing. In Disease Mechanisms in Small Animal Surgery; Bojrab, M.J., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1993; pp. 151–155. [Google Scholar]
- Postlethwait, R.W.; Willigan, D.A.; Ulin, A.W. Human tissue reaction to sutures. Ann. Surg. 1975, 181, 144–150. [Google Scholar] [CrossRef]
- Salthouse, T. Tissue response to sutures. In Biomaterials in Reconstructive Surgery; Rubin, L.R., Ed.; Mosby: St. Louis, MO, USA, 1983; pp. 131–142. [Google Scholar]
- Bezwada, R.S.; Jamiolkowski, D.D.; Lee, I.Y.; Agarwal, V.; Persivale, J.; Trenka-Benthin, S.; Erneta, M.; Suryadevara, J.; Yang, A.; Liu, S. Monocryl suture, a new ultra-pliable absorbable monofilament suture. Biomaterials 1995, 16, 1141–1148. [Google Scholar] [CrossRef]
- Van Winkle, W., Jr.; Hastings, J.C.; Barker, E.; Hines, D.; Nichols, W. Effect of suture materials on healing skin wounds. Surg. Gynecol. Obstet. 1975, 140, 7–12. [Google Scholar] [CrossRef]
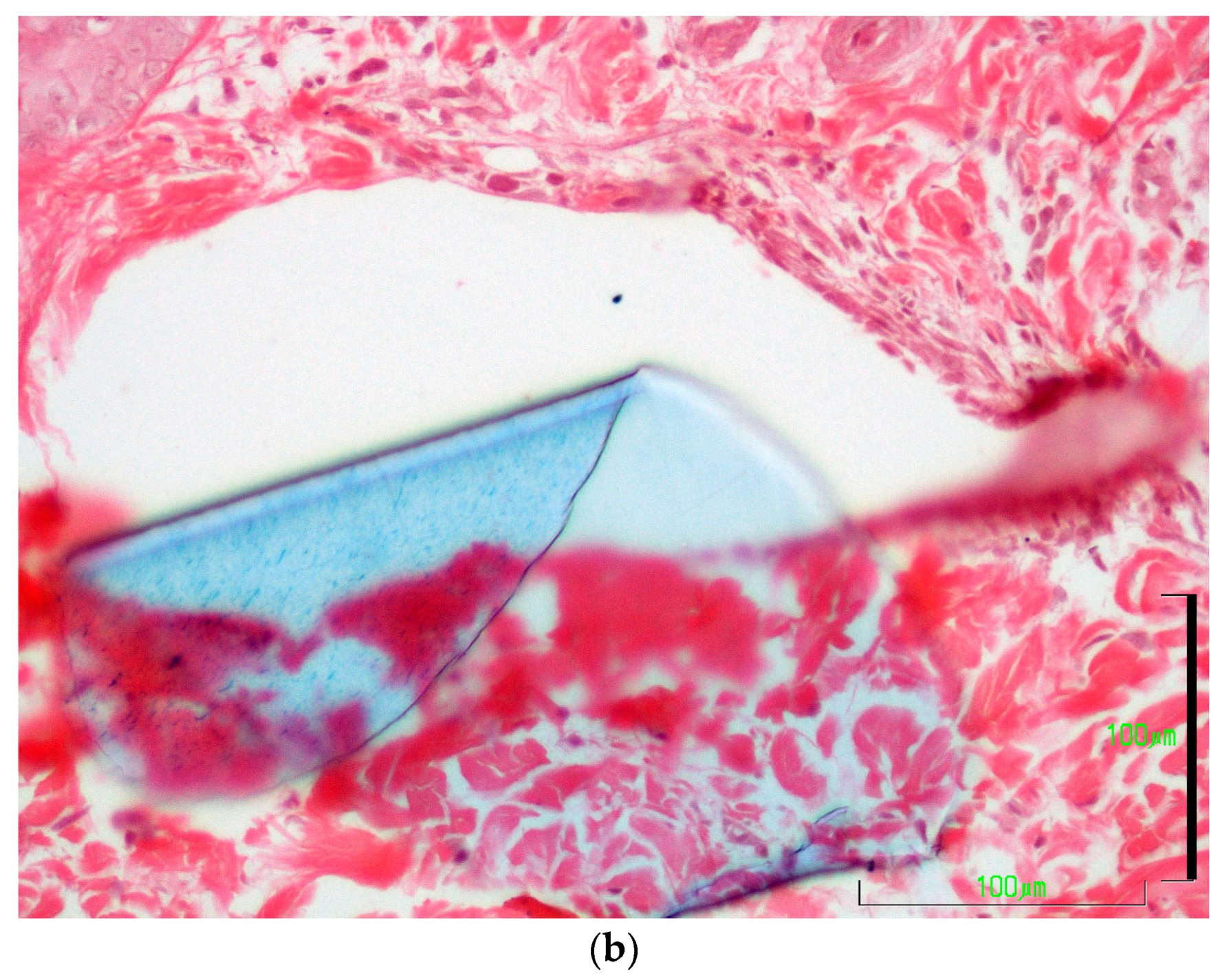

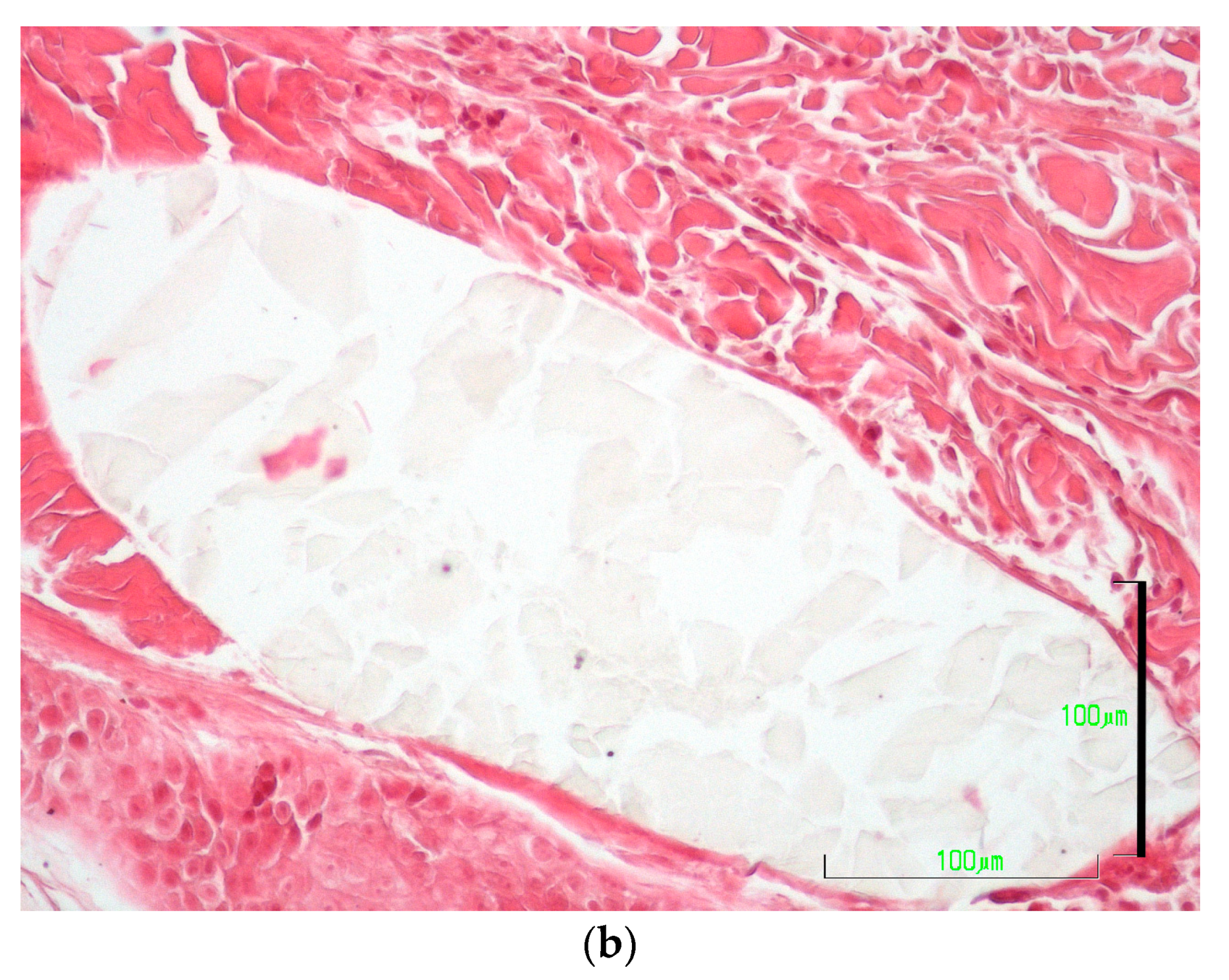
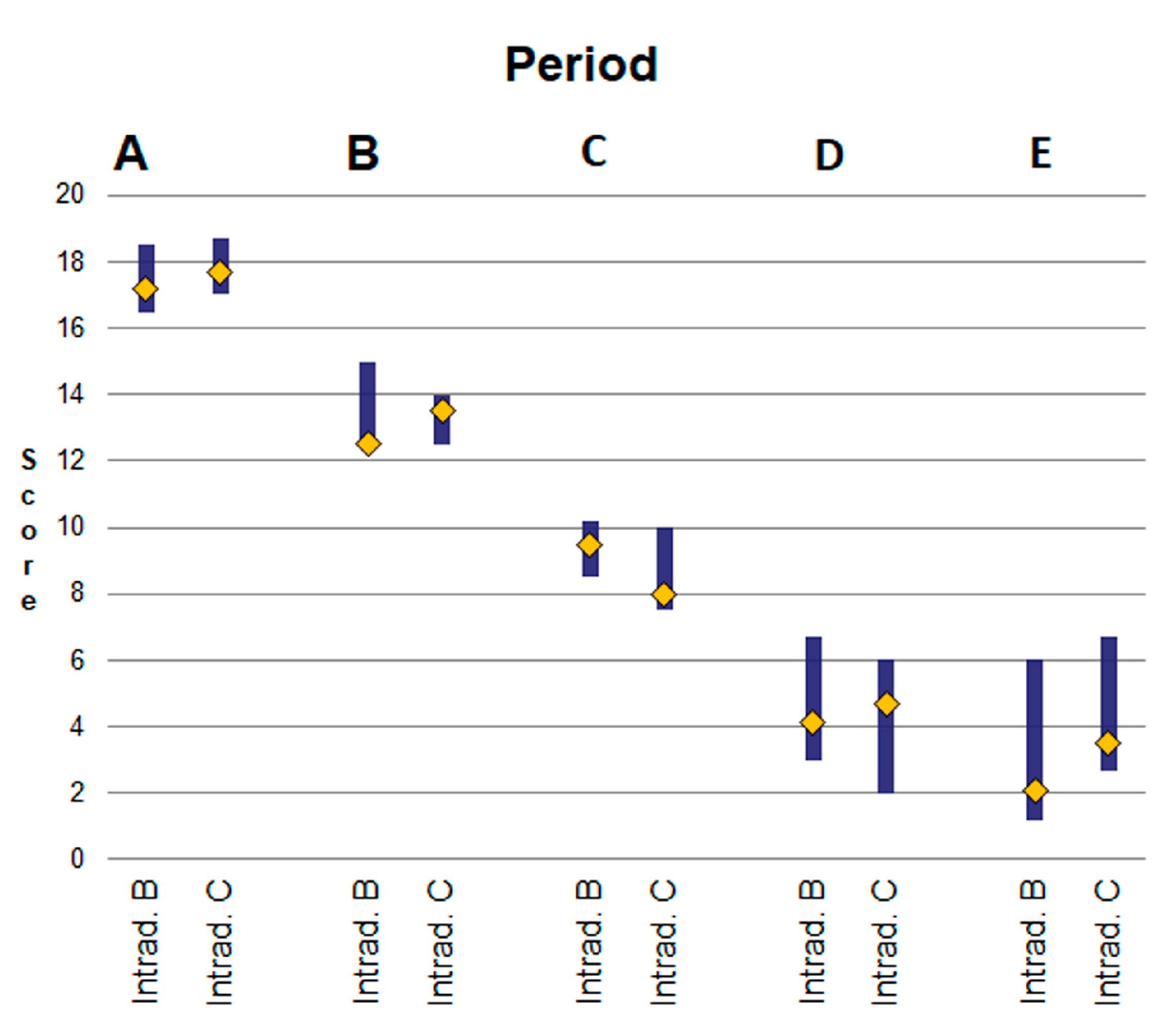
| Technique | Percentile 25 | Median | Percentile 75 | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Intradermal B (poliglecarpone 25) | 15′32″ | 15′37″ | 16′55″ | 16′13″ | 1′30″ |
| Intradermal C (polypropylene) | 13′39″ | 13′48″ | 14′05″ | 13′33″ | 0′44″ |
| Technique | Period A 1st–8th po.d. | Period B 9th–21st po.d. | Period C 22nd–63rd po.d. | Period D 64th–180th po.d. | Period E 181st–365th po.d. |
|---|---|---|---|---|---|
| Intradermal B (poliglecaprone 25) | 4.0 (4.0–4.5) | 2.8 (2.0–3.0) | 2.0 (1.8–3.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Intradermal C (polypropylene) | 4.0 (3.3–4.5) | 2.0 (1.0–3.0) | 1.8 (1.0–2.0) | 1.0 (1.0–2.0) | 1.3 (1.0–2.0) |
| Technique | Period A 1st–8th po.d. | Period B 9th–21st po.d. | Period C 22nd–63rd po.d. | Period D 64th–180th po.d. | Period E 181st–365th po.d. |
|---|---|---|---|---|---|
| Skin thickening (in mm, measured with a skin caliper) | |||||
| Intradermal B (poliglecaprone 25) | 1.53 (1.03–1.85) | 0.65 (0.35–0.75) | 0.38 (0.05–0.75) | 0.0 | 0.0 |
| Intradermal C (polypropylene) | 1.40 (1.0–1.70) | 0.58 (0.30–1.0) | 0.30 (0.10–0.50) | 0.0 (0.0–0.40) | 0 (0.0–0.30) |
| Erythema (in mm, measured with an electronic caliper) | |||||
| Intradermal B (poliglecaprone 25) | 0.30 (0.43–0.60) | 0.0 | 0.0 | 0.0 | 0.0 |
| Intradermal C (polypropylene) | 0.32 (0.0–0.60) | 0.0 | 0.0 | 0.0 | 0.0 |
| Scar width (in mm, measured with an electronic caliper) | |||||
| Intradermal B (poliglecaprone 25) | 0.52 (0.43–0.60) | 0.51 (0.44–0.60) | 0.45 (0.37–0.60) | 0.42 (0.29–0.56) | 0.35 (0.23–0.48) |
| Intradermal C (polypropylene) | 0.56 (0.47–0.67) | 0.53 (0.45–0.60) | 0.41 (0.27–0.57) | 0.56 (0.41–0.66) | 0.30 (0.24–0.48) |
| Hyperpigmentation (score 0–3) | |||||
| Intradermal B (poliglecaprone 25) | 0.0 | 0.0 | 1.0 (0.0–1.0) | 0.00 (0.0–1.0) | 0.50 (0.0–1.0) |
| Intradermal C (polypropylene) | 0.0 | 0.0 | 0.00 (0.0–1.0) | 0.0 (0.0–1.00) | 1.0 (0.0–1.0) |
| Total clinical evaluation (score 0–12) | |||||
| Intradermal B (poliglecaprone 25) | 4 (4–5) | 3 (2–4) | 3 (2–4) | 2 (1–3) | 1 (0–4) |
| Intradermal C (polypropylene) | 4 (4–5) | 3 (2–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) |
| Technique | Period A 1st–8th po.d. | Period B 9th–21st po.d. | Period C 22nd–63rd po.d. | Period D 64th–180th po.d. | Period E 181st–365th po.d. |
|---|---|---|---|---|---|
| Intradermal B (poliglecaprone 25) | 8.60 (7.13–10.31) | 4.93 (4.39–5.74) | 3.23 (2.67–4.26) | 2.15 (1.45–2.90) | 1.45 (0.70–2.21) |
| Intradermal C (polypropylene) | 9.50 (7.87–11.20) | 4.54 (3.55–5.50) | 2.86 (2.20–3.65) | 1.89 (1.42–2.57) | 1.34 (0.80–2.24) |
| Technique | Period A 1st–8th po.d. | Period B 9th–21st po.d. | Period C 22nd–63rd po.d. | Period D 64th–180th po.d. | Period E 181st–365th po.d. |
|---|---|---|---|---|---|
| Inflammation (score 0–3) | |||||
| Intradermal B (poliglecaprone 25) | 2 (2–3) | 2 (2–2) | 1 (0–2) | 0 (0–1) | 0 (0–0) |
| Intradermal C (polypropylene) | 2 (2–3) | 3 (1–3) | 1 (0–1) | 0 (0–0) | 0 (0–0) |
| Epithelial thickness (number of times by which scar epithelial thickness was greater than that of the adjacent healthy epidermis) | |||||
| Intradermal B (poliglecaprone 25) | 2.5 (2.0–3.0) | 2.0 (2.0–3.0) | 1.6 (1.5–2.0) | 1.2 (1.0–1.3) | 1.0 (1.0–1.1) |
| Intradermal C (polypropylene) | 2.5 (2.0–2.5) | 2.0 (2.0–3.0) | 1.4 (1.2–1.5) | 1.1 (1.0–1.3) | 1.0 (1.0–1.2) |
| Scar width (in mm) | |||||
| Intradermal B (poliglecaprone 25) | 0.54 (0.45–0.68) | 0.56 (0.45–0.68) | 0.59 (0.50–0.90) | 0.45 (0.41–0.54) | 0.45 (0.41–0.45) |
| Intradermal C (polypropylene) | 0.68 (0.54–0.68) | 0.63 (0.54–0.81) | 0.52 (0.45–0.54) | 0.45 (0.36–0.68) | 0.45 (0.36–0.45) |
| Total histological evaluation (score 0–15) | |||||
| Intradermal B (poliglecaprone 25) | 7 (6–8) | 5 (5–6) | 3 (2–5) | 1 (0–2) | 0 (0–0) |
| Intradermal C (polypropylene) | 7 (6–8) | 7 (5–7) | 3 (2–4) | 1 (0–2) | 0 (0–3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balomenos, D.B.; Gοuletsοu, P.G.; Galatos, A.D. Comparison of Absorbable and Nonabsorbable Sutures for Intradermal Skin Closure in Dogs. Vet. Sci. 2023, 10, 105. https://doi.org/10.3390/vetsci10020105
Balomenos DB, Gοuletsοu PG, Galatos AD. Comparison of Absorbable and Nonabsorbable Sutures for Intradermal Skin Closure in Dogs. Veterinary Sciences. 2023; 10(2):105. https://doi.org/10.3390/vetsci10020105
Chicago/Turabian StyleBalomenos, Dimitrios B., Pagona G. Gοuletsοu, and Apostolos D. Galatos. 2023. "Comparison of Absorbable and Nonabsorbable Sutures for Intradermal Skin Closure in Dogs" Veterinary Sciences 10, no. 2: 105. https://doi.org/10.3390/vetsci10020105
APA StyleBalomenos, D. B., Gοuletsοu, P. G., & Galatos, A. D. (2023). Comparison of Absorbable and Nonabsorbable Sutures for Intradermal Skin Closure in Dogs. Veterinary Sciences, 10(2), 105. https://doi.org/10.3390/vetsci10020105







