Diagnostic Imaging Studies on Local and Systemic Erythropoietin Application for Promoting Bone Regeneration in Rat Calvarial Defects
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Surgical Procedure
2.4. Clinical Evaluation and Blood Analysis
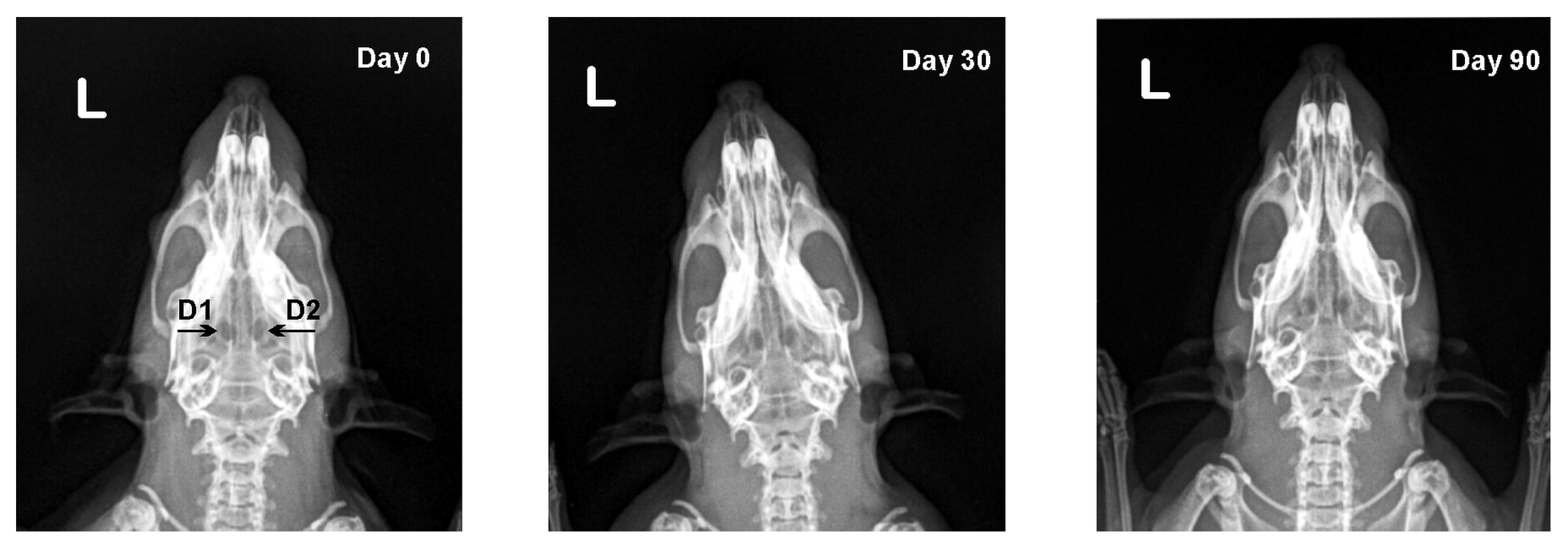
2.5. X-ray Analysis
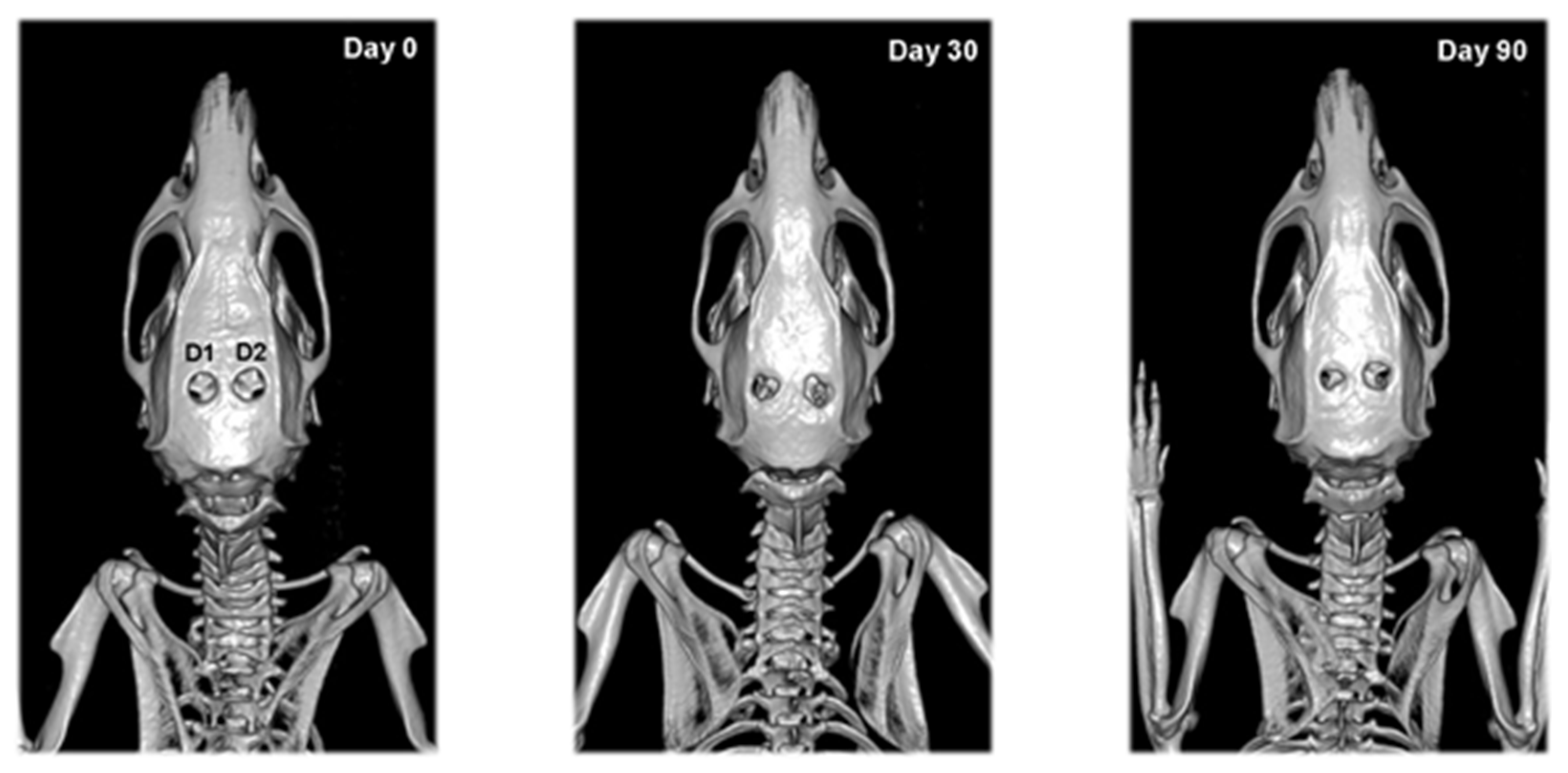
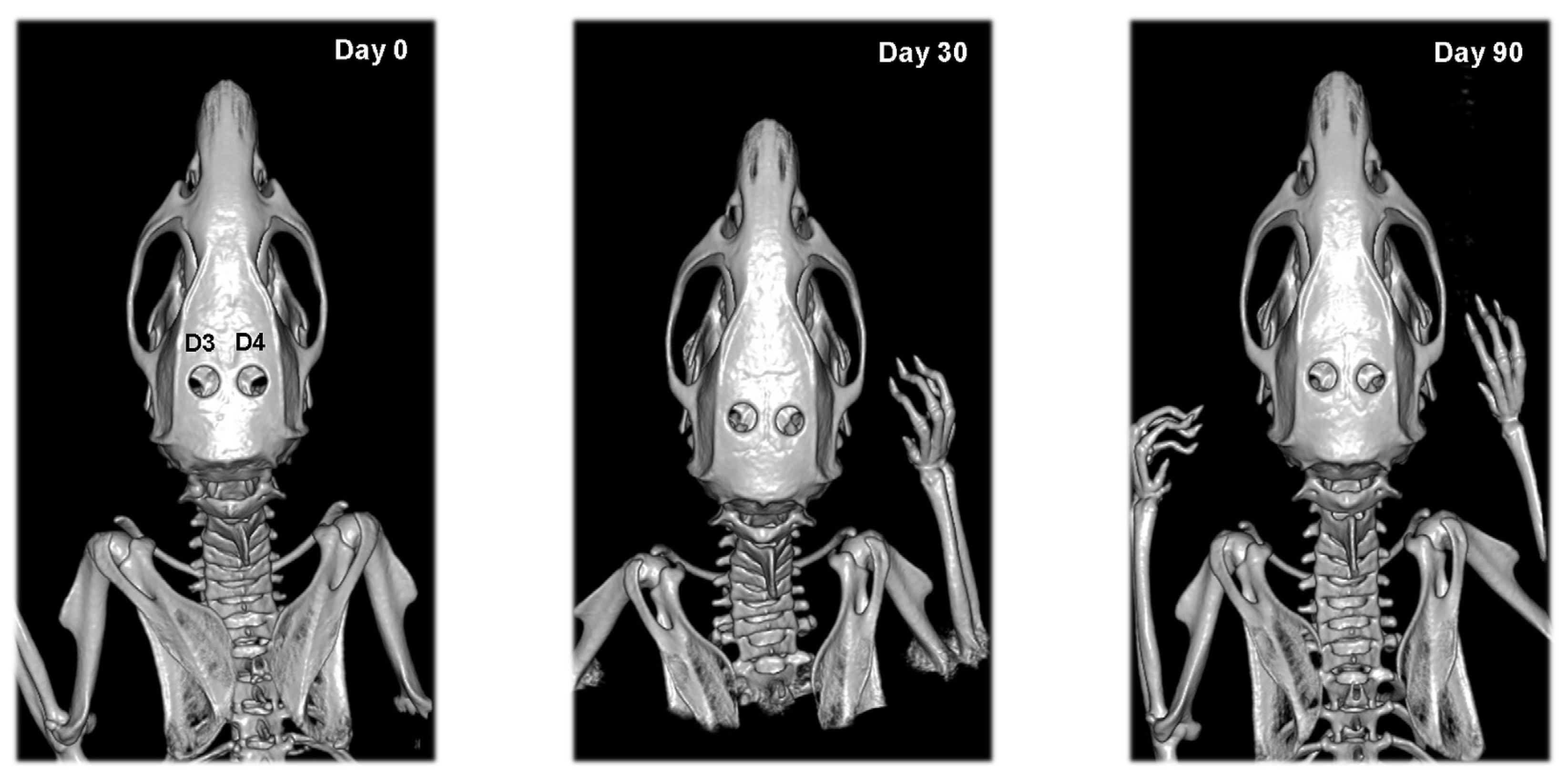
2.6. CT Analysis
2.7. Statistical Analysis
3. Results
3.1. Clinical and Haematological Examinations
3.2. X-ray Results
3.3. CT Results
4. Discussion
5. Conclusions
- Single local dose rhEpo applied on collagen carrier could be used for enhancing bone healing without systemic effect.
- Intraperitoneally-injected rhEpo did not result in bone formation. Simultaneously, it affects the erythropoiesis by increasing the erythrocyte counts, haemoglobin and haematocrit.
- Computed tomography offers a much higher level of detail for visualizing changes during bone healing than conventional radiography.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.; Ramachandran, M. Bone injury, healing and grafting. In Book Basic Orthopaedic Sciences. The Stanmore Guide; Ramachandran, M., Ed.; Hodder Arnold: London, UK, 2007; pp. 123–134. [Google Scholar]
- Strelzow, J.A.; Grewal, R. Chapter 22—Predicting Union of Scaphoid Fractures. In Scaphoid Fractures: Evidence-Based Management; Buijze, G.A., Jupiter, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 199–208. [Google Scholar]
- Niikura, T.; Lee, S.Y.; Sakai, Y.; Nishida, K.; Kuroda, R.; Kurosaka, M. Causative factors of fracture nonunion: The proportions of mechanical, biological, patient-dependent, and patient-independent factors. J. Orthop. Sci. 2014, 19, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Gaston, M.S.; Simpson, A.H. Inhibition of fracture healing. J. Bone Jt. Surg. Br. 2007, 89, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Coleman, T.; Brines, M. Science review: Recombinant human erythropoietin in critical illness: A role beyond anemia? Crit. Care 2004, 8, 337–341. [Google Scholar] [CrossRef][Green Version]
- Debeljak, N.; Solar, P.; Sytkowski, A. Erythropoietin and cancer: The unintended consequences of anemia correction. Front. Immunol. 2014, 5, 563. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M. Erythropoietin and co.: Intrinsic structure and functional disorder. Mol. BioSyst. 2017, 13, 56–72. [Google Scholar] [CrossRef]
- Ganz, T. Erythropoietin and iron—A conflicted alliance? Kidney Int. 2018, 94, 851–853. [Google Scholar] [CrossRef]
- Perreault, A.A.; Venters, B.J. Integrative view on how erythropoietin signaling controls transcription patterns in erythroid cells. Curr. Opin. Hematol. 2018, 25, 189–195. [Google Scholar] [CrossRef]
- Rцlfing, J. The effect of erythropoietin on bone. Acta Orthop. 2014, 85, 1–29. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, F.; He, Q.; Tsang, W.P.; Lu, L.; Li, Q.; Wu, Z.; Qui, G.; Zhou, G.; Wan, C. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS ONE 2014, 9, e102010. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Kenanidis, E.I.; MacFarlane, R.J.; Michail, T.; Potoupnis, M.E.; Heliotis, M.; Tsiridis, E. Investigational drugs for fracture healing: Preclinical & clinical data. Expert Opin. Investig. Drugs 2016, 25, 585–596. [Google Scholar] [CrossRef]
- Eggold, J.T.; Rankin, E.B. Erythropoiesis, EPO, macrophages, and bone. Bone 2019, 119, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Speidel, V.; Scheuer, C.; Laschke, M.W.; Holstein, J.H.; Histing, T.; Pohleman, T.; Menger, M.D. Low dose erythropoietin stimulates bone healing in mice. J. Orthop. Res. 2011, 29, 165–172. [Google Scholar] [CrossRef]
- Omlor, G.W.; Kleinschmidt, K.; Gantz, S.; Speicher, A.; Guehring, T.; Richter, W. Increased bone formation in a rabbit long-bone defect model after single local and single systemic application of erythropoietin. Acta Orthop. 2016, 87, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Abou Elmagd, I.; Khairy, M.; Fahmi, A. Effects of Erythropoietin on The Healing of Calvarial Bone Defect. Egypt. Dent. J. 2019, 65, 3283–3294. [Google Scholar] [CrossRef][Green Version]
- Mihmanli, A.; Dolanmaz, D.; Avunduk, M.C.; Erdemli, E. Effects of recombinant human erythropoietin on mandibular distraction osteogenesis. J. Oral Maxillofac. Surg. 2009, 67, 2337–2343. [Google Scholar] [CrossRef]
- Guo, L.; Luo, T.; Fang, Y.; Yang, L.; Wang, L.; Liu, J.; Shi, B. Effects of erythropoietin on osteoblast proliferation and function. Clin. Exp. Med. 2014, 14, 69–76. [Google Scholar] [CrossRef]
- Bozlar, M.; Kalaci, A.; Aslan, B.; Baktiroglu, L.; Yanat, A.N.; Tasci, A. Effects of erythropoietin on fracture healing in rats. Saudi Med. J. 2006, 27, 1267–1269. [Google Scholar]
- Rцlfing, J.; Bendtsen, M.; Jensen, J.; Stiehler, M.; Foldager, C.B.; Hellfritzsch, M.B.; Bьnger, C. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J. Orthop. Res. 2012, 30, 1083–1088. [Google Scholar] [CrossRef]
- Bakhshi, H.; Kazemian, G.; Emami, M.; Nemati, A.; Karimi Yarandi, H.; Safdari, F. Local erythropoietin injection in tibiofibular fracture healing. Trauma Mon. 2013, 17, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Nazeman, P.; Rad, M.R.; Khojasteh, A. Topical erythropoietin as a novel preventive and therapeutic agent in bisphosphonate-related osteonecrosis of the jaw. Dent. Hypotheses 2016, 7, 56–60. [Google Scholar]
- Kimбkovб, P.; Solбr, P.; Solбrovб, Z.; Komel, R.; Debeljak, N. Erythropoietin and Its Angiogenic Activity. Int. J. Mol. Sci. 2017, 18, 1519. [Google Scholar] [CrossRef]
- Cervellini, I.; Sacre, S.; Ghezzi, P.; Mengozzi, M. Erythropoietin does not affect TNF and IL-6 production directly. J. Biol. Regul. Homeost. Agents 2013, 27, 189–196. [Google Scholar]
- Xu, T.; Jin, H.; Lao, Y.; Wang, P.; Zhang, S.; Ruan, H.; Mao, Q.; Zhou, L.; Xiao, L.; Tong, P.; et al. Administration of erythropoietin prevents bone loss in osteonecrosis of the femoral head in mice. Mol. Med. Rep. 2007, 16, 8755–8762. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the critical size in calvarial bone defects: Revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 2010, 125, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Book Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Wang, J.; Lee, C.H.; Sud, S.; et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coating 2020, 10, 971. [Google Scholar] [CrossRef]
- Sun, H.; Jung, Y.; Shiozawa, Y.; Taichmann, R.S.; Krebsbach, P.H. Erythropoietin modulates the structure of bone morphogenetic protein 2-engineered cranial bone. Tissue Eng. Part A 2012, 18, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Pharoah, M.J. Oral Radiology: Principles and Interpretation, 5th ed.; Mosby: Philadelphia, PA, USA, 2003. [Google Scholar]
- Grigoryan, M.; Lynch, J.A.; Fierlinger, A.L.; Guermazi, A.; Fan, B.; MacLean, D.B.; MacLean, A.; Genant, H.K. Quantitative and qualitative assessment of closed fracture healing using computed tomography and conventional radiography. Acad. Radiol. 2003, 10, 1267–1273. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; De Groot, K.; Spandau, J.M.; Landry, A.L.; Hertel, B.; Duckert, T.; Boehm, S.M.; Menne, J.; Haller, H.; Fliser, D. Erythropoietin regulates endothelial progenitor cells. Blood 2004, 103, 921–926. [Google Scholar] [CrossRef]
- Holstein, J.H.; Menger, M.D.; Scheuer, C.; Meier, C.; Culemann, U.; Wirbel, R.J.; Garcia, P.; Pohlemann, T. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007, 80, 893–900. [Google Scholar] [CrossRef]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tami, A. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 1–14. [Google Scholar] [CrossRef]


| Day 0 | Day 30 | Day 90 | ||
|---|---|---|---|---|
| Erythrocyte count, T/L | Group I | 6.22 (5.36–8.24) | 6.74 (6.29–9.09) | 6.77 (6.22–8.28) |
| Group II | 5.63 (5.03–8.00) | 6.25 (5.66–7.04) *** | 7.50 (7.22–7.87) *** | |
| Haemoglobin content, g/L | Group I | 139 (113–157) | 142 (134–185) | 148 (138–164) ** |
| Group II | 117 (105–137) | 142 (112–158) * | 158 (150–163) *** | |
| Haematocrit, % | Group I | 34.2 (31.4–44.1) | 37.6 (34.1–47.7) | 37.9 (35.5–44.7) |
| Group II | 30.6 (26.9–36.0) | 35.7 (30.5–42.0) | 42.6 (38.8–44.1) *** |
| Defect | Day 0 | Day 30 | Day 90 |
|---|---|---|---|
| D1 | 0.92 ± 0.02 | 0.92 ± 0.03 | 0.93 ± 0.03 |
| D2 | 0.93 ± 0.02 | 0.93 ± 0.04 | 0.95 ± 0.04 |
| D3 | 0.92 ± 0.02 | 0.91 ± 0.03 | 0.90 ± 0.03 |
| D4 | 0.93 ± 0.01 | 0.92 ± 0.02 | 0.90 ± 0.02 * |
| Defect | Day 0 | Day 30 | Day 90 |
|---|---|---|---|
| D1 | 0 (0–0) | 1 (0–2) **# | 2 (1–2) ** |
| D2 | 0 (0–0) | 2 (2–3) *** | 2 (2–3) *** |
| D3 | 0 (0–0) | 0 (0–2) | 0 (0–2) |
| D4 | 0 (0–0) | 0 (0–2) | 1 (0–2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaprazov, T.; Vasileva, R.; Atliev, K.; Firkova, E. Diagnostic Imaging Studies on Local and Systemic Erythropoietin Application for Promoting Bone Regeneration in Rat Calvarial Defects. Vet. Sci. 2022, 9, 578. https://doi.org/10.3390/vetsci9100578
Chaprazov T, Vasileva R, Atliev K, Firkova E. Diagnostic Imaging Studies on Local and Systemic Erythropoietin Application for Promoting Bone Regeneration in Rat Calvarial Defects. Veterinary Sciences. 2022; 9(10):578. https://doi.org/10.3390/vetsci9100578
Chicago/Turabian StyleChaprazov, Tsvetan, Radina Vasileva, Kiril Atliev, and Elena Firkova. 2022. "Diagnostic Imaging Studies on Local and Systemic Erythropoietin Application for Promoting Bone Regeneration in Rat Calvarial Defects" Veterinary Sciences 9, no. 10: 578. https://doi.org/10.3390/vetsci9100578
APA StyleChaprazov, T., Vasileva, R., Atliev, K., & Firkova, E. (2022). Diagnostic Imaging Studies on Local and Systemic Erythropoietin Application for Promoting Bone Regeneration in Rat Calvarial Defects. Veterinary Sciences, 9(10), 578. https://doi.org/10.3390/vetsci9100578






