Gastroprotective Effects of Oral Glycosaminoglycans with Sodium Alginate in an Indomethacin-Induced Gastric Injury Model in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Prototype Administration and Induction of Gastric Lesions
2.3. Drugs
2.4. Macroscopic Evaluation
2.5. Microscopic Evaluation
2.6. Statistical Analysis
3. Results
3.1. Establishment of an In Vivo Model of Indomethacin-Induced Gastric Damage
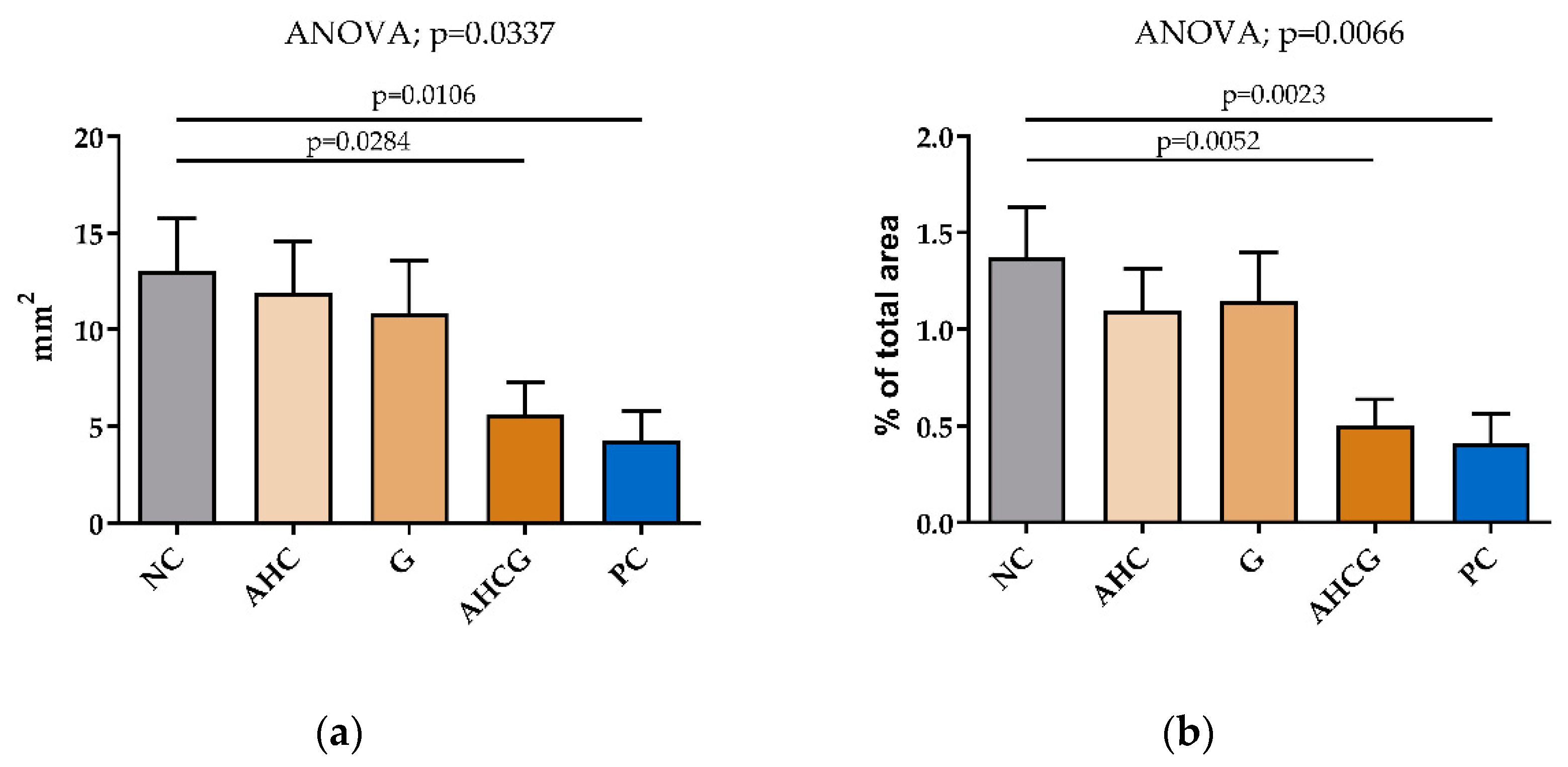
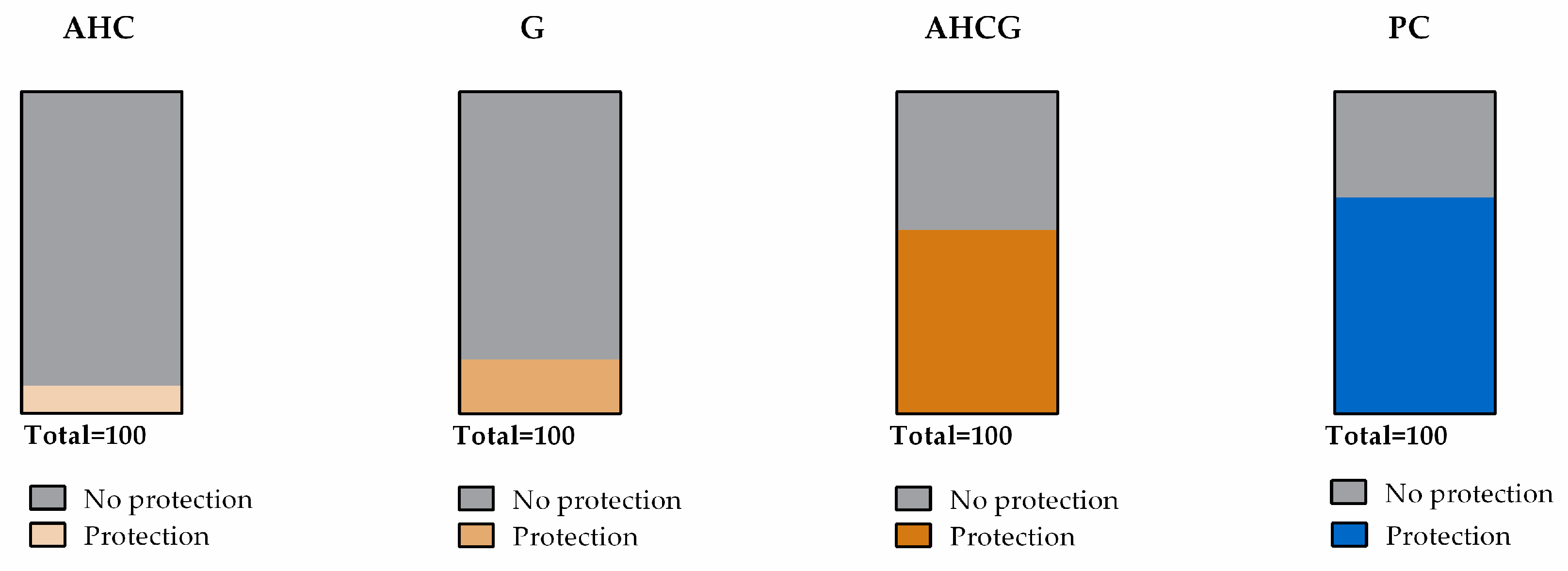
3.2. Macroscopic Results
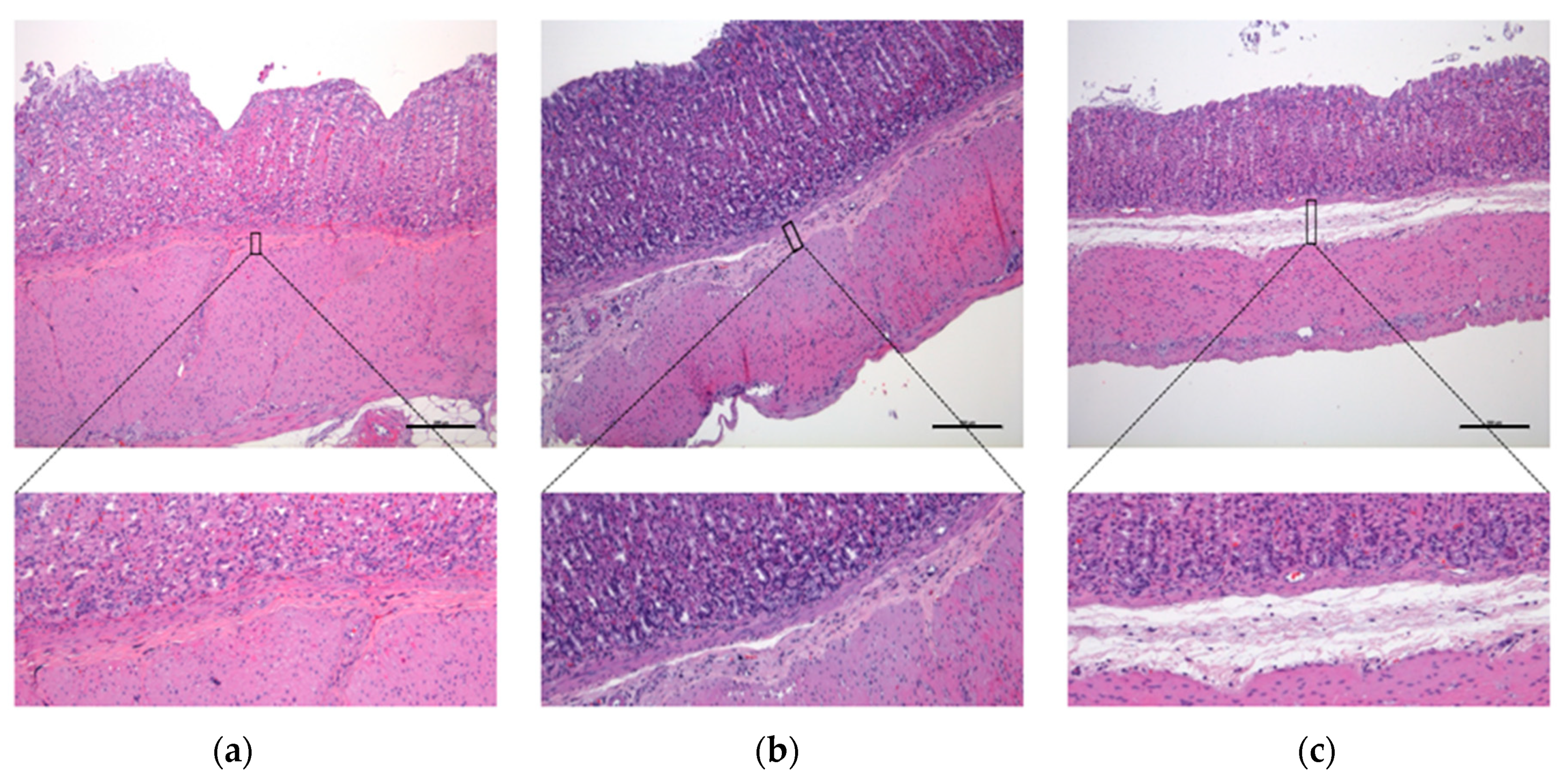
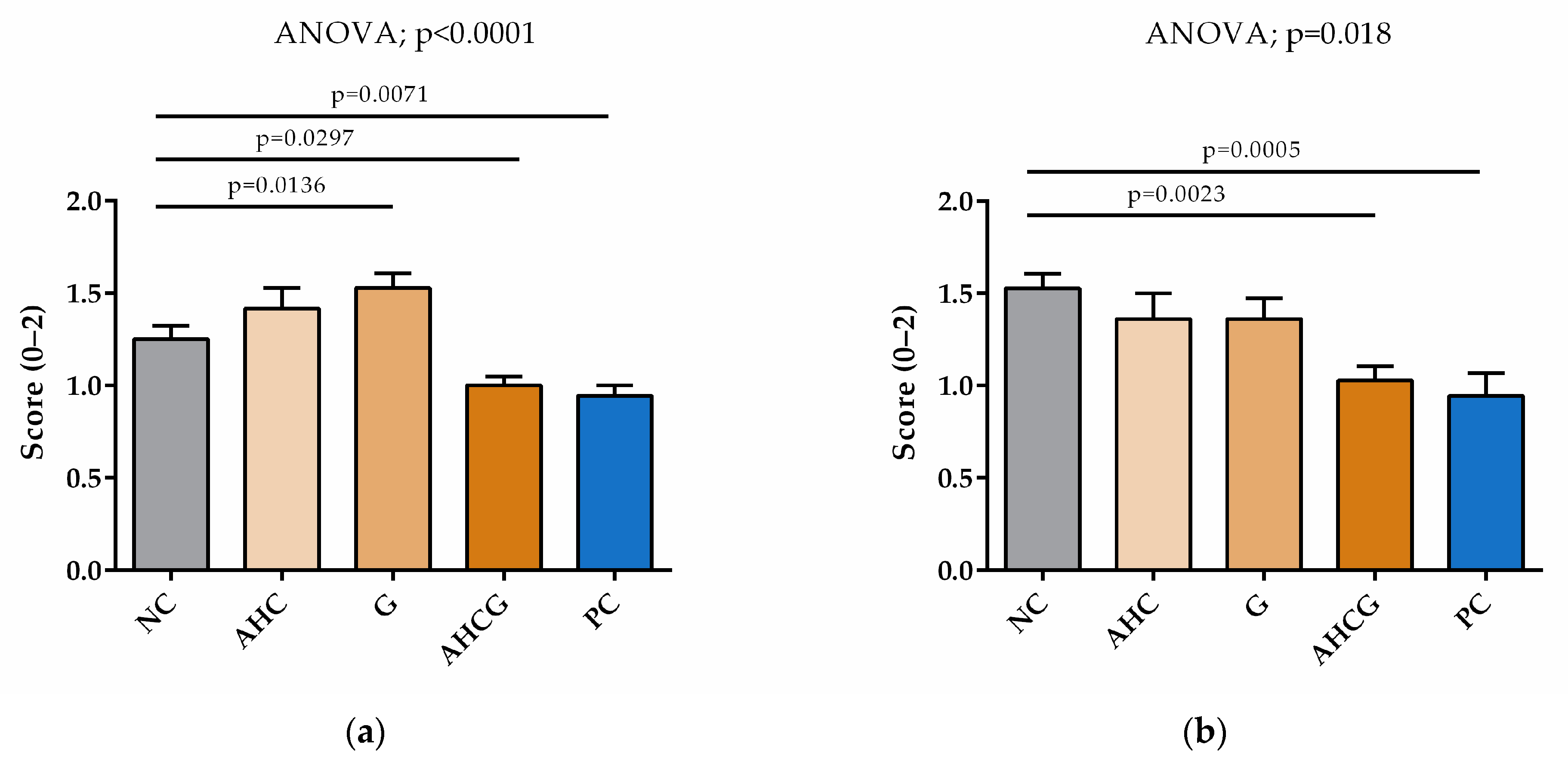
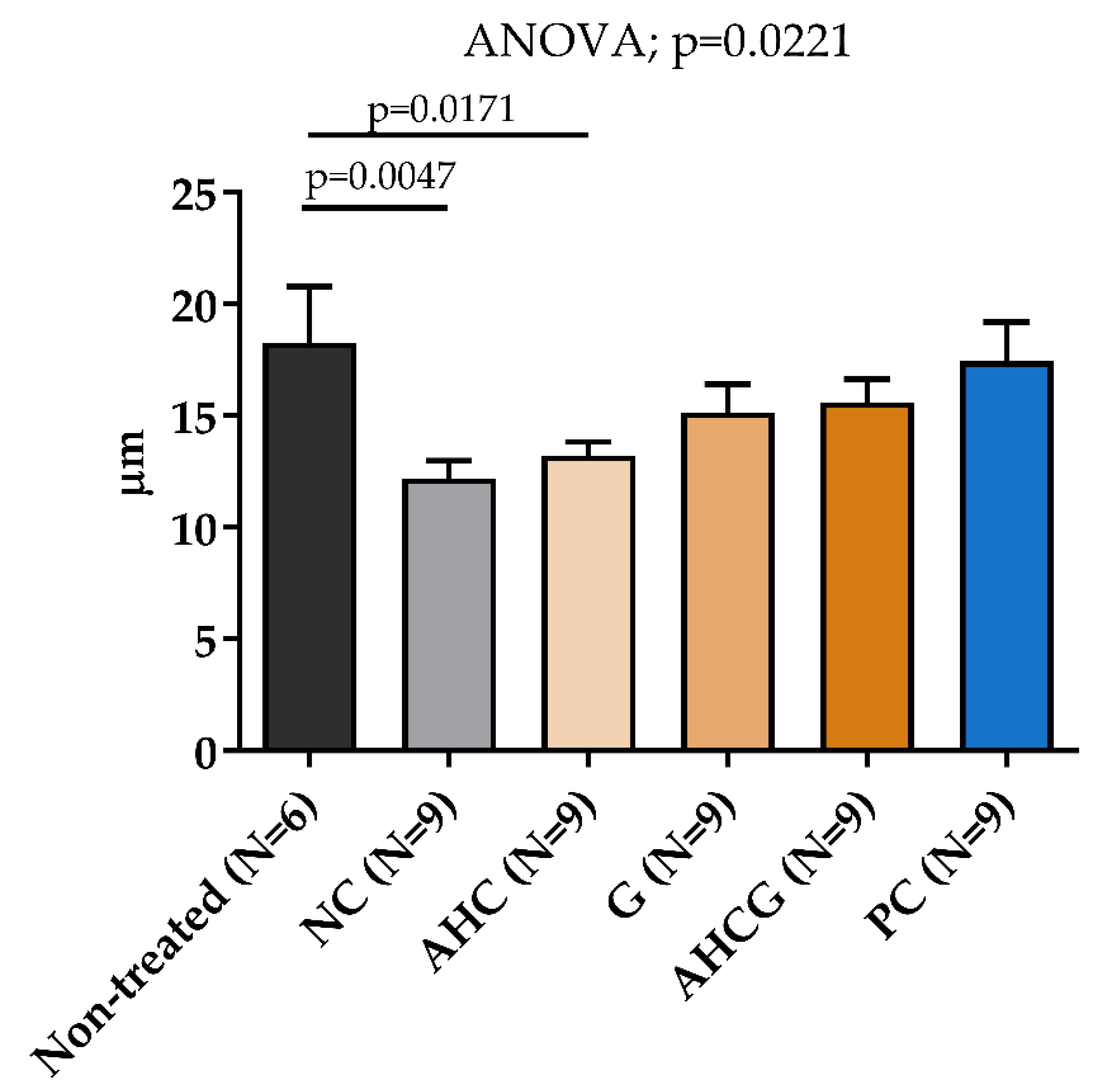
3.3. Microscopic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marks, S.L.; Kook, P.H.; Papich, M.G.; Tolbert, M.K.; Willard, M.D. ACVIM consensus statement: Support for rational administration of gastrointestinal protectants to dogs and cats. J. Vet. Intern. Med. 2018, 32, 1823–1840. [Google Scholar] [CrossRef]
- Stanton, M.; Bright, R. Gastroduodenal Ulceration in Dogs. Retrospective study of 43 cases and literature review. J. Vet. Intern. Med. 1989, 3, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, R.; Ohno, R.; Tanigami, M.; Tanaka, A.; Takeuchi, K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J. Pharmacol. Exp. Ther. 2006, 318, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.; Hill, T.; Tolbert, M. Prevalence of gastrointestinal lesions in dogs chronically treated with nonsteroidal anti-inflammatory drugs. J. Vet. Intern. Med. 2021, 35, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Opekun, A.; Willingham, F.; Qureshi, W. Visible Small-Intestinal Mucosal Injury in Chronic NSAID Users. Clin. Gastroenterol. Hepatol. 2005, 3, 55–59. [Google Scholar] [CrossRef]
- DuBois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Putte, L.B.A.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Lucas, S. The Pharmacology of Indomethacin. Headache 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Simões, S.; Lopes, R.; Campos, M.; Marruz, M.; da Cruz, M.; Corvo, L. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Anim. Model. Exp. Med. 2019, 2, 121–126. [Google Scholar] [CrossRef]
- Morini, G.; Grandi, D. Methods to measure gastric mucosal lesions in the rat. Curr. Protoc. Toxicol. 2010, 43, 21.2.1–21.2.15. [Google Scholar] [CrossRef]
- Adinortey, M.; Ansah, C.; Galyuon, I.; Nyarko, A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Di Simone, M.P.; Baldi, F.; Vasina, V.; Scorrano, F.; Bacci, M.L.; Ferrieri, A.; Poggioli, G. Barrier effect of Esoxx® on esophageal mucosal damage: Experimental study on ex-vivo swine model. Clin. Exp. Gastroenterol. 2012, 5, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Pace, F.; Scarpignato, C.; the Esoxx Study Group. Randomised clinical trial: Mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease—Efficacy of Esoxx, a hyaluronic acid—Chondroitin sulphate based bioadhesive formulation. Aliment. Pharmacol. Ther. 2017, 45, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Haruma, K.; Ito, M.; Takahashi, N.; Takasugi, H.; Wada, Y.; Nakata, H.; Katoh, T.; Miyamoto, M.; Tanaka, S. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: A randomized clinical trial. Dis. Esophagus 2012, 25, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Mizuno, S.; Rikitake, Y. Preventative effects of sodium alginate on indomethacin-induced small-intestinal injury in mice. Int. J. Med. Sci. 2016, 13, 653–663. [Google Scholar] [CrossRef]
- Yamamoto, A.; Itoh, T.; Nasu, R.; Kajiwara, E.; Nishida, R. Sodium Alginate Inhibits Methotrexate-Induced Gastrointestinal Mucositis in Rats. Biol. Pharm. Bull. 2013, 36, 1528–1534. [Google Scholar] [CrossRef]
- Yamamoto, A.; Itoh, T.; Nasu, R.; Nishida, R. Sodium alginate ameliorates indomethacin-induced gastrointestinal mucosal injury via inhibiting translocation in rats. World J. Gastroenterol. 2014, 20, 2641–2652. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Souich, P.D.; García, A.; Vergés, J.; Montell, E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J. Cell. Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef]
- Lyons, K.; Andrews, F.; Comper, W.; O’Brien, P. Changes in sulfated macromolecules produced in vivo during normal and indomethacin-delayed ulcer healing in rats. Dig. Dis. Sci. 1997, 42, 1755–1764. [Google Scholar] [CrossRef]
- Lim, Y.; Phan, T.; Dial, E.; Graham, D.; Lichtenberger, L. In vitro and in vivo protection against indomethacin-induced small intestinal injury by proton pump inhibitors, acid pump antagonists, or indomethacin-phosphatidylcholine. Digestion 2012, 86, 171–177. [Google Scholar] [CrossRef]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice-Part 1. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; de la Motte, C. The Role of Hyaluronan Treatment in Intestinal Innate Host Defense. Front. Immunol. 2020, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Isa IL, M.; Rasala, S.; Demir, S.; Singh, R.; Baby, B.V.; Swamy, S.K.; Dockery, P.; Jala, V.R.; Rochev, Y.; et al. Modulation of Gut Barrier Functions in Ulcerative Colitis by Hyaluronic Acid System. Adv. Sci. 2022, 9, e2103189. [Google Scholar] [CrossRef] [PubMed]
- Shmagel, A.; Demmer, R.; Knights, D.; Butler, M.; Langsetmo, L.; Lane, N.E.; Ensrud, K. The effects of glucosamine and chondroitin sulfate on gut microbial composition: A systematic review of evidence from animal and human studies. Nutrients 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Kessler, S.; Rho, H.; Cowman, M.; De La Motte, C. Specific-sized hyaluronan fragments promote expression of human β-defensin 2 in intestinal epithelium. J. Biol. Chem. 2012, 287, 30610–30624. [Google Scholar] [CrossRef]
- Hill, D.R.; Rho, H.K.; Kessler, S.P.; Amin, R.; Homer, C.R.; McDonald, C.; Cowman, M.K.; de la Motte, C.A. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J. Biol. Chem. 2013, 288, 29090–29104. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Liu, L.; Guo, L.; Deng, Y.; Liu, J. N-acetyl glucosamine improves intestinal mucosal barrier function in rat. Bangladesh J. Pharmacol. 2012, 7, 281–284. [Google Scholar] [CrossRef]
- Burge, K.; Hannah, L.; Eckert, J.; Gunasekaran, A.; Chaaban, H. The Protective Influence of Chondroitin Sulfate, a Component of Human Milk, on Intestinal Bacterial Invasion and Translocation. J. Hum. Lact. 2019, 35, 538–549. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Mills, D. Release and utilization of N-acetyl-d-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 2012, 18, 430–435. [Google Scholar] [CrossRef]
- Chaaban, H.; Burge, K.; Eckert, J.; Trammell, M.; Dyer, D.; Keshari, R.S.; Silasi, R.; Regmi, G.; Lupu, C.; Good, M.; et al. Acceleration of small intestine development and remodeling of the microbiome following hyaluronan 35 kda treatment in neonatal mice. Nutrients 2021, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sugihara, K.; Gillilland, M.; Jon, S.; Kamada, N.; Moon, J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Kim, Y.J.; Do, K.H.; Kim, J.K.; Ham, J.S.; Lee, W.K. Effects of queso blanco cheese containing bifidobacterium longum kacc 91563 on the intestinal microbiota and short chain fatty acid in healthy companion dogs. Korean J. Food Sci. Anim. Resour. 2018, 38, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Pohl, K.; Moodley, P.; Dhanda, A. The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1498–1506. [Google Scholar] [CrossRef]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef]
- Wang, K.; Bai, F.; Zhou, X.; Wang, J.; Li, Y.; Xu, H.; Gao, R.; Wu, H.; Liu, K.; Zhao, Y. Characterization of chondroitin sulfates isolated from large hybrid sturgeon cartilage and their gastroprotective activity against ethanol-induced gastric ulcers. Food Chem. 2021, 363, 130436. [Google Scholar] [CrossRef]
- Iliev, I.; Loidl, A. A 61-year-old woman with chronic iron deficiency anemia due to a cameron lesion and a response to oral application of combined poloxamer 407 with hyaluronic acid and chondroitin sulfate following single treatment with pantoprazole: A case report. Am. J. Case Rep. 2021, 22, e928021-1–e928021-9. [Google Scholar] [CrossRef]
- Sicard, J.-F.; Vogeleer, P.; Le Bihan, G.; Olivera, Y.R.; Beaudry, F.; Jacques, M.; Harel, J. N-Acetyl-glucosamine influences the biofilm formation of Escherichia coli. Gut Pathog. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Zhu, A.; Patel, I.; Hidalgo, M.; Gandhi, V. N-Acetylglucosamine for Treatment of Inflammatory Bowel Disease. Nat. Med. J. 2015, 7, 2015-04. [Google Scholar]


| Group | Composition |
|---|---|
| AHC | Sodium alginate, Chondroitin sulfate, Hyaluronic Acid |
| AHCG | Sodium alginate, Chondroitin sulfate, Hyaluronic Acid, N-acetylglucosamine |
| G | N-acetylglucosamine, Xanthan gum (E-415), Potassium sorbate (E-202), Citric acid (E-330) |
| NC | Xanthan gum (E-415), Potassium sorbate (E-202), Citric acid (E-330) |
| PC | Sucrose sulfate complex with aluminum hydroxide gel (Sucralfate) |
| Scale | Submucosal Edema |
|---|---|
| 0 | Normal appearance |
| 0.5 | Mild separation of the mucosa and muscular layers. |
| The submucosal tissue maintains its normal aspect. Submucosal vessels surrounded by submucosal connective tissue. | |
| 1 | Moderate swelling of the submucosal layer (edema) separating the mucosa and muscular layers. |
| The submucosal tissue maintains its normal appearance. Submucosal vessels surrounded by submucosal connective tissue. | |
| 1.5 | Important and significant swelling of the submucosal layer (edema). Notable separation of the mucosa and muscular layers. |
| Submucosal vessels isolated from other structures. | |
| 2 | Severe swelling and expansion of the submucosal layer (edema). Maximum separation of the mucosa and muscular layers. |
| Submucosal vessels completely isolated from other structures. |
| Scale | Vascular Engorgement |
|---|---|
| 0 | Normal appearance |
| 0.5 | Microvessels (capillaries) become slightly tortuous, erythrocytes (RBCs) visible inside. |
| Mild increase in the total quantity of erythrocytes. | |
| Submucosal vessels are not engorged. | |
| 1 | Tortuous microvessels, RBC visible inside. |
| Moderately increased RBC. | |
| Mild engorgement of the capillaries. | |
| Some submucosal vessels are mildly engorged. | |
| 1.5 | Tortuous microvessels (capillaries), RBC visible inside. Important increase in the number of RBC. |
| Focal areas of moderately engorged capillaries in the surface layers of the mucosa. | |
| Some deep engorged microvessels penetrate to the superficial layers. | |
| Submucosal vessels are engorged. | |
| 2 | Tortuous microvessels, RBC visible inside, and some extravasation of RBC can be seen. |
| Severe increase in the number of RBC. | |
| Severely engorged capillaries concentrated in the superficial layers of the mucosa. Some deep engorged microvessels penetrate to the more superficial layers. | |
| Submucosal vessels are engorged. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traserra, S.; Cuerda, H.; Vallejo, A.; Segarra, S.; Sabata, R.; Jimenez, M. Gastroprotective Effects of Oral Glycosaminoglycans with Sodium Alginate in an Indomethacin-Induced Gastric Injury Model in Rats. Vet. Sci. 2023, 10, 667. https://doi.org/10.3390/vetsci10120667
Traserra S, Cuerda H, Vallejo A, Segarra S, Sabata R, Jimenez M. Gastroprotective Effects of Oral Glycosaminoglycans with Sodium Alginate in an Indomethacin-Induced Gastric Injury Model in Rats. Veterinary Sciences. 2023; 10(12):667. https://doi.org/10.3390/vetsci10120667
Chicago/Turabian StyleTraserra, Sara, Héctor Cuerda, Adriana Vallejo, Sergi Segarra, Roger Sabata, and Marcel Jimenez. 2023. "Gastroprotective Effects of Oral Glycosaminoglycans with Sodium Alginate in an Indomethacin-Induced Gastric Injury Model in Rats" Veterinary Sciences 10, no. 12: 667. https://doi.org/10.3390/vetsci10120667
APA StyleTraserra, S., Cuerda, H., Vallejo, A., Segarra, S., Sabata, R., & Jimenez, M. (2023). Gastroprotective Effects of Oral Glycosaminoglycans with Sodium Alginate in an Indomethacin-Induced Gastric Injury Model in Rats. Veterinary Sciences, 10(12), 667. https://doi.org/10.3390/vetsci10120667








