Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Intestine Histology and Histometry for Trophic Activity
2.3. Intestine Histochemistry: AB-PAS
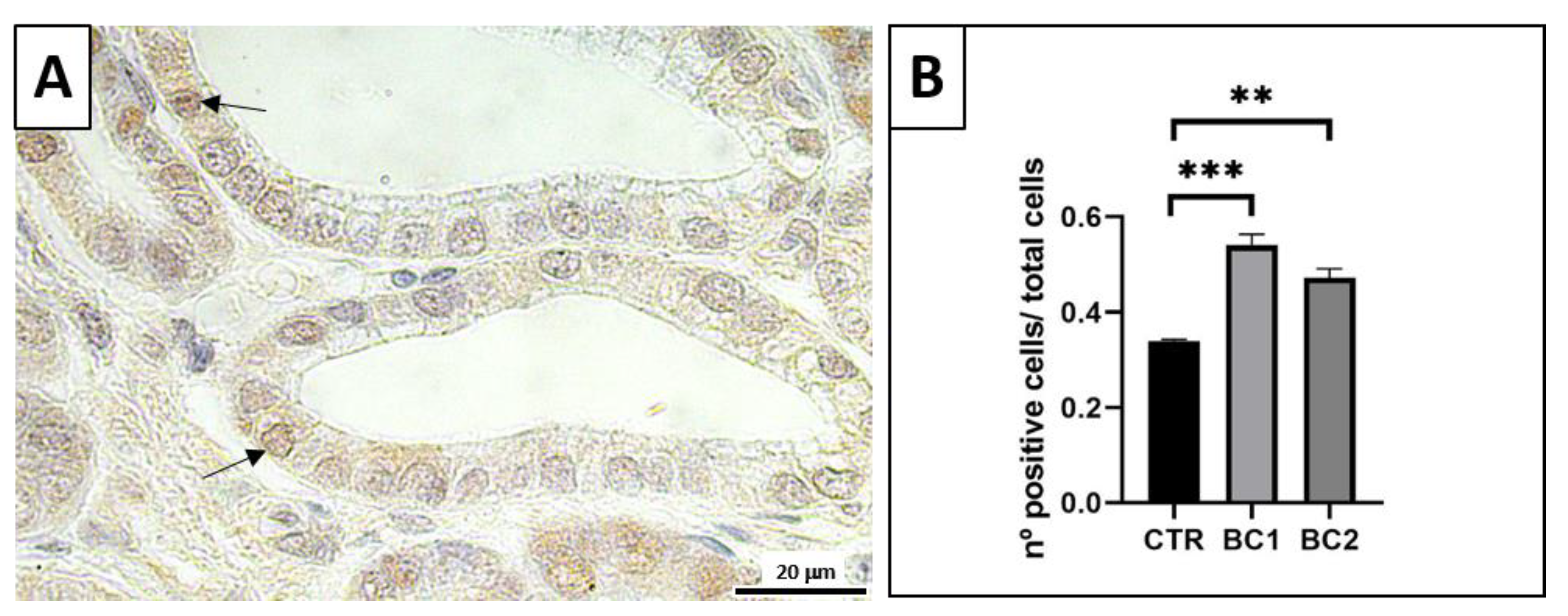
2.4. Immunohistochemistry (PCNA for Trophic Activity)
2.5. Immunofluorescence
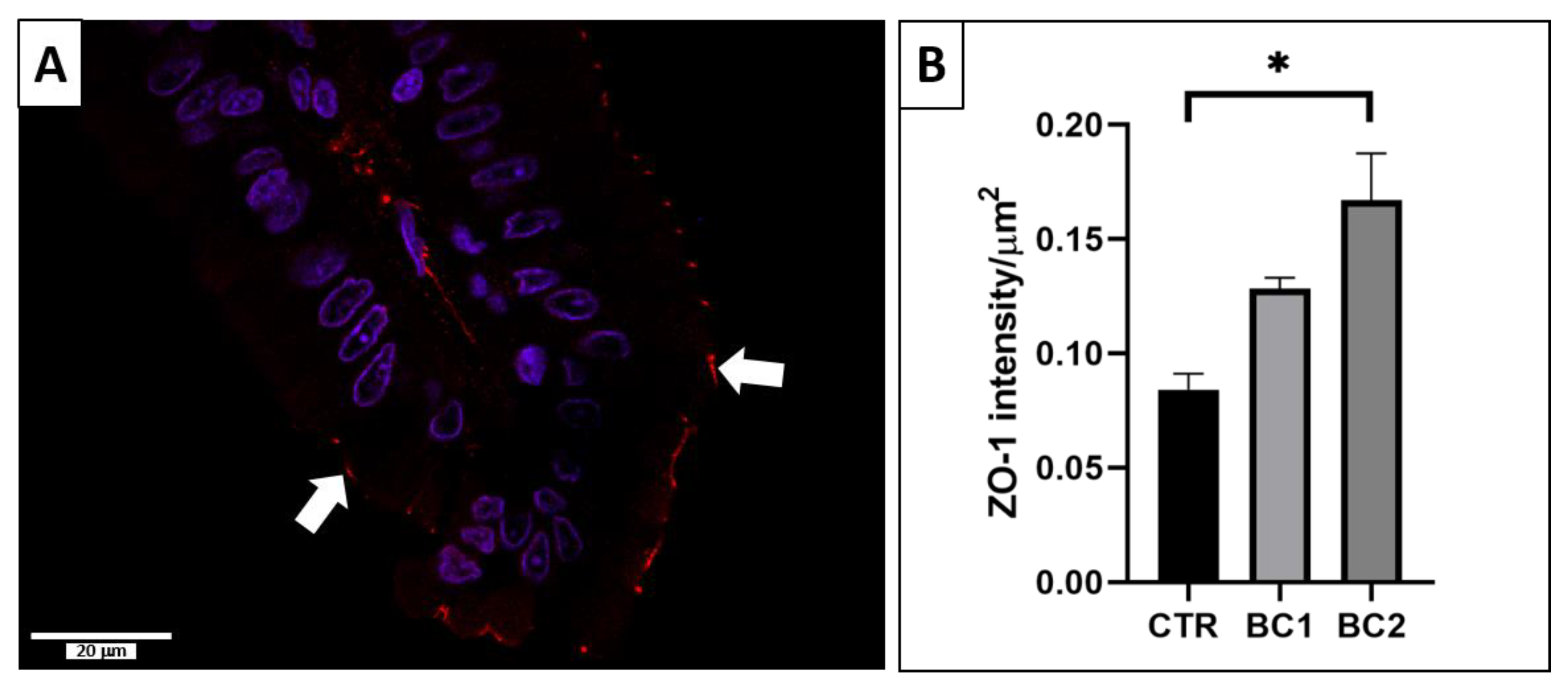
2.5.1. Single Immunofluorescence: Zonulin-1 for Intestinal Permeability
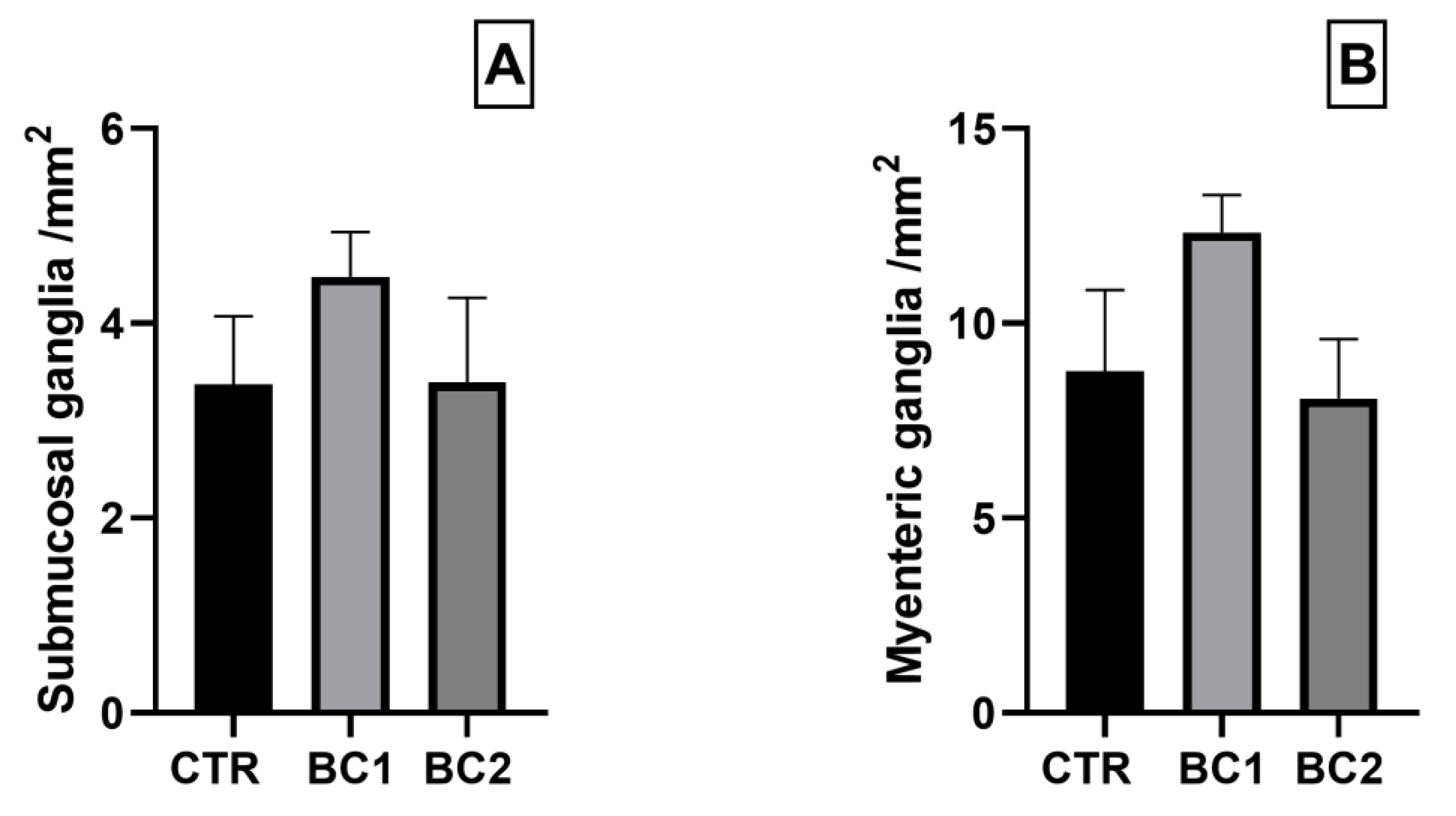
2.5.2. Double Immunofluorescence: PGP9.5 and GFAP for Inflammation and Neuronal Plasticity
2.6. Statistical Analyses
3. Results
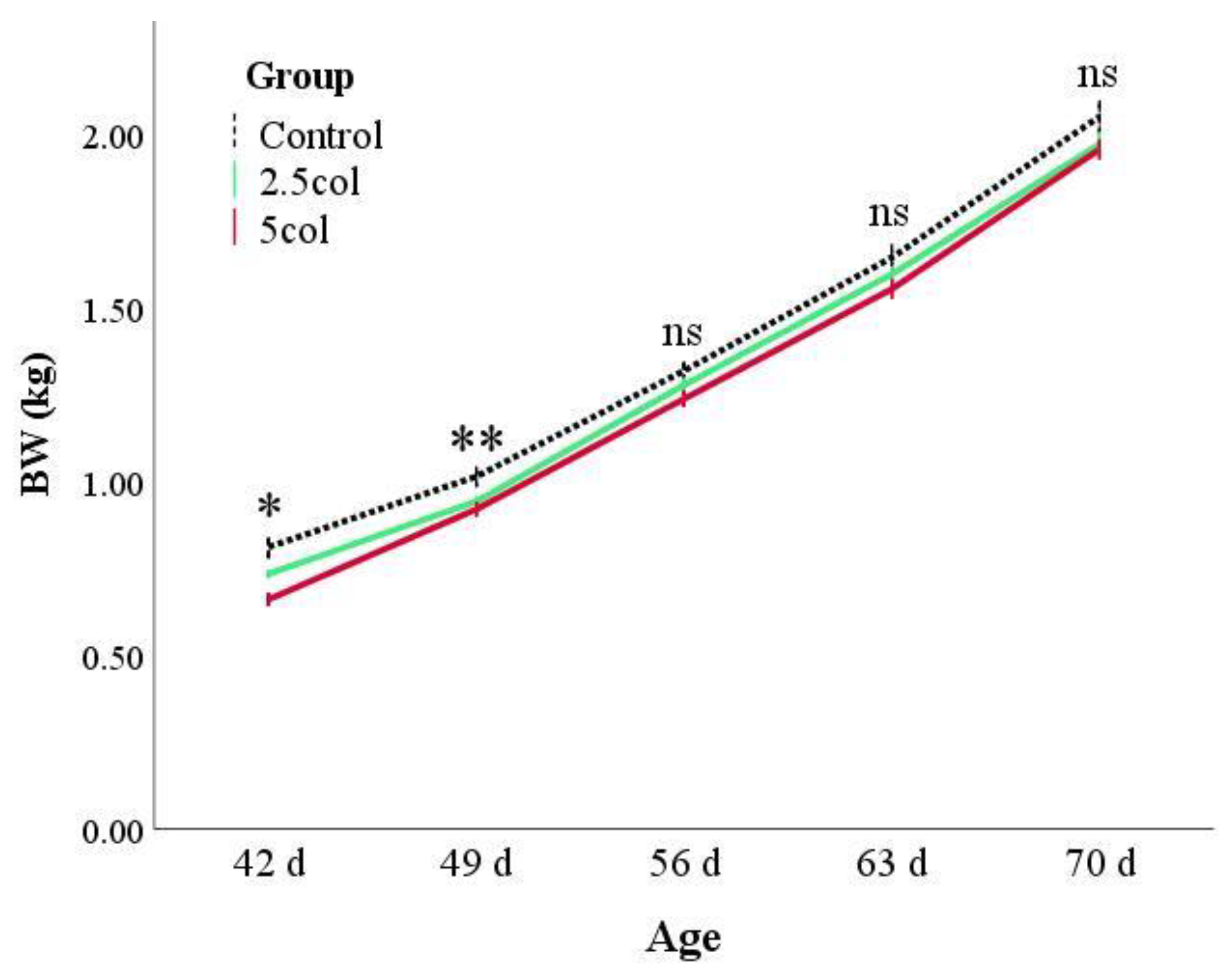
3.1. Zootechnical Parameters—Rabbits Growth
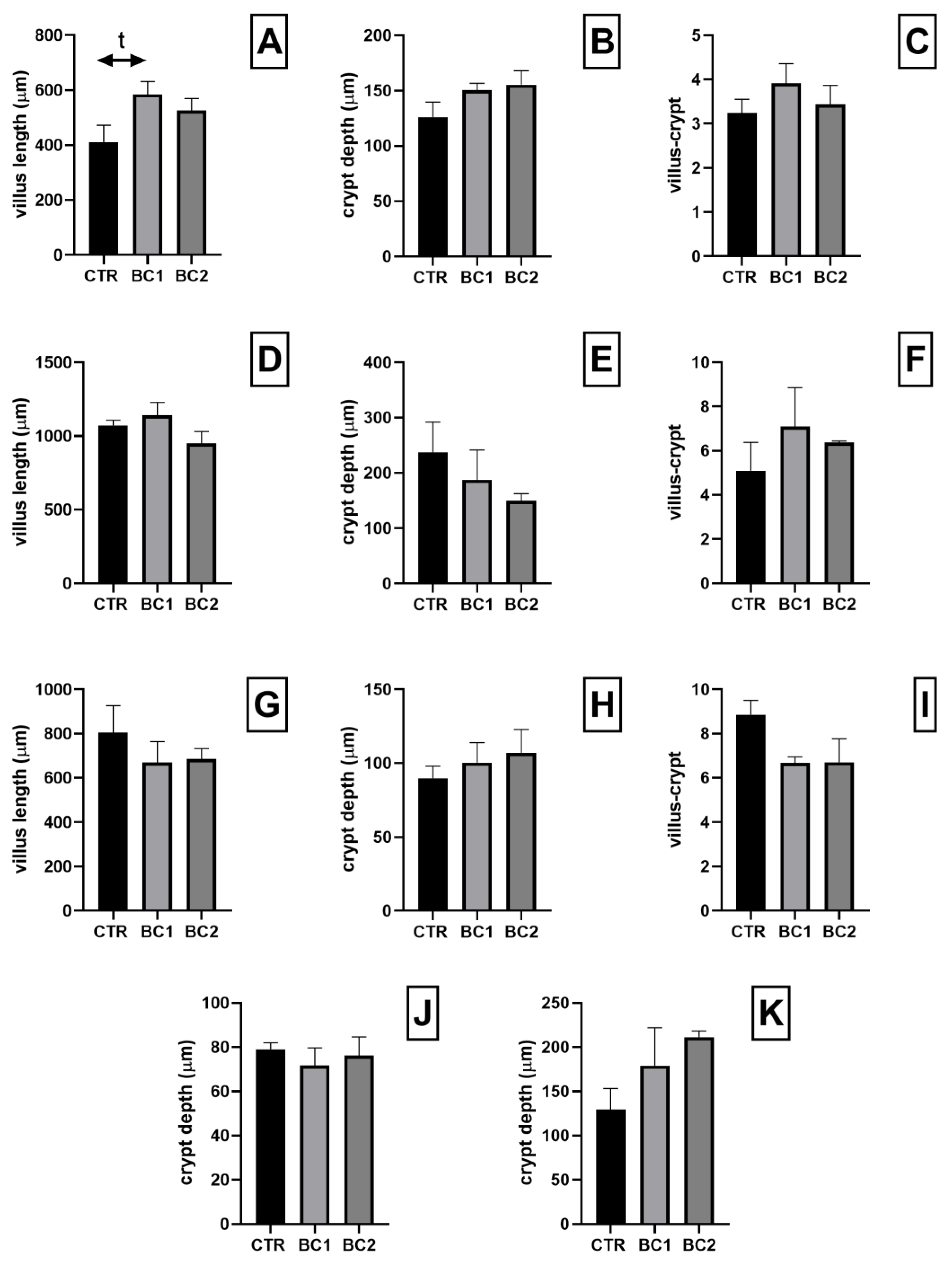
3.2. Small and Large Intestine Histology and Histometry
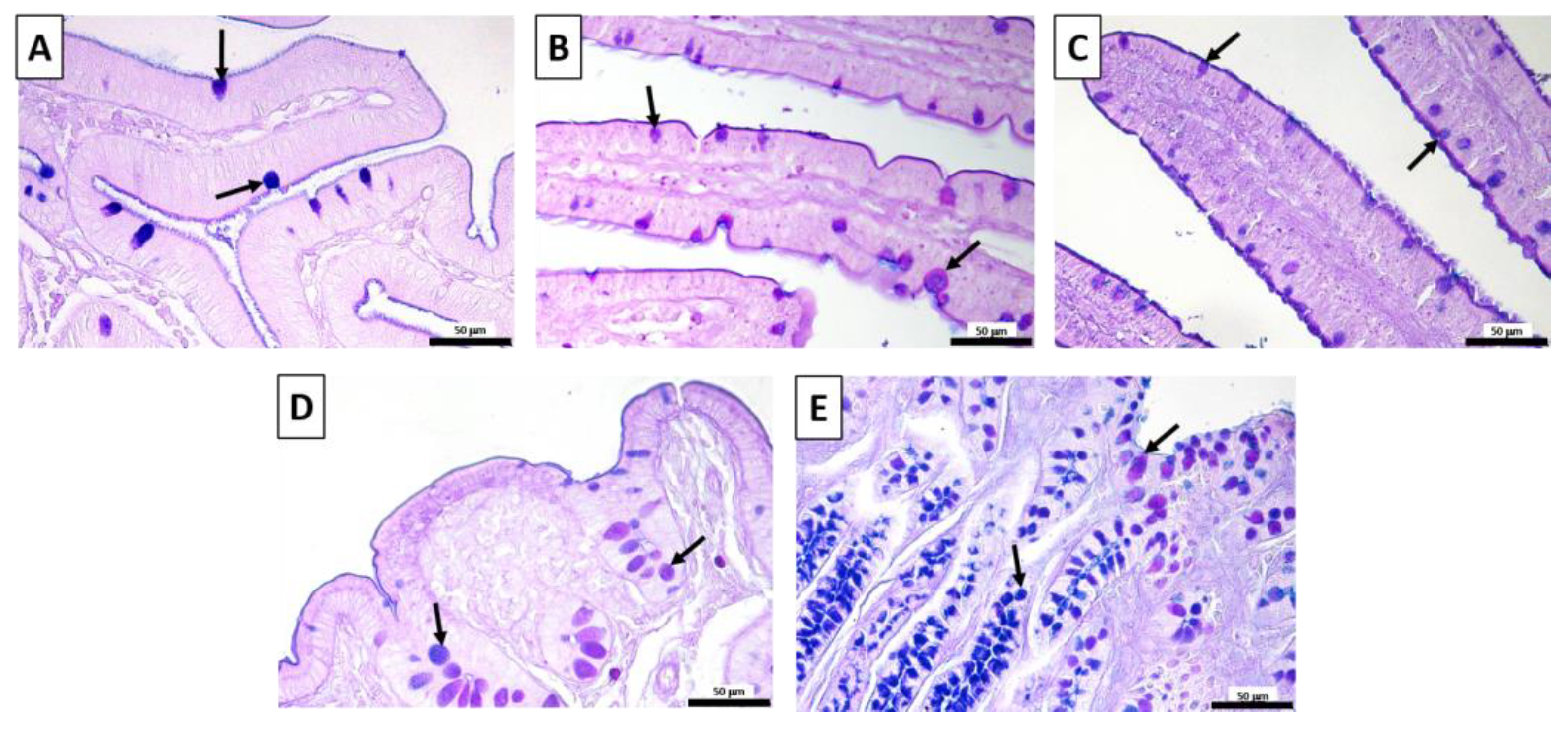
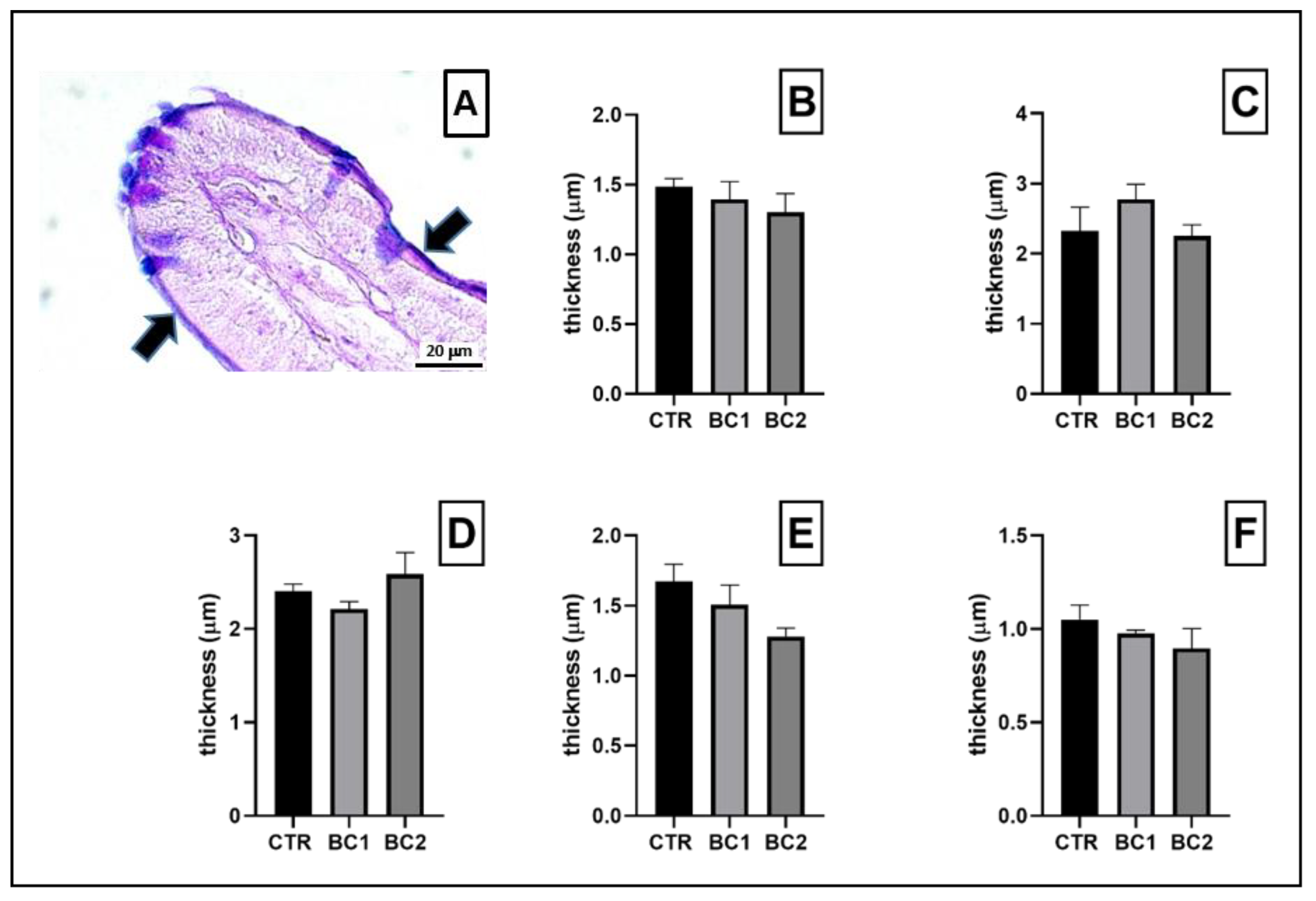
3.3. Small and Large Intestine Histochemistry: AB-PAS
3.4. Duodenum Immunohistochemistry (PCNA for Trophic Activity)
3.5. Immunofluorescence
3.5.1. Single Immunofluorescence: Zonulin-1 for Duodenum Permeability
3.5.2. Duodenum Double Immunofluorescence: PGP9.5 and GFAP for Inflammation and Neuronal Plasticity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gidenne, T.; Fortun-Lamothe, L. Feeding Strategy for Young Rabbits around Weaning: A Review of Digestive Capacity and Nutritional Needs. Anim. Sci. 2002, 75, 169–184. [Google Scholar] [CrossRef]
- Modina, S.C.; Polito, U.; Rossi, R.; Corino, C.; Di Giancamillo, A. Nutritional Regulation of Gut Barrier Integrity in Weaning Piglets. Animals 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Oglesbee, B.L.; Lord, B. Gastrointestinal Diseases of Rabbits. Ferrets Rabbit. Rodents 2020, 174–187. [Google Scholar] [CrossRef]
- Bivolarski, B.L.; Vachkova, E.G. Morphological and Functional Events Associated to Weaning in Rabbits. J. Anim. Physiol. Anim. Nutr. 2014, 98, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of Commensal and Pathogenic Microorganisms with the Intestinal Mucosal Barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2023, 1–19. [Google Scholar] [CrossRef]
- Fasano, A. All Disease Begins in the (Leaky) Gut: Role of Zonulin-Mediated Gut Permeability in the Pathogenesis of Some Chronic Inflammatory Diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Furness, J. The Enteric Nervous System. In The Enteric Nervous System; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–8. ISBN 978-0-470-98875-6. [Google Scholar]
- Pham, T.D.; Gershon, M.D.; Rothman, T.P. Time of Origin of Neurons in the Murine Enteric Nervous System: Sequence in Relation to Phenotype. J. Comp. Neurol. 1991, 314, 789–798. [Google Scholar] [CrossRef]
- Faure, C.; Chalazonitis, A.; Rhéaume, C.; Bouchard, G.; Sampathkumar, S.-G.; Yarema, K.J.; Gershon, M.D. Gangliogenesis in the Enteric Nervous System: Roles of the Polysialylation of the Neural Cell Adhesion Molecule and Its Regulation by Bone Morphogenetic Protein-4. Dev. Dyn. 2007, 236, 44–59. [Google Scholar] [CrossRef]
- Gershon, M.D. The Play Is Still Being Written on Opening Day: Postnatal Maturation of Enteric Neurons May Provide an Opening for Early Life Mischief. J. Physiol. 2012, 590, 2185–2186. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B Protein in the Gut: The Evidence for Enteroglial-Sustained Intestinal Inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential Benefits of Colostrum in Gastrointestinal Diseases. FBS 2016, 8, 331–351. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Agradi, S.; Curone, G.; Vigo, D.; Pastorelli, G.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Serra, V.; et al. Effect of Bovine Colostrum Dietary Supplementation on Rabbit Meat Quality. Foods 2022, 11, 3433. [Google Scholar] [CrossRef]
- Filipescu, I.E.; Leonardi, L.; Menchetti, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Piro, F.; Quattrone, A.; Barbato, O.; Brecchia, G. Preventive Effects of Bovine Colostrum Supplementation in TNBS-Induced Colitis in Mice. PLoS ONE 2018, 13, e0202929. [Google Scholar] [CrossRef]
- Kindlein, L.; Moretti, D.B.; Pauletti, P.; Bagaldo, A.R.; Rodrigues, A.P.O.; Machado-Neto, R. Bovine Colostrum Enriched with Lyophilized Bovine Colostrum Stimulates Intestinal Epithelium Renewal of Holstein Calves in the First Days of Life. J. Anim. Physiol. Anim. Nutr. 2018, 102, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, L.; Dar, A.; Pandey, N.; Mondal, D. Bovine Colostrum as Immunomodulator for Prevention of Escherichia coli Diarrhea in Weaned Rabbits. Afr. J. Agric. Res. 2011, 6, 5066–5072. [Google Scholar]
- Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Balzaretti, C.M.; De Bellis, R.; et al. Antioxidant Activity of Different Tissues from Rabbits Fed Dietary Bovine Colostrum Supplementation. Animals 2023, 13, 850. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Wu, T.; Qi, Y.; Huang, D.; Han, R.; Chen, S.; Tang, J.; Ren, M.; Zhao, X. Bovine Colostrum Promoted Ileal Health in Newborn Lambs at 24 h after Birth: Insight from Intestinal Morphology and Innate Immunity. Animal 2022, 16, 100592. [Google Scholar] [CrossRef]
- Sugiharto, S.; Poulsen, A.-S.R.; Canibe, N.; Lauridsen, C. Effect of Bovine Colostrum Feeding in Comparison with Milk Replacer and Natural Feeding on the Immune Responses and Colonisation of Enterotoxigenic Escherichia coli in the Intestinal Tissue of Piglets. Br. J. Nutr. 2015, 113, 923–934. [Google Scholar] [CrossRef]
- Buttar, H.S.; Bagwe, S.M.; Bhullar, S.K.; Kaur, G. Health Benefits of Bovine Colostrum in Children and Adults. In Dairy in Human Health and Disease Across the Lifespan; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 1; pp. 3–20. ISBN 978-0-12-809868-4. [Google Scholar]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A Survey of Bovine Colostrum Composition and Colostrum Management Practices on Pennsylvania Dairy Farms1. J. Dairy Sci. 2007, 90, 4108–4116. [Google Scholar] [CrossRef]
- Simmen, F.A.; Simmen, R.C.M.; Reinhart, G. Maternal and Neonatal Somatomedin C/Insulin-like Growth Factor-I (IGF-I) and IGF Binding Proteins during Early Lactation in the Pig. Dev. Biol. 1988, 130, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chandwe, K.; Kelly, P. Colostrum Therapy for Human Gastrointestinal Health and Disease. Nutrients 2021, 13, 1956. [Google Scholar] [CrossRef]
- Burrin, D.G.; Davis, T.A.; Ebner, S.; Schoknecht, P.A.; Fiorotto, M.L.; Reeds, P.J. Colostrum Enhances the Nutritional Stimulation of Vital Organ Protein Synthesis in Neonatal Pigs. J. Nutr. 1997, 127, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Vaishnavi, C.; Ray, P.; Singh, M.; Kochhar, R. Preliminary Investigation on the Effect of Lactobacillus and Epidermal Growth Factor on Tight Junction Proteins in Experimental Clostridium Difficile Infection. Adv. Microbiol. 2014, 2014, 425–435. [Google Scholar] [CrossRef][Green Version]
- Banan, A.; Zhang, L.J.; Shaikh, M.; Fields, J.Z.; Farhadi, A.; Keshavarzian, A. Inhibition of Oxidant-Induced Nuclear Factor-κB Activation and Inhibitory-κBα Degradation and Instability of F-Actin Cytoskeletal Dynamics and Barrier Function by Epidermal Growth Factor: Key Role of Phospholipase-γ Isoform. J. Pharmacol. Exp. Ther. 2004, 309, 356–368. [Google Scholar] [CrossRef]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 Restores TGF-Β1 Signaling in Chronic Inflammatory Bowel Disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef]
- Sabatino, A.D.; Pickard, K.M.; Rampton, D.; Kruidenier, L.; Rovedatti, L.; Leakey, N.A.B.; Corazza, G.R.; Monteleone, G.; MacDonald, T.T. Blockade of Transforming Growth Factor β Upregulates T-Box Transcription Factor T-Bet, and Increases T Helper Cell Type 1 Cytokine and Matrix Metalloproteinase-3 Production in the Human Gut Mucosa. Gut 2008, 57, 605–612. [Google Scholar] [CrossRef]
- Johnson, T.; Jacobson, B.T.; Jones, K.; Mosdal, C.; Jones, S.; Vitkovic, M.; Kruppenbacher, S.; Sebrell, A.; Bimczok, D. Transfer and Persistence of Bovine Immunoglobulins in Lambs Fed a Colostrum Replacer. Vet. Rec. 2022, 191, e1974. [Google Scholar] [CrossRef]
- Yang, M.; Zou, Y.; Wu, Z.H.; Li, S.L.; Cao, Z.J. Colostrum Quality Affects Immune System Establishment and Intestinal Development of Neonatal Calves. J. Dairy Sci. 2015, 98, 7153–7163. [Google Scholar] [CrossRef]
- Bridger, J.C.; Brown, J.F. Development of Immunity to Porcine Rotavirus in Piglets Protected from Disease by Bovine Colostrum. Infect. Immun. 1981, 31, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Roshan, M.; Ghoreishi, S.M.; Akhlaghi, A.; Kanani, M.; Shams-Abadi, A.R.A.; Ghaffari, M.H. Extended Colostrum Feeding for 2 Weeks Improves Growth Performance and Reduces the Susceptibility to Diarrhea and Pneumonia in Neonatal Holstein Dairy Calves. J. Dairy Sci. 2020, 103, 8130–8142. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Pan, X.; Nguyen, D.N.; Brunse, A.; Bojesen, A.M.; Rudloff, S.; Mortensen, M.S.; Burrin, D.G.; Sangild, P.T. Human Milk Fortification with Bovine Colostrum Is Superior to Formula-Based Fortifiers to Prevent Gut Dysfunction, Necrotizing Enterocolitis, and Systemic Infection in Preterm Pigs. JPEN J. Parenter. Enteral. Nutr. 2019, 43, 252–262. [Google Scholar] [CrossRef]
- Støy, A.C.F.; Heegaard, P.M.H.; Thymann, T.; Bjerre, M.; Skovgaard, K.; Boye, M.; Stoll, B.; Schmidt, M.; Jensen, B.B.; Sangild, P.T. Bovine Colostrum Improves Intestinal Function Following Formula-Induced Gut Inflammation in Preterm Pigs. Clin. Nutr. 2014, 33, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Nguyen, D.N.; Ren, S.; Moodley, A.; Sangild, P.T. Bovine Colostrum before or after Formula Feeding Improves Systemic Immune Protection and Gut Function in Newborn Preterm Pigs. Front. Immunol. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine Colostrum: A Source of Bioactive Compounds for Prevention and Treatment of Gastrointestinal Disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients 2022, 14, 2512. [Google Scholar] [CrossRef]
- Davison, G. The Use of Bovine Colostrum in Sport and Exercise. Nutrients 2021, 13, 1789. [Google Scholar] [CrossRef]
- Ismail, R.I.H.; Awad, H.A.; Imam, S.S.; Gad, G.I.; Aboushady, N.M.; Abdou, R.M.; Eissa, D.S.; Azzam, N.T.; Barakat, M.M.; Yassin, M.M.; et al. Gut Priming with Bovine Colostrum and T Regulatory Cells in Preterm Neonates: A Randomized Controlled Trial. Pediatr. Res. 2021, 90, 650–656. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Szymańska, P.; Fichna, J. Supplementation of Bovine Colostrum in Inflammatory Bowel Disease: Benefits and Contraindications. Adv. Nutr. 2021, 12, 533–545. [Google Scholar] [CrossRef]
- Han, G.; Cho, H.; Kim, H.; Jang, Y.; Jang, H.; Kim, D.E.; Kim, E.S.; Kim, E.H.; Hwang, K.Y.; Kim, K.; et al. Bovine Colostrum Derived-Exosomes Prevent Dextran Sulfate Sodium-Induced Intestinal Colitis via Suppression of Inflammation and Oxidative Stress. Biomater. Sci. 2022, 10, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Halasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Brecchia, G.; Menchetti, L.; Cardinali, R.; Castellini, C.; Polisca, A.; Zerani, M.; Maranesi, M.; Boiti, C. Effects of a Bacterial Lipopolysaccharide on the Reproductive Functions of Rabbit Does. Anim. Reprod. Sci. 2014, 147, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Canali, C.; Castellini, C.; Boiti, C.; Brecchia, G. The Different Effects of Linseed and Fish Oil Supplemented Diets on Insulin Sensitivity of Rabbit Does during Pregnancy. Res. Vet. Sci. 2018, 118, 126–133. [Google Scholar] [CrossRef]
- Agradi, S.; Cremonesi, P.; Menchetti, L.; Balzaretti, C.; Severgnini, M.; Riva, F.; Castiglioni, B.; Draghi, S.; Di Giancamillo, A.; Castrica, M.; et al. Bovine Colostrum Supplementation Modulates the Intestinal Microbial Community in Rabbits. Animals 2023, 13, 976. [Google Scholar] [CrossRef]
- Menchetti, L.; Curone, G.; Filipescu, I.E.; Barbato, O.; Leonardi, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Riva, F.; Casano, A.B.; et al. The Prophylactic Use of Bovine Colostrum in a Murine Model of TNBS-Induced Colitis. Animals 2020, 10, 492. [Google Scholar] [CrossRef]
- Antonio, J.; Sanders, M.S.; Van Gammeren, D. The Effects of Bovine Colostrum Supplementation on Body Composition and Exercise Performance in Active Men and women. Nutrition 2001, 17, 243–247. [Google Scholar] [CrossRef]
- Mero, A.; Nykänen, T.; Keinänen, O.; Knuutinen, J.; Lahti, K.; Alen, M.; Rasi, S.; Leppäluoto, J. Protein Metabolism and Strength Performance after Bovine Colostrum Supplementation. Amino Acids 2005, 28, 327–335. [Google Scholar] [CrossRef]
- Rossi, L.; Di Giancamillo, A.; Reggi, S.; Domeneghini, C.; Baldi, A.; Sala, V.; Dell’Orto, V.; Coddens, A.; Cox, E.; Fogher, C. Expression of Verocytotoxic Escherichia coli Antigens in Tobacco Seeds and Evaluation of Gut Immunity after Oral Administration in Mouse Model. J. Vet. Sci. 2013, 14, 263–270. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Vitari, F.; Pastorelli, G.; Corino, C.; Domeneghini, C. Dietary Conjugated Linoleic Acids Decrease Leptin in Porcine Adipose Tissue. J. Nutr. 2009, 139, 1867–1872. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Vitari, F.; Bosi, G.; Savoini, G.; Domeneghini, C. The Chemical Code of Porcine Enteric Neurons and the Number of Enteric Glial Cells Are Altered by Dietary Probiotics. Neurogastroenterol. Motil. 2010, 22, e271–e278. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of Barrier Function in Injured Intestinal Mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef]
- Pácha, J. Development of Intestinal Transport Function in Mammals. Physiol. Rev. 2000, 80, 1633–1667. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Kong, Y.; Kleinstein, S.H.; Subramanian, S.; Ahern, P.P.; Gordon, J.I.; Medzhitov, R. Analysis of Gene–Environment Interactions in Postnatal Development of the Mammalian Intestine. Proc. Natl. Acad. Sci. USA 2015, 112, 1929–1936. [Google Scholar] [CrossRef]
- le Huërou-Luron, I.; Huguet, A.; Callarec, J.; Leroux, T.; le Dividich, J. Supplementation of a weaning diet with bovine colostrum increases feed intake and growth of weaned piglets. Journées Rech. Porc. Fr. 2004, 36, 33–38. [Google Scholar]
- Burrin, D.G.; Davis, T.A.; Ebner, S.; Schoknecht, P.A.; Fiorotto, M.L.; Reeds, P.J.; Mcavoy, S. Nutrient-Independent and Nutrient-Dependent Factors Stimulate Protein Synthesis in Colostrum-Fed Newborn Pigs. Pediatr. Res. 1995, 37, 593–599. [Google Scholar] [CrossRef][Green Version]
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing Cell Renewal in the Intestine: Stem Cells, Signals and Combinatorial Control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Woliński, J.; Słupecka, M.; Weström, B.; Prykhodko, O.; Ochniewicz, P.; Arciszewski, M.; Ekblad, E.; Szwiec, K.; Skibo, U.G.; Kovalenko, T.; et al. Effect of Feeding Colostrum versus Exogenous Immunoglobulin G on Gastrointestinal Structure and Enteric Nervous System in Newborn Pigs. J. Anim. Sci. 2012, 90, 327–330. [Google Scholar] [CrossRef]
- Donovan, S.M.; Mcneil, L.K.; Jiménez-flores, R.; Odle, J. Insulin-Like Growth Factors and Insulin-Like Growth Factor Binding Proteins in Porcine Serum and Milk throughout Lactation. Pediatr. Res. 1994, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.A.; Lamar, C.H.; Bottoms, G.D.; Cline, T.R. Growth-Stimulating Substances in Porcine Milk. Am. J. Vet. Res. 1987, 48, 1531–1533. [Google Scholar] [PubMed]
- Huguet, A.; Sève, B.; Le Dividich, J.; Le Huërou-Luron, I. Effects of a Bovine Colostrum-Supplemented Diet on Some Gut Parameters in Weaned Piglets. Reprod. Nutr. Dev. 2006, 46, 167–178. [Google Scholar] [CrossRef]
- Neunlist, M.; Toumi, F.; Oreschkova, T.; Denis, M.; Leborgne, J.; Laboisse, C.L.; Galmiche, J.-P.; Jarry, A. Human ENS Regulates the Intestinal Epithelial Barrier Permeability and a Tight Junction-Associated Protein ZO-1 via VIPergic Pathways. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G1028–G1036. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a Newly Discovered Modulator of Intestinal Permeability, and Its Expression in Coeliac Disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Neunlist, M.; Van Landeghem, L.; Bourreille, A.; Savidge, T. Neuro-Glial Crosstalk in Inflammatory Bowel Disease. J. Intern. Med. 2008, 263, 577–583. [Google Scholar] [CrossRef]
- Savidge, T.C.; Sofroniew, M.V.; Neunlist, M. Starring Roles for Astroglia in Barrier Pathologies of Gut and Brain. Lab. Investig. 2007, 87, 731–736. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric Glia Regulate Intestinal Barrier Function and Inflammation Via Release of S-Nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Da Cunha Franceschi, R.; Nardin, P.; Machado, C.V.; Tortorelli, L.S.; Martinez-Pereira, M.A.; Zanotto, C.; Gonçalves, C.-A.; Zancan, D.M. Enteric Glial Reactivity to Systemic LPS Administration: Changes in GFAP and S100B Protein. Neurosci. Res. 2017, 119, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.D.; Rossi, R.; Martino, P.A.; Aidos, L.; Maghin, F.; Domeneghini, C.; Corino, C. Copper Sulphate Forms in Piglet Diets: Microbiota, Intestinal Morphology and Enteric Nervous System Glial Cells. Anim. Sci. J. 2018, 89, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schäfer, K.-H. Enteric Glia: S100, GFAP, and Beyond. Anat. Rec. 2019, 302, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Neunlist, M.; Aubert, P.; Bonnaud, S.; Van Landeghem, L.; Coron, E.; Wedel, T.; Naveilhan, P.; Ruhl, A.; Lardeux, B.; Savidge, T.; et al. Enteric Glia Inhibit Intestinal Epithelial Cell Proliferation Partly through a TGF-Β1-Dependent Pathway. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G231–G241. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal Microbiota Shapes Gut Physiology and Regulates Enteric Neurons and Glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aidos, L.; Pallaoro, M.; Mirra, G.; Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Riva, F.; Balzaretti, C.M.; et al. Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum. Vet. Sci. 2023, 10, 657. https://doi.org/10.3390/vetsci10110657
Aidos L, Pallaoro M, Mirra G, Serra V, Castrica M, Agradi S, Curone G, Vigo D, Riva F, Balzaretti CM, et al. Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum. Veterinary Sciences. 2023; 10(11):657. https://doi.org/10.3390/vetsci10110657
Chicago/Turabian StyleAidos, Lucia, Margherita Pallaoro, Giorgio Mirra, Valentina Serra, Marta Castrica, Stella Agradi, Giulio Curone, Daniele Vigo, Federica Riva, Claudia Maria Balzaretti, and et al. 2023. "Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum" Veterinary Sciences 10, no. 11: 657. https://doi.org/10.3390/vetsci10110657
APA StyleAidos, L., Pallaoro, M., Mirra, G., Serra, V., Castrica, M., Agradi, S., Curone, G., Vigo, D., Riva, F., Balzaretti, C. M., De Bellis, R., Pastorelli, G., Brecchia, G., Modina, S. C., & Di Giancamillo, A. (2023). Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum. Veterinary Sciences, 10(11), 657. https://doi.org/10.3390/vetsci10110657












