Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Conventional Treatment of the Most Common Musculoskeletal Pathologies in Equine Medicine
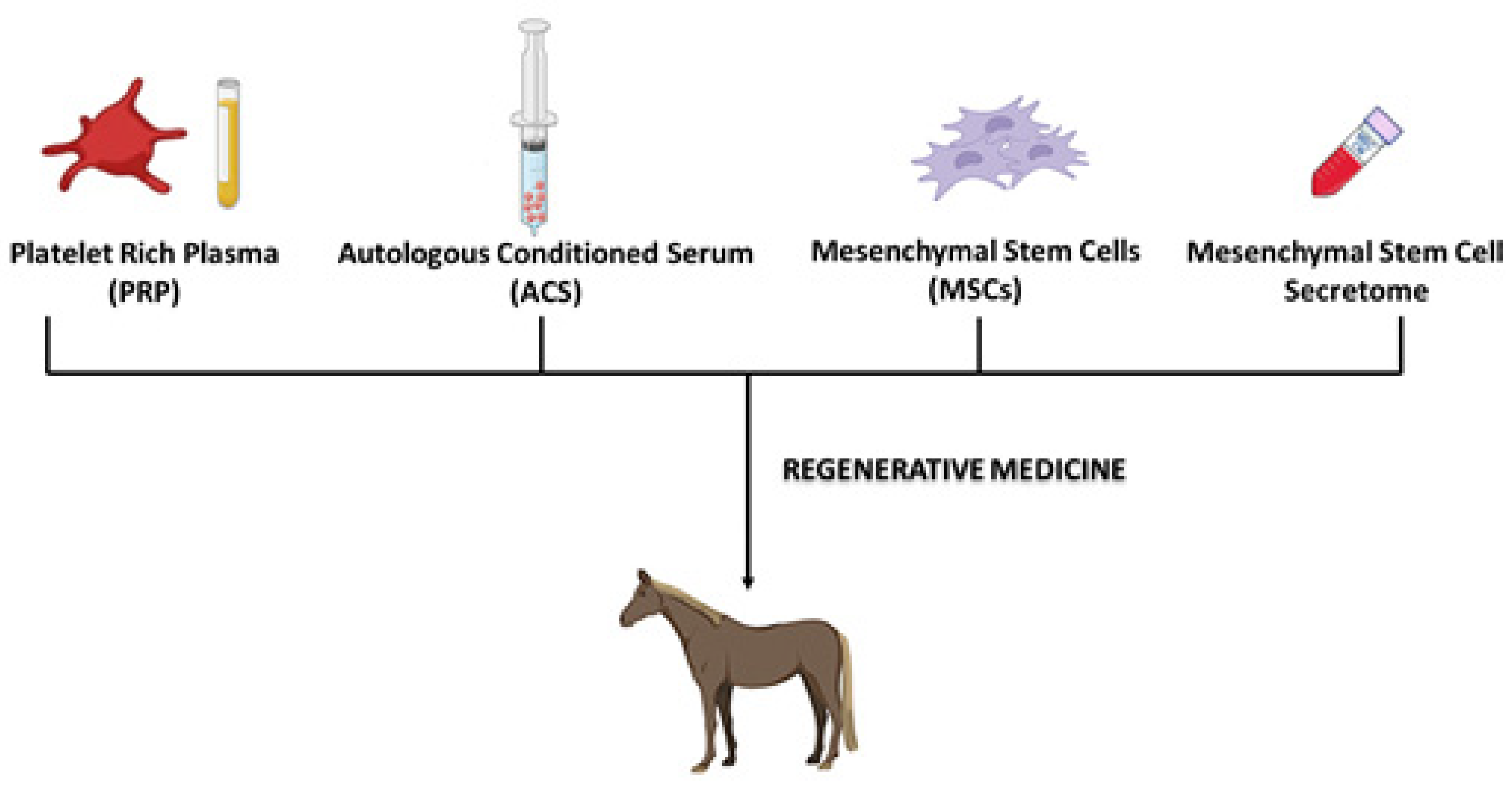
1.2. Regenerative Medicine
1.2.1. Platelet-Rich Plasma (PRP)
1.2.2. Autologous Conditioned Serum (ACS)
1.2.3. Autologous Protein Solution (APS)
1.2.4. Mesenchymal Stem Cells (MSCs)
1.2.5. MSCs-Derived Secretome
2. Materials and Methods
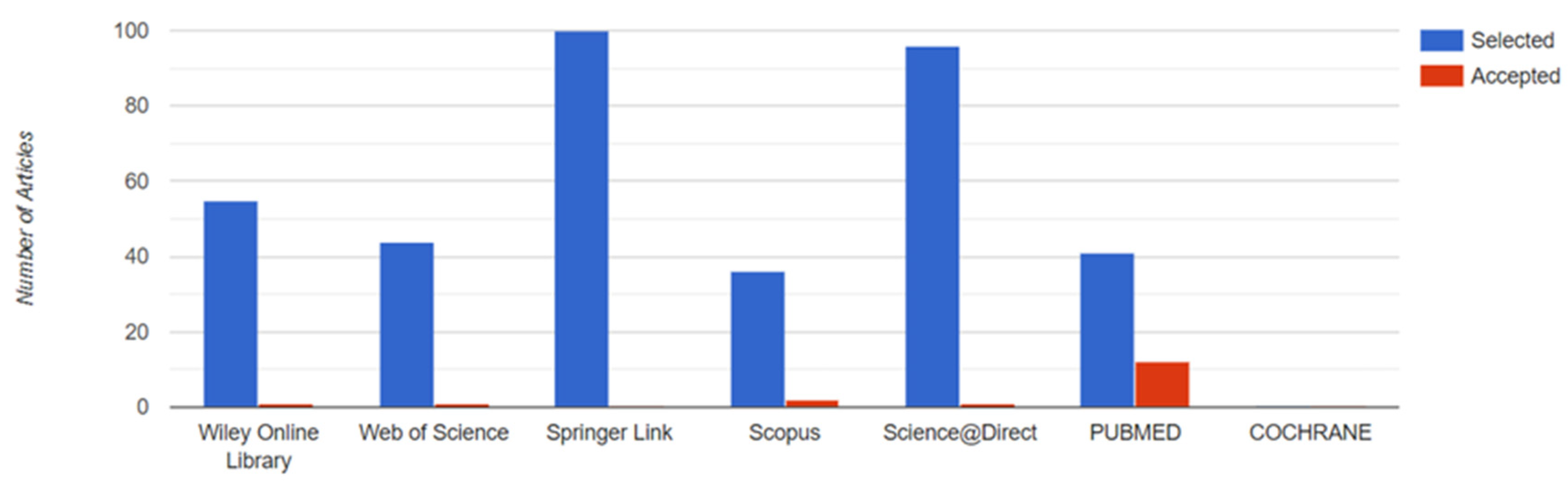
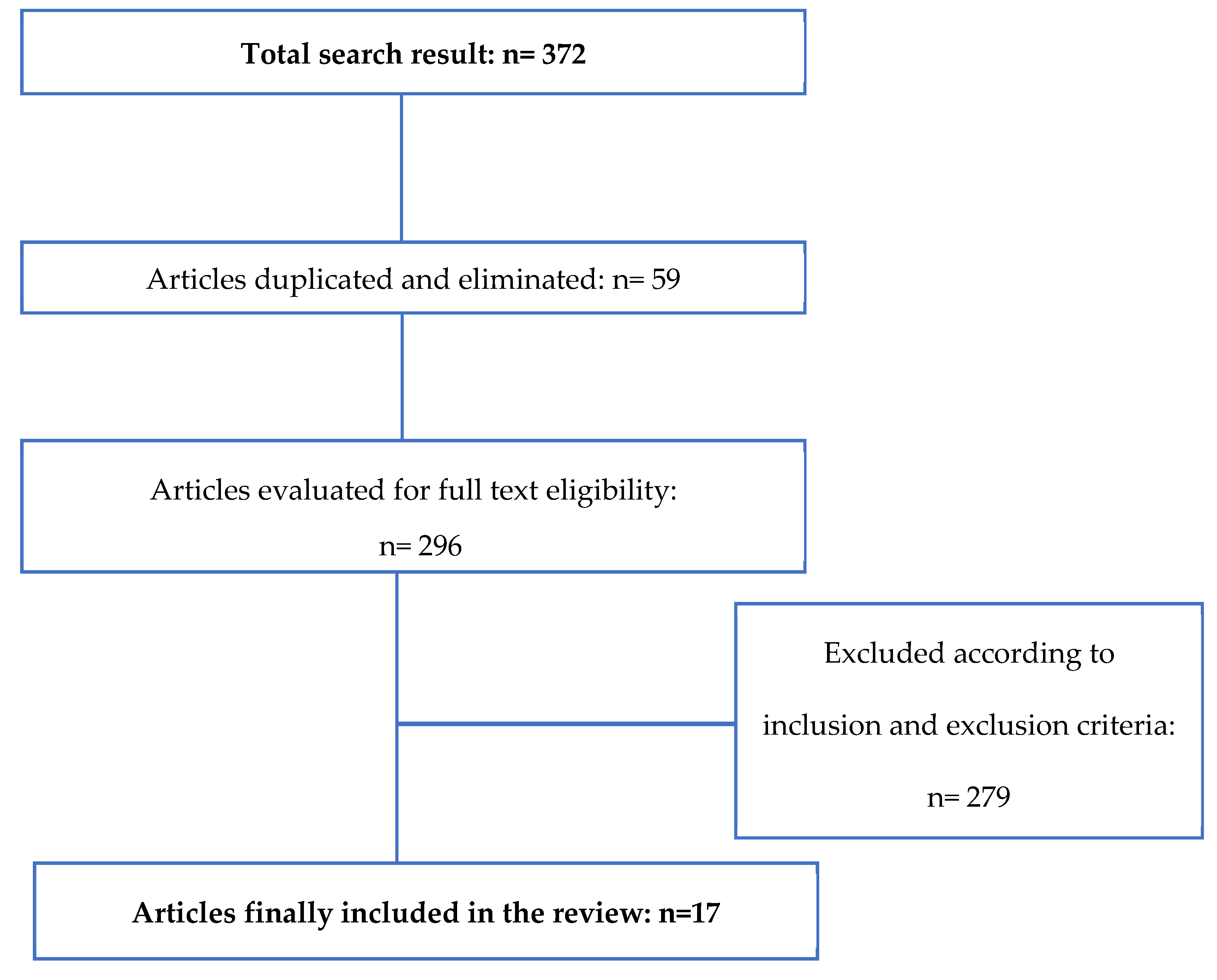
2.1. Search Strategy
2.1.1. PICOC Question
- Population: horses with musculoskeletal pathologies;
- Intervention: regenerative medicine or cell therapy;
- Comparison: conventional therapy in musculoskeletal pathologies;
- Outcome: regenerative and anti-inflammatory effects;
- Context: academic.
2.1.2. Search Questions
- Is regenerative medicine based on cell therapy effective in horses?
- What types of therapies are most used in regenerative medicine in horses?
- What types of pathologies can be treated with regenerative medicine in horses?
- When is regenerative medicine the best option for treating musculoskeletal pathologies?
- Is the secretome of MSCs used in horses?
2.1.3. Search String
2.1.4. Databases
- COCHRANE (https://www.cochranelibrary.com/); accessed on 1 January 2022.
- PUBMED (https://pubmed.ncbi.nlm.nih.gov/); accessed on 15 January 2022.
- Science Direct (http://www.sciencedirect.com); accessed on 15 January 2022.
- Web of Science (http://wos.fecyt.es/); accessed on 1 February 2022.
- Scopus (http://scopus.fecyt.es/); accessed on 2 February 2022.
- Springer Link (http://link.springer.com); accessed on 21 January 2022.
- Wiley Online Library (https://onlinelibrary.wiley.com/) accessed on 19 February 2022.
2.2. Eligibility Criteria
- Articles in Spanish and English;
- Full text availability;
- Clinical trials;
- Published within the last 10 years.
- Other pathologies unrelated to osteoarticular issues;
- Studies made on other species different from horses, including human beings;
- No use of regenerative medicine.
3. Results and Discussion
| References | Study Design | Number of Equine Patients | Therapy | Lesion Treated |
|---|---|---|---|---|
| Torricelli et al., 2011 [3] | Controlled experimental study | 13 | PRP associated with bone marrow mononucleated cells | Lameness |
| Scala et al., 2014 [39] | Controlled experimental study | 150 | No-gelled platelet concentrate | Teno-desmic injuries |
| Yamada et al., 2016 [40] | Controlled experimental study | 12 | PRP gel associated with stem cells from adipose tissue | Chondral defects |
| Geburek et al., 2016 [24] | Controlled experimental study | 10 | PRP concentrate | Tendinopathy of the superficial digital flexor tendon |
| Beerts et al., 2017 [41] | Controlled experimental study | 104 | PRP and allogenic tenogenically induced mesenchymal stem cells | Tendon and ligament injuries |
| References | Study Design | Number of Equine Patients | Therapy | Lesion Treated |
|---|---|---|---|---|
| Frisbie et al., 2007 [25] | Controlled experimental study | 16 | ACS | Osteoarthritis |
| Georg et al., 2010 [42] | Controlled experimental study | 7 | Autologous conditioned plasma (ACP) | Severe tendinitis of the superficial digital flexor tendon, deep digital flexor tendon, or desmitis of the inferior check ligament |
| Geburek et al., 2015 [43] | Controlled experimental study | 15 | ACS | Tendinitis of the superficial digital flexor tendon |
| Marques-Smith et al., 2020 [44] | Controlled experimental study | 20 | ACS | Articular lameness |
| References | Study Design | Number of Equine Patients | Therapy | Lesion Treated |
|---|---|---|---|---|
| Mokbel et al., 2011 [45] | Controlled experimental study | 27 donkeys | BMSCs | Osteoarthritis |
| Carvalho et al., 2013 [46] | Controlled experimental study | 8 | ASCs and PRP concentrates | Tendinitis of the superficial digital flexor tendon |
| Renzi et al., 2013 [47] | Clinical trial | 33 | BMSCs | Tendonitis and desmitis |
| Broeckx et al., 2014 [48] | Controlled experimental study | 20 | PRP and peripheral blood mononuclear cells (PBMCs) | Degenerative joint disease |
| González-Fernández et al., 2016 [49] | Controlled randomized and experimental study | 6 | ASCs and BMSCs | Meniscal defects |
| Villatoro et al., 2018 [50] | Clinical trial | 80 | ASCs | Osteoarthritis |
| References | Study Design | Number of Horses | Therapy | Lesion Treated |
|---|---|---|---|---|
| Arévalo-Turrubiarte et al., 2022 [53] | In vitro experiments with randomized complete block design | - | BMSC, ASC, and synovial fluid-derived secretome | Inflammation model of osteoarthritis |
| Kearney et al., 2022 [34] | Randomized positively and negatively controlled experimental study | 8 | BMSCs-derived secretome | Joint inflammation |
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Weeren, P.R.; Back, W. Musculoskeletal Disease in Aged Horses and Its Management. Vet. Clin. N. Am.-Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef]
- Wayne Mcilwraith, C.; Fortier, L.A.; Frisbie, D.D.; Nixon, A.J. Equine Models of Articular Cartilage Repair. Cartilage 2011, 2, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Fini, M.; Filardo, G.; Tschon, M.; Pischedda, M.; Pacorini, A.; Kon, E.; Giardino, R. Regenerative Medicine for the Treatment of Musculoskeletal Overuse Injuries in Competition Horses. Int. Orthop. 2011, 35, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of Bone Marrow-Derived Mesenchymal Stem Cells Demonstrates Improved Outcome in Horses with Overstrain Injury of the Superficial Digital Flexor Tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Smith, R.K.W. Clinical Update on the Use of Mesenchymal Stem Cells in Equine Orthopaedics. Equine Vet. J. 2010, 42, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Bogers, S.H. Cell-Based Therapies for Joint Disease in Veterinary Medicine: What We Have Learned and What We Need to Know. Front. Vet. Sci. 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Baccarin, R.Y.A.; Seidel, S.R.T.; Michelacci, Y.M.; Tokawa, P.K.A.; Oliveira, T.M. Osteoarthritis: A Common Disease That Should Be Avoided in the Athletic Horse’s Life. Anim. Front. 2022, 12, 25–36. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K.W. Resolving an Inflammatory Concept: The Importance of Inflammation and Resolution in Tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Arlt, S.P.; Belshaw, Z.; Buckley, L.; Corah, L.; Doit, H.; Fajt, V.R.; Grindlay, D.J.C.; Moberly, H.K.; Morrow, L.D.; et al. Critically Appraised Topics (CATs) in Veterinary Medicine: Applying Evidence in Clinical Practice. Front. Vet. Sci. 2020, 7, 314. [Google Scholar] [CrossRef]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Menè, P.; Savarino, V.; Fornasari, D. Management of Osteoarthritis: Expert Opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.D. Managing Chronic Arthritis. Vet. Clin. N. Am.-Equine Pract. 2002, 18, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Fendrick, A.M.; Greenberg, B.P. A Review of the Benefits and Risks of Nonsteroidal Anti-Inflammatory Drugs in the Management of Mild-to-Moderate Osteoarthritis. Osteopath. Med. Prim. Care 2009, 3, 1. [Google Scholar] [CrossRef]
- Esteves, C.L.; Sheldrake, T.A.; Mesquita, S.P.; Pesántez, J.J.; Menghini, T.; Dawson, L.; Péault, B.; Donadeu, F.X. Isolation and Characterization of Equine Native MSC Populations. Stem Cell Res. Ther. 2017, 8, 80. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A Brief Definition of Regenerative Medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Chow, L.; Griffenhagen, G.M.; Bass, L.; Goodrich, L.R.; Impastato, R.; Dow, S. Distinct Differences in Immunological Properties of Equine Orthobiologics Revealed by Functional and Transcriptomic Analysis Using an Activated Macrophage Readout System. Front. Vet. Sci. 2023, 10, 1109473. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Choudhary, S. Stem Cells in Veterinary Science; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9789811634642. [Google Scholar]
- Kawase, T. Platelet-Rich Plasma and Its Derivatives as Promising Bioactive Materials for Regenerative Medicine: Basic Principles and Concepts Underlying Recent Advances. Odontology 2015, 103, 126–135. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Orive, G.; Mujika, I.; Andia, I. Platelet-Rich Therapies in the Treatment of Orthopaedic Sport Injuries. Sports Med. 2009, 39, 345–354. [Google Scholar] [CrossRef]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports Medicine Applications of Platelet Rich Plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef]
- Geburek, F.; Gaus, M.; van Schie, H.T.M.; Rohn, K.; Stadler, P.M. Effect of Intralesional Platelet-Rich Plasma (PRP) Treatment on Clinical and Ultrasonographic Parameters in Equine Naturally Occurring Superficial Digital Flexor Tendinopathies—A Randomized Prospective Controlled Clinical Trial. BMC Vet. Res. 2016, 12, 191. [Google Scholar] [CrossRef]
- Wehling, P.; Moser, C.; Frisbied, D.; McIlwraith, C.W.; Kawcak, C.E.; Krauspe, R.; Reinecke, J.A. Autologous Conditioned Serum in the Treatment of Orthopedic Diseases: The Orthokine® Therapy. BioDrugs 2007, 21, 323–332. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; Morris, M.J. A Comparative Review of Autologous Conditioned Serum and Autologous Protein Solution for Treatment of Osteoarthritis in Horses. Front. Vet. Sci. 2021, 8, 602978. [Google Scholar] [CrossRef] [PubMed]
- Linardi, R.L.; Dodson, M.E.; Moss, K.L.; King, W.J.; Ortved, K.F. The Effect of Autologous Protein Solution on the Inflammatory Cascade in Stimulated Equine Chondrocytes. Front. Vet. Sci. 2019, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Romero, A.; Zaragoza, P.; Rodellar, C.; Vázquez, F.J. Practical Considerations for Clinical Use of Mesenchymal Stem Cells: From the Laboratory to the Horse. Vet. J. 2018, 238, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gittel, C.; Brehm, W.; Burk, J.; Juelke, H.; Staszyk, C.; Ribitsch, I. Isolation of Equine Multipotent Mesenchymal Stromal Cells by Enzymatic Tissue Digestion or Explant Technique: Comparison of Cellular Properties. BMC Vet. Res. 2013, 9, 22. [Google Scholar] [CrossRef][Green Version]
- Smith, R.K.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and Implantation of Autologous Equine Mesenchymal Stem Cells from Bone Marrow into the Superficial Digital Flexor Tendon as a Potential Novel Treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef]
- Barker, W.H.J.; Smith, M.R.W.; Minshall, G.J.; Wright, I.M. Soft tissue injuries of the tarsocrural joint: A retrospective analysis of 30 cases evaluated arthroscopically. Equine Vet. J. 2013, 45, 435–441. [Google Scholar] [CrossRef]
- Nicpoń, J.; Marycz, K.; Grzesiak, J. Therapeutic Effect of Adipose-Derived Mesenchymal Stem Cell Injection in Horses Suffering from Bone Spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef]
- Kearney, C.M.; Khatab, S.; van Buul, G.M.; Plomp, S.G.M.; Korthagen, N.M.; Labberté, M.C.; Goodrich, L.R.; Kisiday, J.D.; Van Weeren, P.R.; van Osch, G.J.V.M.; et al. Treatment Effects of Intra-Articular Allogenic Mesenchymal Stem Cell Secretome in an Equine Model of Joint Inflammation. Front. Vet. Sci. 2022, 9, 907616. [Google Scholar] [CrossRef]
- Lavoie, J.R.; Rosu-Myles, M. Uncovering the Secretes of Mesenchymal Stem Cells. Biochimie 2013, 95, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 1–9. [Google Scholar] [CrossRef]
- Mayet, A.; Zablotski, Y.; Roth, S.P.; Brehm, W.; Troillet, A. Systematic Review and Meta-Analysis of Positive Long-Term Effects after Intra-Articular Administration of Orthobiologic Therapeutics in Horses with Naturally Occurring Osteoarthritis. Front. Vet. Sci. 2023, 10, 1125695. [Google Scholar] [CrossRef] [PubMed]
- Camargo Garbin, L.; Lopez, C.; Carmona, J.U. A Critical Overview of the Use of Platelet-Rich Plasma in Equine Medicine Over the Last Decade. Front. Vet. Sci. 2021, 8, 641818. [Google Scholar] [CrossRef]
- Scala, M.; Lenarduzzi, S.; Spagnolo, F.; Trapasso, M.; Ottonello, C.; Muraglia, A.; Barla, A.; Strada, P. Regenerative Medicine for the Treatment of Teno-Desmic Injuries of the Equine. A Series of 150 Horses Treated with Platelet-Derived Growth Factors. Vivo 2014, 28, 1119–1124. [Google Scholar]
- Yamada, A.L.M.; Alvarenga, M.L.; Brandão, J.S.; Watanabe, M.J.; Rodrigues, C.A.; Hussni, C.A.; Alves, A.L.G. Arcabouço de PRP-Gel Associado a Células Tronco Mesenquimais: Uso Em Lesões Condrais Em Modelo Experimental Equino. Pesqui. Vet. Bras. 2016, 36, 461–467. [Google Scholar] [CrossRef][Green Version]
- Beerts, C.; Suls, M.; Broeckx, S.Y.; Seys, B.; Vandenberghe, A.; Declercq, J.; Duchateau, L.; Vidal, M.A.; Spaas, J.H. Tenogenically Induced Allogeneic Peripheral Blood Mesenchymal Stem Cells in Allogeneic Platelet-Rich Plasma: 2-Year Follow-up after Tendon or Ligament Treatment in Horses. Front. Vet. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Georg, R.; Maria, C.; Gisela, A.; Bianca, C. Autologous Conditioned Plasma as Therapy of Tendon and Ligament Lesions in Seven Horses. J. Vet. Sci. 2010, 11, 173–175. [Google Scholar] [CrossRef]
- Geburek, F.; Lietzau, M.; Beineke, A.; Rohn, K.; Stadler, P.M. Effect of a Single Injection of Autologous Conditioned Serum (ACS) on Tendon Healing in Equine Naturally Occurring Tendinopathies. Stem Cell Res. Ther. 2015, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Marques-Smith, P.; Kallerud, A.S.; Johansen, G.M.; Boysen, P.; Jacobsen, A.M.; Reitan, K.M.; Henriksen, M.M.; Löfgren, M.; Fjordbakk, C.T. Is Clinical Effect of Autologous Conditioned Serum in Spontaneously Occurring Equine Articular Lameness Related to ACS Cytokine Profile? BMC Vet. Res. 2020, 16, 181. [Google Scholar] [CrossRef]
- Mokbel, A.N.; El Tookhy, O.S.; Shamaa, A.A.; Rashed, L.A.; Sabry, D.; El Sayed, A.M. Homing and Reparative Effect of Intra-Articular Injection of Autologus Mesenchymal Stem Cells in Osteoarthritic Animal Model. BMC Musculoskelet. Disord. 2011, 12, 259. [Google Scholar] [CrossRef]
- Carvalho, A.D.M.; Badial, P.R.; Álvarez, L.E.C.; Yamada, A.L.M.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Garcia Alves, A.L. Equine Tendonitis Therapy Using Mesenchymal Stem Cells and Platelet Concentrates: A Randomized Controlled Trial. Stem Cell Res. Ther. 2013, 4, 85. [Google Scholar] [CrossRef]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.; Zimmerman, M.; Crocetti, S.; Suls, M.; Mariën, T.; Ferguson, S.J.; Chiers, K.; Duchateau, L.; Franco-Obregón, A.; Wuertz, K.; et al. Regenerative Therapies for Equine Degenerative Joint Disease: A Preliminary Study. PLoS ONE 2014, 9, e85917. [Google Scholar] [CrossRef]
- González-Fernández, M.L.; Pérez-Castrillo, S.; Sánchez-Lázaro, J.A.; Prieto-Fernández, J.G.; López-González, M.E.; Lobato-Pérez, S.; Colaço, B.J.; Olivera, E.R.; Villar-Suárez, V. Assessment of Regeneration in Meniscal Lesions by Use of Mesenchymal Stem Cells Derived from Equine Bone Marrow and Adipose Tissue. Am. J. Vet. Res. 2016, 77, 779–788. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Hermida-Prieto, M.; Fernández, V.; Fariñas, F.; Alcoholado, C.; Isabel Rodríguez-García, M.; Mariñas-Pardo, L.; Becerra, J. Allogeneic Adipose-Derived Mesenchymal Stem Cells (Horse Allo 20) for the Treatment of Osteoarthritis-Associated Lameness in Horses: Characterization, Safety, and Efficacy of Intra-Articular Treatment. Stem Cells Dev. 2018, 27, 1147–1160. [Google Scholar] [CrossRef]
- Xing, D.; Kwong, J.; Yang, Z.; Hou, Y.; Zhang, W.; Ma, B.; Lin, J. Intra-Articular Injection of Mesenchymal Stem Cells in Treating Knee Osteoarthritis: A Systematic Review of Animal Studies. Osteoarthr. Cartil. 2018, 26, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem Cells: Insights into the Secretome. Biochim. Biophys. Acta 2013, 1834, 2380–2384. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Turrubiarte, M.; Baratta, M.; Ponti, G.; Chiaradia, E.; Martignani, E. Extracellular Vesicles from Equine Mesenchymal Stem Cells Decrease Inflammation Markers in Chondrocytes in Vitro. Equine Vet. J. 2022, 54, 1133–1143. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Fraile, A.; González-Cubero, E.; Martínez-Flórez, S.; Olivera, E.R.; Villar-Suárez, V. Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review. Vet. Sci. 2023, 10, 666. https://doi.org/10.3390/vetsci10120666
Pérez Fraile A, González-Cubero E, Martínez-Flórez S, Olivera ER, Villar-Suárez V. Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review. Veterinary Sciences. 2023; 10(12):666. https://doi.org/10.3390/vetsci10120666
Chicago/Turabian StylePérez Fraile, Andrea, Elsa González-Cubero, Susana Martínez-Flórez, Elías R. Olivera, and Vega Villar-Suárez. 2023. "Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review" Veterinary Sciences 10, no. 12: 666. https://doi.org/10.3390/vetsci10120666
APA StylePérez Fraile, A., González-Cubero, E., Martínez-Flórez, S., Olivera, E. R., & Villar-Suárez, V. (2023). Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review. Veterinary Sciences, 10(12), 666. https://doi.org/10.3390/vetsci10120666








