Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Materials
2.2.1. Erythropoietin
2.2.2. Collagen Matrix
2.2.3. Bone Substitute
2.2.4. Metal Implants
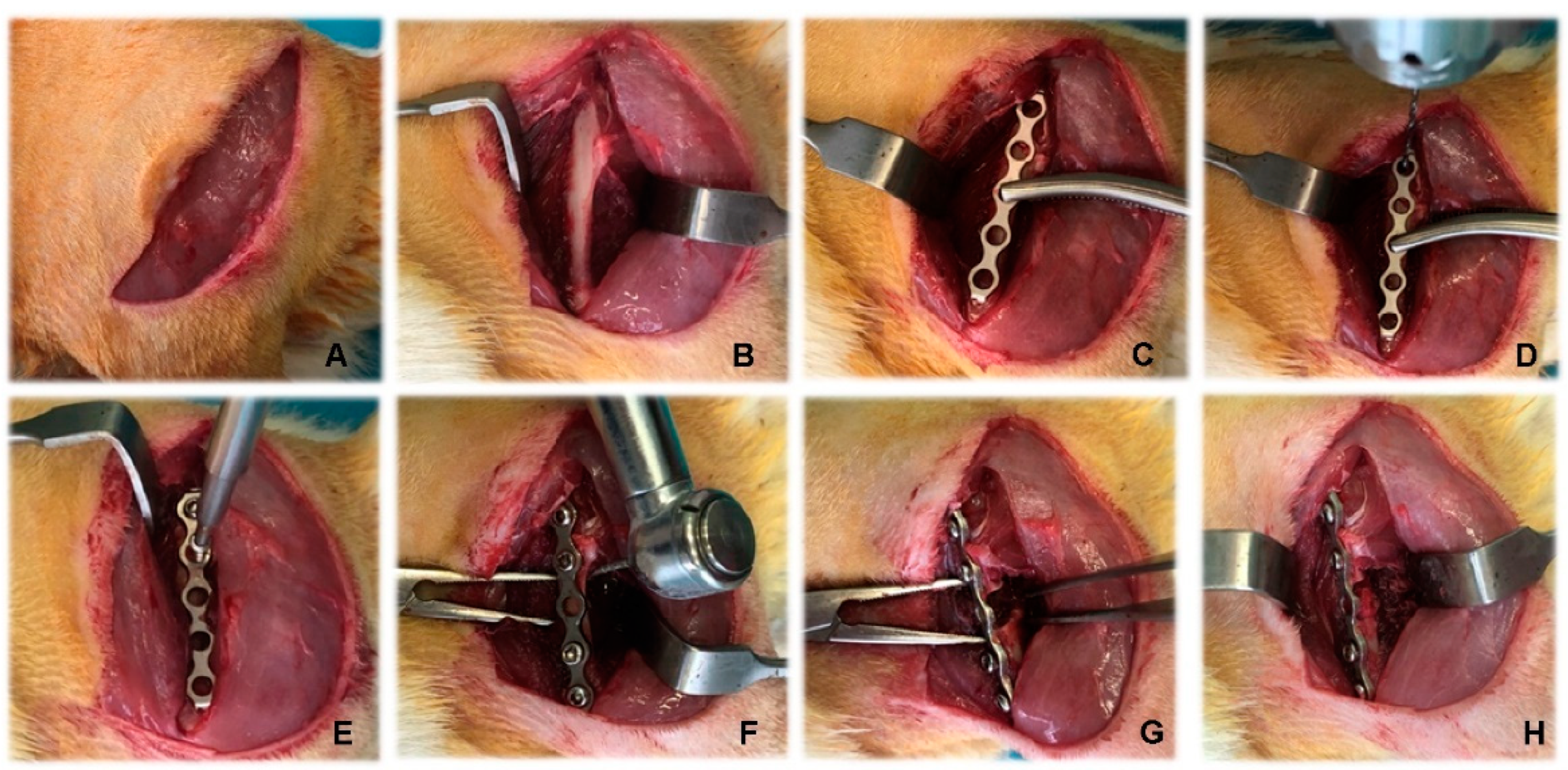
2.3. Anaesthesiological and Surgical Protocol
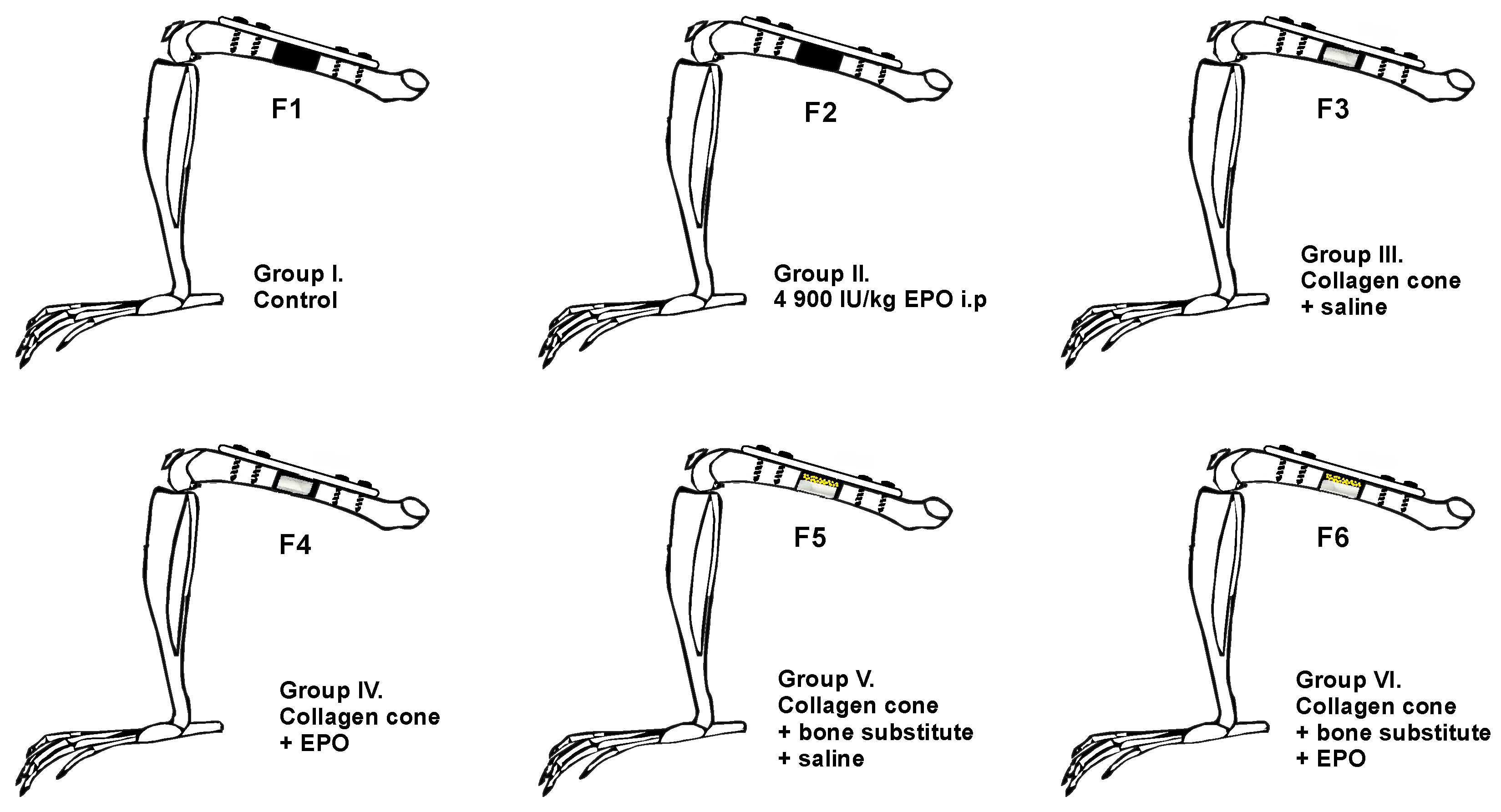
- Group F1 (n = 10): the defect was left empty.
- Group F2 (n = 10): the defect was left empty, and the rats received a single intraperitoneal EPO injection at a dose of 4900 IU/kg.
- Group F3 (n = 8): collagen cone soaked with physiological saline.
- Group F4 (n = 10): collagen cone soaked with 49 IU EPO.
- Group F5 (n = 8): bone substitute combined with collagen cone soaked with physiological saline.
- Group F6 (n = 10): bone substitute combined with collagen cone soaked with 49 IU EPO.
2.4. Radiography
2.5. Bone Densitometry
2.6. Histological Examinations
2.7. Statistical Analysis
3. Results
3.1. Clinical Examinations
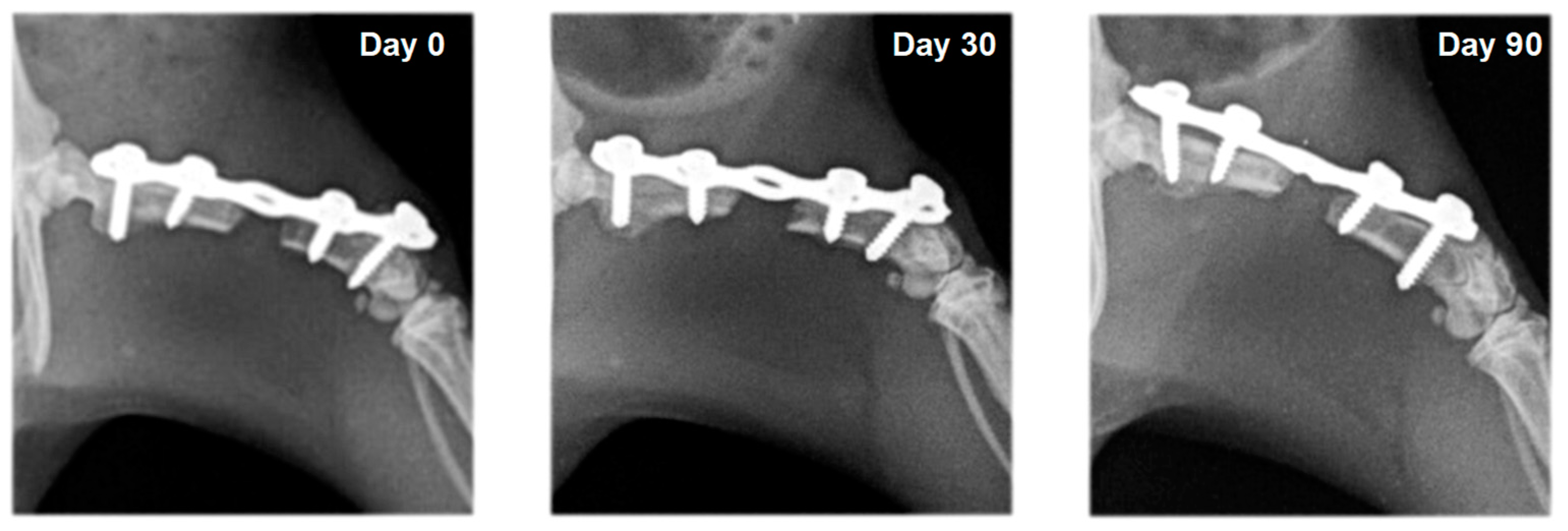
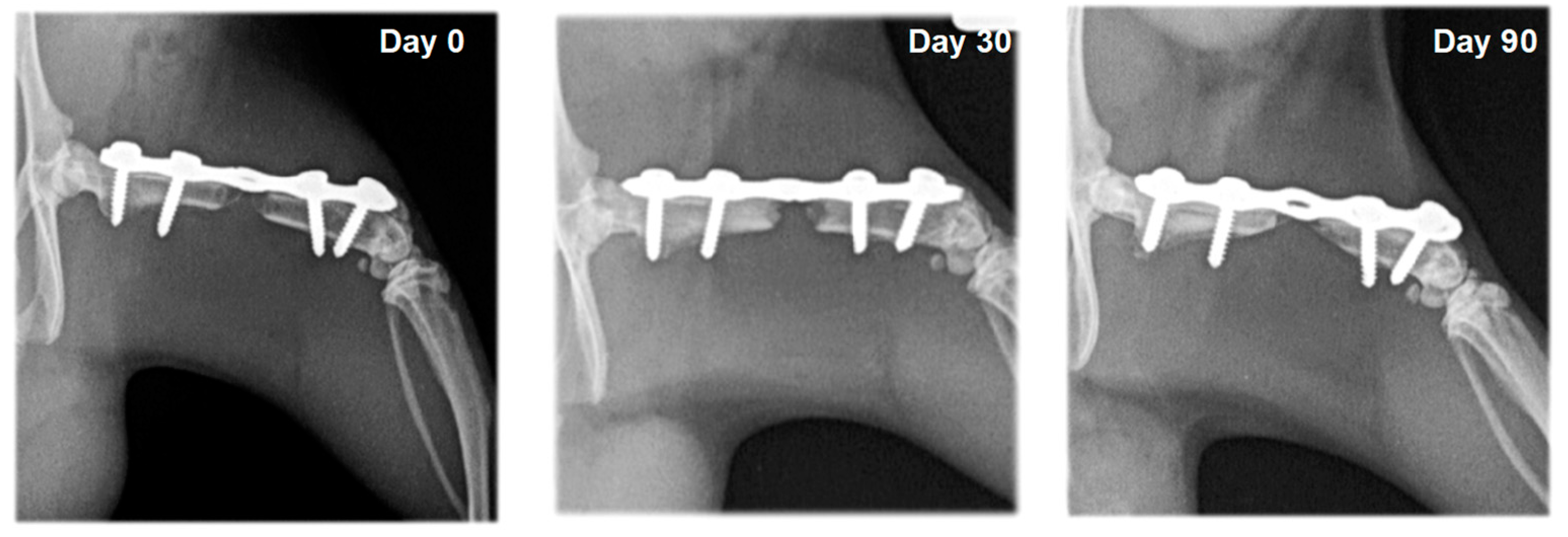
3.2. Radiography
3.3. Bone Densitometry
3.4. Histology
3.4.1. Microscopic Histological Pattern on Post-Operative Day 30
3.4.2. Microscopic Histological Pattern on Post-Operative Day 90
4. Discussion
5. Conclusions
- Erythropoietin, applied locally on a collagen scaffold, is capable of inducing bone healing, whereas a single high dose applied intraperitoneally has only an insignificant effect on bone formation.
- Erythropoietin results in a more rapid integration of spongy xenograft granules to the host bone.
- Histologically, the regeneration of rat femoral critical-size defects occurred via endochondral ossification.
- Osteodensitometry, a non-invasive technique of quantifying bone mineral density, may be used to assess the strength of the newly formed bone callus.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma. 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.W.; Bhandari, M.; Guyatt, G.; Heels-Ansdell, D.; Schemitsch, E.H.; Swiontkowski, M.; Tornetta, P.; Walter, S. Critical-sized defect in the tibia: Is it critical? Results from the SPRINT trial. J. Orthop. Trauma. 2014, 28, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Pederson, W.C.; Person, D.W. Long bone reconstruction with vascularized bone grafts. Orthop. Clin. N. Am. 2007, 38, 23–35. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A. Bone morphogenetic proteins in musculoskeletal medicine. Injury 2009, 40, S1–S3. [Google Scholar] [CrossRef]
- Mocini, D.; Leone, T.; Tubaro, M.; Santini, M.; Penco, M. Structure, production and function of erythropoietin: Implications for therapeutical use in cardiovascular disease. Curr. Med. Chem. 2007, 14, 2278–2287. [Google Scholar] [CrossRef]
- Zubareva, E.V.; Nadezhdin, S.V.; Burda, Y.E.; Nadezhdina, N.A.; Gashevskaya, A.S. Pleiotropic effects of erythropoietin. Influence of erythropoietin on processes of mesenchymal stem cells differentiation. Res. Results Pharmacol. 2019, 55, 53–66. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Wang, J.; Lee, C.H.; Sud, S.; et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef] [PubMed]
- Bozlar, M.; Kalaci, A.; Aslan, B.; Baktiroglu, L.; Yanat, A.N.; Tasci, A. Effects of erythropoietin on fracture healing in rats. Saudi Med. J. 2006, 27, 1267–1269. [Google Scholar] [PubMed]
- Holstein, J.H.; Menger, M.D.; Scheuer, C.; Meier, C.; Culemann, U.; Wirbel, R.J.; Garcia, P.; Pohlemann, T. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007, 80, 893–900. [Google Scholar] [CrossRef]
- Garcia, P.; Speidel, V.; Scheuer, C.; Laschke, M.W.; Holstein, J.H.; Histing, T.; Pohleman, T.; Menger, M.D. Low dose erythropoietin stimulates bone healing in mice. J. Orthop. Res. 2011, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Diker, N.; Sarican, H.; Cumbul, A.; Kilic, E. Effects of systemic erythropoietin treatment and heterogeneous xenograft in combination on bone regeneration of a critical-size defect in an experimental model. J. Craniomaxillofac. Surg. 2018, 46, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, N.; Reshetov, I.; Zelianin, A.; Philippov, V.; Sergeeva, N.; Sviridova, I.; Komlev, V.; Andreeva, U.; Kuznecova, O. Three-dimensional TCP scaffolds enriched with Erythropoietin for stimulation of vascularization and bone formation. Electron J. Gen. Med. 2019, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, R. Local application of erythropoietin and xenograft for enhancing bone regeneration. Tradit. Mod. Vet. Med. 2022, 1, 38–43. [Google Scholar] [CrossRef]
- Steinbrech, D.S.; Mehrara, B.J.; Saadeh, P.B.; Greenwald, J.A.; Spector, J.A.; Gittes, G.K.; Longaker, M.T. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am. J. Physiol. Cell Physiol. 2000, 278, C853–C860. [Google Scholar] [CrossRef]
- Jaquet, K.; Krause, K.; Tawakol-Khodai, M.; Geidel, S.; Kuck, K.H. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc. Res. 2002, 64, 326–333. [Google Scholar] [CrossRef]
- Alper, G.; Bernick, S.; Yazdi, M.; Nimni, M.N. Osteogenesis in bone defects in rats: The effects of hydroxyapatite and demineralized bone matrix. Am. J. Med. Sci. 1989, 298, 371. [Google Scholar] [CrossRef]
- Roshan-Ghias, A.; Lambers, F.M.; Gholam-Rezaee, M.; Müller, R.; Pioletti, D.P. In vivo loading increases mechanical properties of scaffold by affecting bone formation and bone resorption rates. Bone 2011, 49, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, H.; Hashimoto, J.; Crawford, E.; Manske, P.; Lou, J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J. Orthop. Res. 2003, 21, 44. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Göckelmann, M.; Schoen, M.; Ignatius, A.; Willie, B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Morshed, S.; Corrales, L.; Genant, H.; Miclau, T., III. Outcome assessment in clinical trials of fracture-healing. J. Bone Joint Surg. Am. 2008, 90, 62–67. [Google Scholar] [CrossRef]
- Corrales, L.A.; Morshed, S.; Bhandari, M.; Miclaus, T. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Joint Surg. Am. 2008, 90, 1862–1868. [Google Scholar] [CrossRef]
- Jamsa, T.; Koivukangas, A.; Kippo, K.; Hannuniemi, R.; Jalovaara, P.; Tuukkanen, J. Comparison of radiographic and pQCT analyses of healing rat tibial fractures. Calcif. Tissue Int. 2000, 66, 288–291. [Google Scholar] [CrossRef]
- Grigoryan, M.; Lynch, J.A.; Fierlinger, A.L.; Guermazi, A.; Fan, B.; MacLean, D.B.; MacLean, A.; Genant, H.K. Quantitative and qualitative assessment of closed fracture healing using computed tomography and conventional radiography. Acad. Radiol. 2003, 10, 1267–1273. [Google Scholar] [CrossRef]
- Markel, M.D.; Bogdanske, J.J.; Xiang, Z.; Klohnen, A. Atrophic nonunion can be predicted with dual energy X-ray absorptiometry in a canine ostectomy model. J. Orthop. Res. 1995, 13, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J.; den Boer, F.C.; Bramer, J.A.; van Lingen, A.; Roos, J.C.; Bakker, F.C.; Patka, P.; Haarman, H.J. Evaluation of Strength of Healing Fractures With Dual Energy Xray Absorptiometry. Clin. Orthop. Relat. Res. 2000, 380, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, G.F.; Xu, S.W.; Wang, J.W.; Zhou, Y.F.; Liu, J. Role of dual energy X-ray absorptiometry in monitoring fracture healing in a rat femoral fracture model. Chin. J. Traumatol. 2005, 8, 121–125. [Google Scholar] [PubMed]
- Glatt, V.; Matthys, R. Adjustable stiffness, external fixator for the rat femur osteotomy and segmental bone defect models. J. Vis. Exp. 2014, 92, e51558. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef] [PubMed]
- AVMA (American Veterinary Medical Association). AVMA Guidelines for the Euthanasia of Animals, 2020th ed.; AMVA: Schaumburg, IL, USA, 2020; p. 28. [Google Scholar]
- Muschler, G.F.; Raut, V.; Patterson, T.; Wenke, J.; Hollinger, J. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Eastaugh-Waring, S.J.; Joslin, C.C.; Hardy, J.R.; Cunningham, J.L. Quantification of fracture healing from radiographs using the maximum callus index. Clin. Orthop. Relat. Res. 2009, 467, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tami, A. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef]
- Omlor, G.W.; Kleinschmidt, K.; Gantz, S.; Speicher, A.; Guehring, T.; Richter, W. Increased bone formation in a rabbit long-bone defect model after single local and single systemic application of erythropoietin. Acta Orthop. 2016, 87, 425–431. [Google Scholar] [CrossRef]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-Oss® in osteoblastic differentiation and matrix synthesis. Clin. Oral Implants Res. 2004, 15, 315–324. [Google Scholar] [CrossRef]
- Bosco, A.F.; Faleiros, P.L.; Carmona, L.R.; Garcia, V.G.; Theodoro, L.H.; de Araujo, N.G.; Nagata, M.J.H.; de Almeida, J.M. Effects of low-level laser therapy on bone healing of critical-size defects treated with bovine bone graft. J. Photochem. Photobiol. 2016, 163, 303–310. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C.; Clawson, L.D.; La Fontaine, J. Incorporation of bovine-based structural bone grafts used in reconstructive foot surgery. J. Foot Ankle Surg. 2012, 51, 30–33. [Google Scholar] [CrossRef]
- Ledford, C.K.; Nunley, J.A., 2nd; Viens, N.A.; Lark, R.K. Bovine xenograft failures in pediatric foot reconstructive surgery. J. Pediatr. Orthop. 2013, 33, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Karalashvili, L.; Chichua, N.; Menabde, G.; Atskvereli, L.; Grdzelidze, T.; Machavariani, A.; Zurmukhtashvili, M.; Gogilashvili, K.; Kakabadze, Z.; Chichua, Z. Decellularized Bovine Bone Graft for Zygomatic Bone Reconstruction. Med. Case Rep. 2017, 4, 52. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, J.; Park, I.H. Efficacy of Dual Energy X-ray Absorptiometry for Evaluation of Biomechanical Properties: Bone Mineral Density and Actual Bone Strength. J. Bone Metab. 2014, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
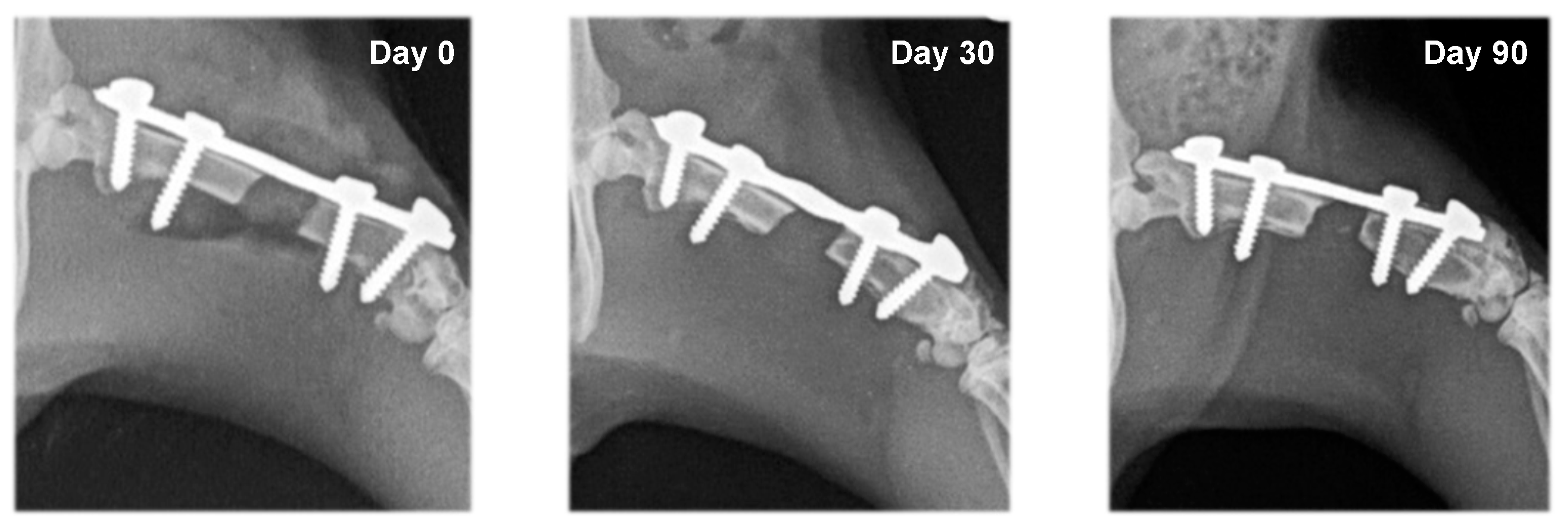
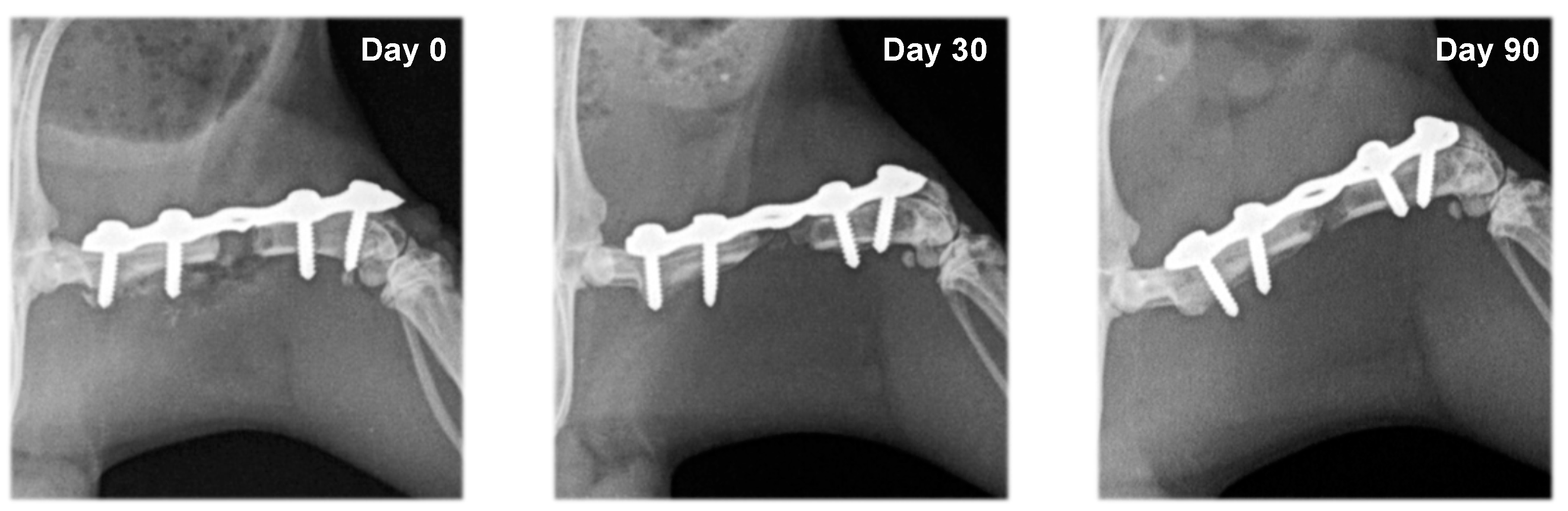


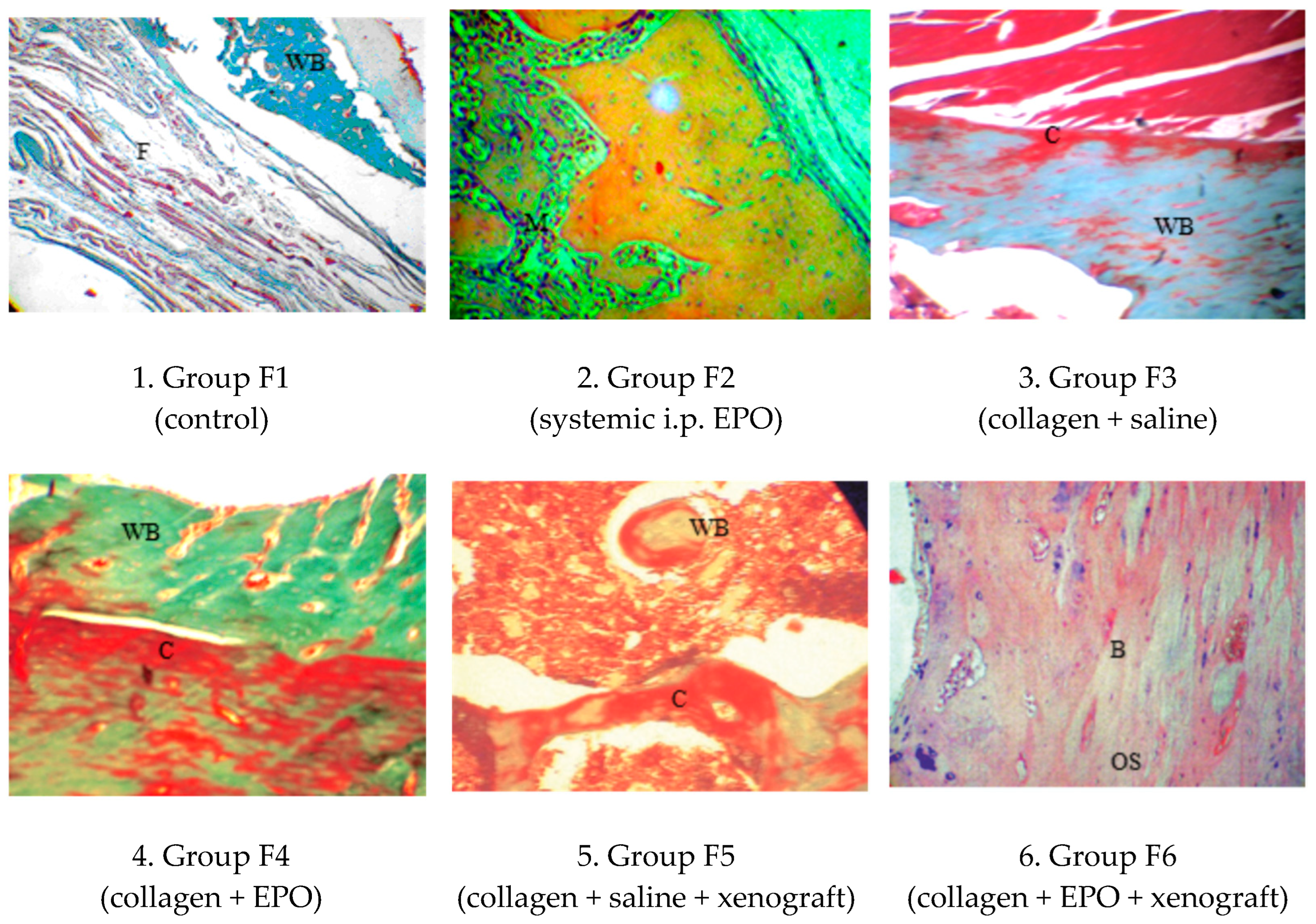
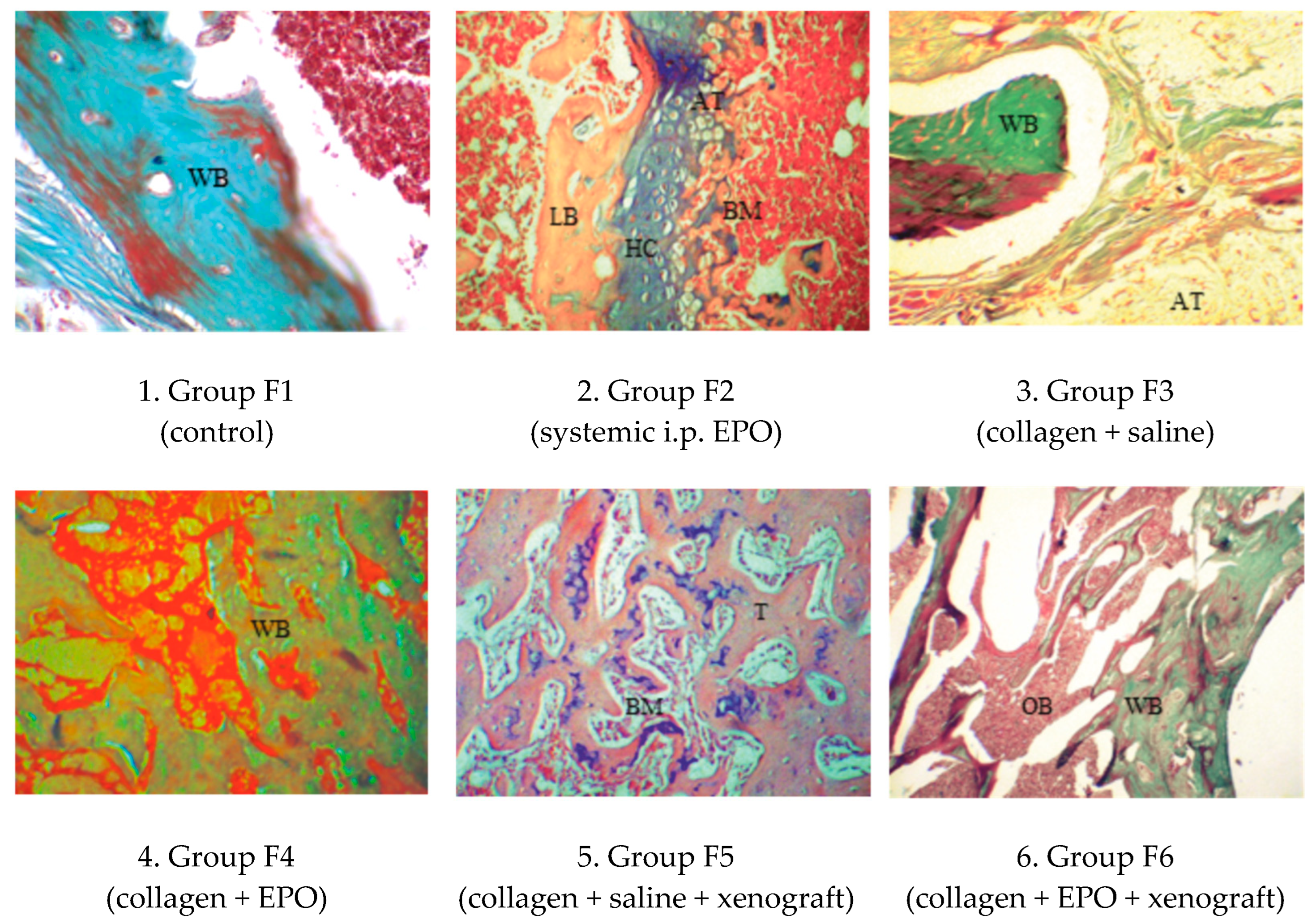
| Group | Day 0 | Day 30 | Day 90 |
|---|---|---|---|
| F1 | n = 10 | n = 10 | n = 5 |
| 9 (9–9) | 11 (11–11) a*** | 11 (11–11) a** | |
| F2 | n = 10 | n = 10 | n = 5 |
| 9 (9–9) | 11 (11–11) a*** | 11 (11–11) a*** | |
| F3 | n = 8 | n = 8 | n = 4 |
| 9 (9–9) | 15 (11–16) a** | 16.5 (14–19) a** b* | |
| F4 | n = 10 | n = 10 | n = 5 |
| 9 (9–9) | 16.5 (11–25) a** | 22 (21–32) a*** | |
| F5 | n = 8 | n = 8 | n = 4 |
| 11 (11–11) | 25.5 (22–28) a*** c* | 24.5 (21–30) a** | |
| F6 | n = 10 | n = 10 | n = 5 |
| 11 (11–11) | 28 (25–34) a*** | 32 (25–34) a** |
| Group | Day 0 | Day 30 | Day 90 |
|---|---|---|---|
| F1 | n = 10 | n = 10 | n = 5 |
| 0.60 (0.47–0.63) | 0.66 (0.64–0.71) a*** | 0.75 (0.73–0.77) a*** b*** | |
| F2 | n = 10 | n = 10 | n = 5 |
| 0.52 (0.43–0.63) | 0.71 (0.69–0.73) a*** | 0.76 (0.73–0.78) a*** b*** | |
| F3 | n = 8 | n = 8 | n = 4 |
| 0.66 (0.54–0.68) | 0.70 (0.65–0.74) a*** | 0.75 (0.73–0.77) a*** b** | |
| F4 | n = 10 | n = 10 | n = 5 |
| 0.65 (0.60–0.69) | 0.72 (0.68–0.76) a*** | 0.85 (0.77–0.86) a*** b*** | |
| F5 | n = 8 | n = 8 | n = 4 |
| 0.86 (0.74–0.88) | 0.87 (0.84–0.94) | 0.92 (0.87–0.96) | |
| F6 | n = 10 | n = 10 | n = 5 |
| 0.87 (0.75–0.90) | 0.92 (0.84–0.95) | 0.98 (0.90–0.99) |
| Group F4 n = 5 | Group F5 n = 4 | Group F6 n = 5 | |
|---|---|---|---|
| Right femur | 0.303 (0.217–0.396) | 0.318 (0.240–0.384) | 0.320 * (0.266–0.396) |
| Left femur | 0.378 (0.335–0.408) | 0.400 (0.311–0.420) | 0.430 (0.380–0.513) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, R.; Chaprazov, T. Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft. Vet. Sci. 2023, 10, 196. https://doi.org/10.3390/vetsci10030196
Vasileva R, Chaprazov T. Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft. Veterinary Sciences. 2023; 10(3):196. https://doi.org/10.3390/vetsci10030196
Chicago/Turabian StyleVasileva, Radina, and Tzvetan Chaprazov. 2023. "Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft" Veterinary Sciences 10, no. 3: 196. https://doi.org/10.3390/vetsci10030196
APA StyleVasileva, R., & Chaprazov, T. (2023). Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft. Veterinary Sciences, 10(3), 196. https://doi.org/10.3390/vetsci10030196






