Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Animals
Inclusion Criteria
2.2. Methods
2.2.1. Sample Collection
Endometrial Swab and Cytology Brush
Uterine Low-Volume Lavage
Endometrial Biopsy
Negative Control
2.3. DNA Extraction, Sequencing and Metagenomic Analyses
3. Results
3.1. Sequencing Results
3.2. Alpha Diversity
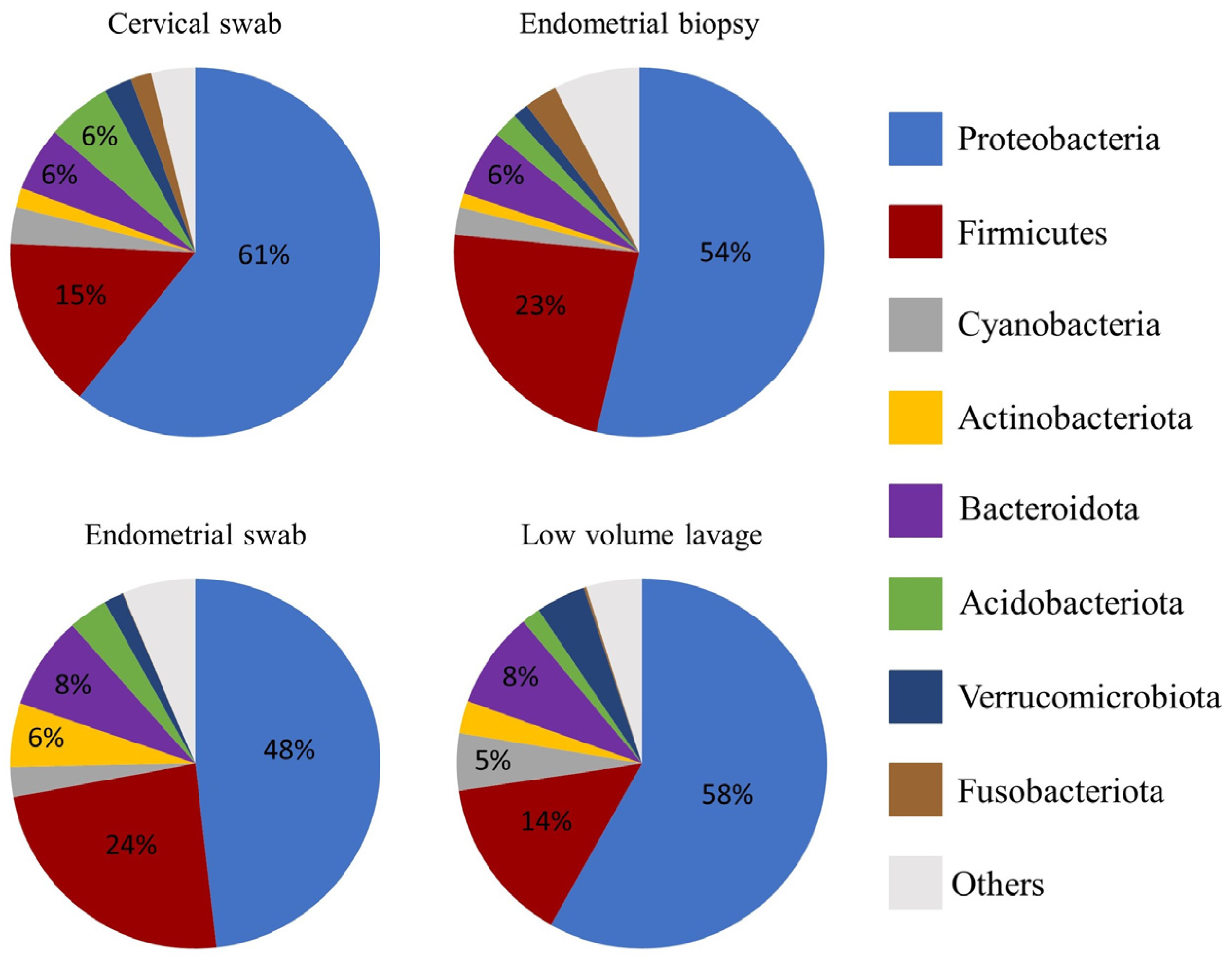
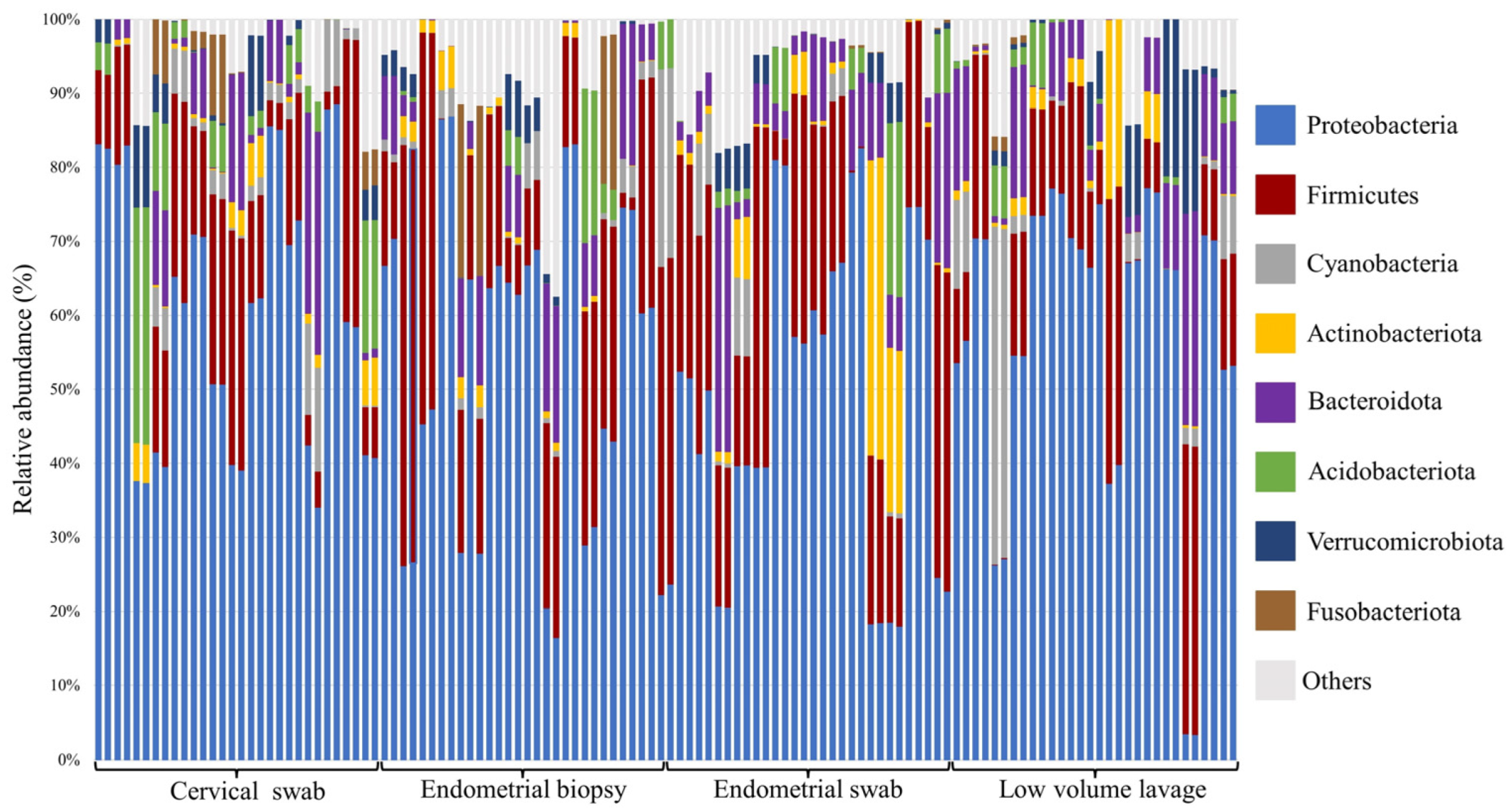
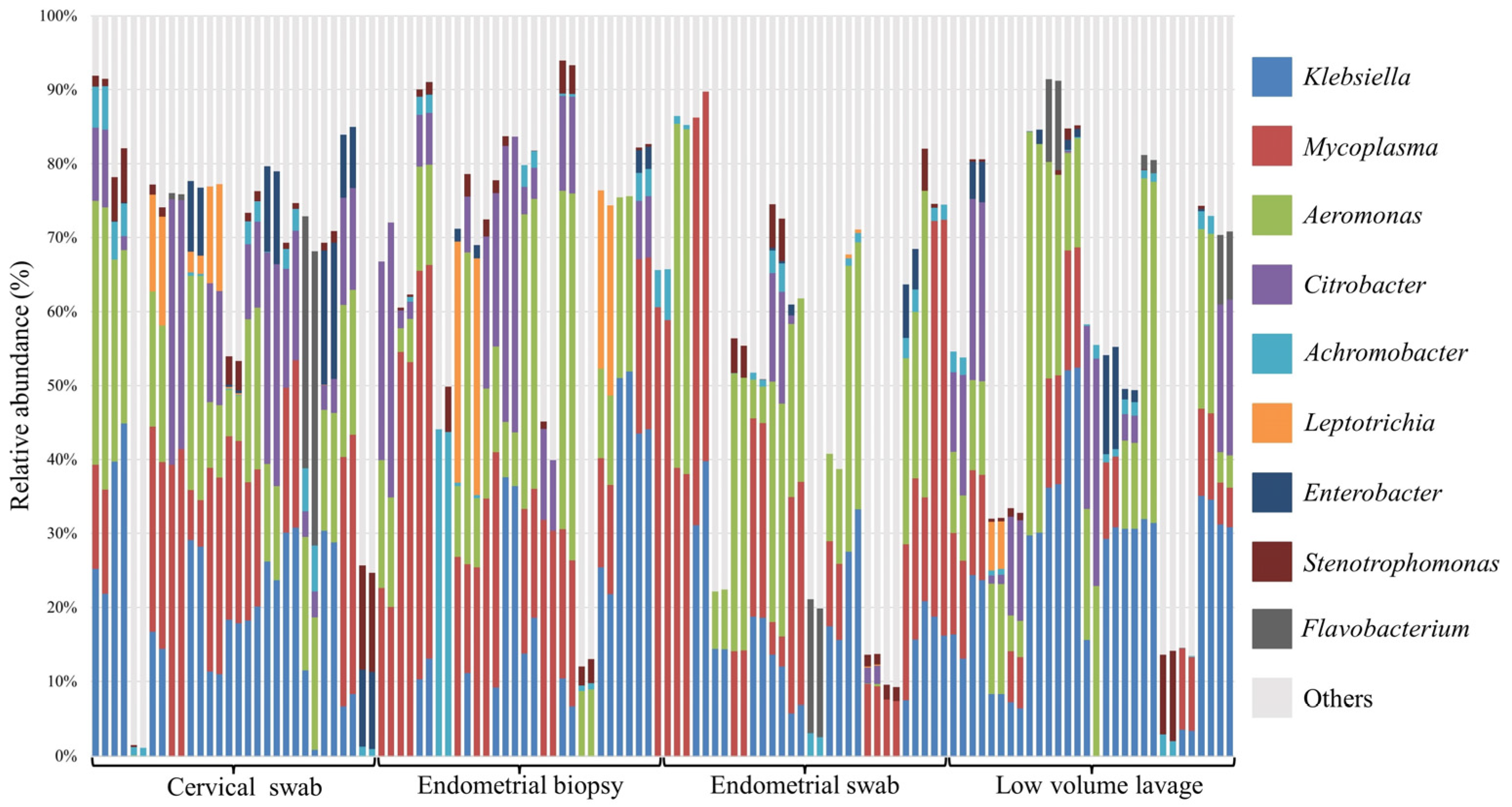
3.3. Relative Abundance at Phyla and Genus Level
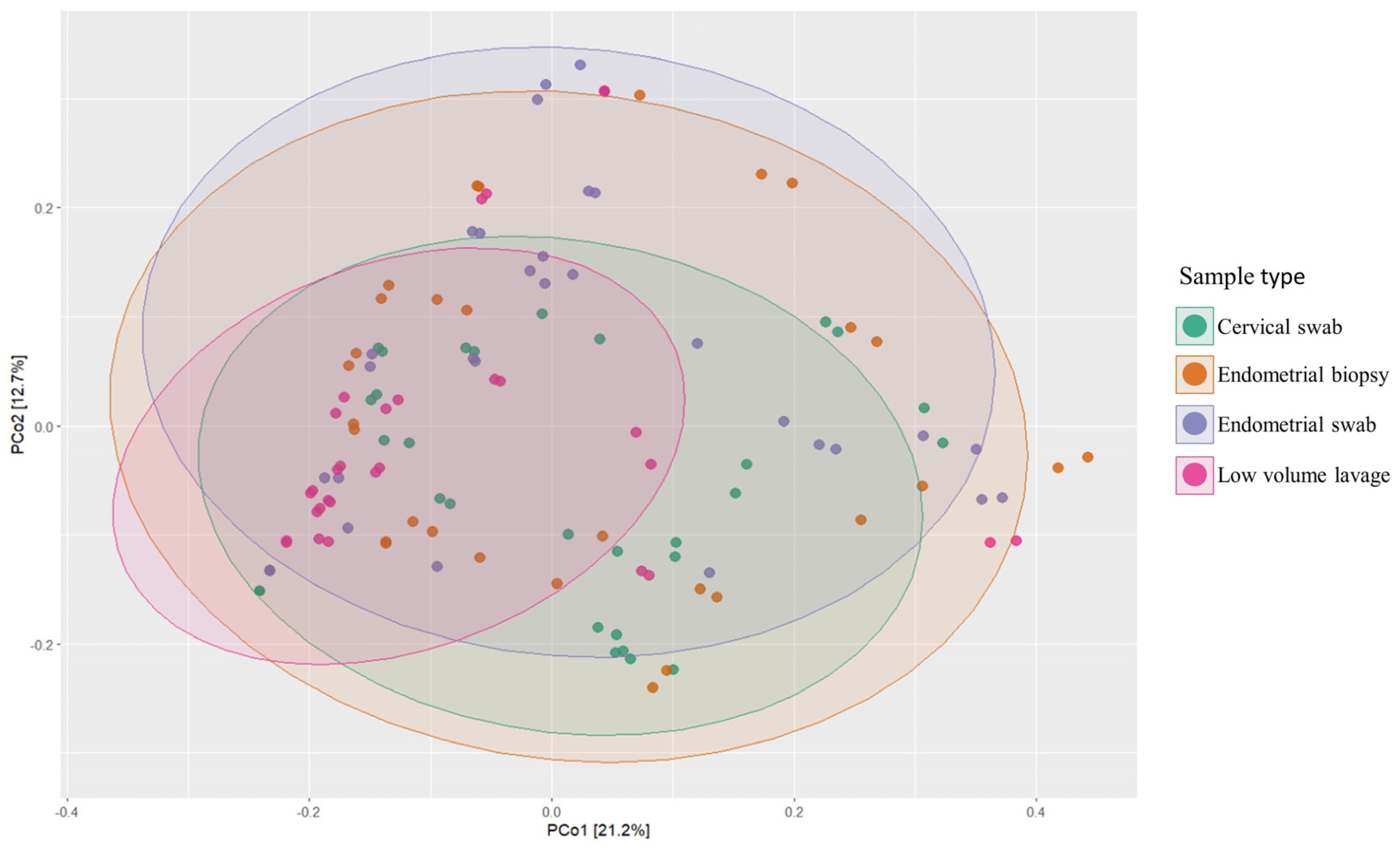
3.4. Analysis of Similarity (ANOSIM)
3.5. Beta Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Loncar, K.D.; Hennet, M.L.; Borlee, B.R. In Vitro Efficacy of Nonantibiotic Treatments on Biofilm Disruption of Gram-Negative Pathogens and an In Vivo Model of Infectious Endometritis Utilizing Isolates from the Equine Uterus. J. Clin. Microbiol. 2016, 54, 631–639. [Google Scholar] [CrossRef]
- LeBlanc, M.; Causey, R. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.H.; McCue, P.M.; Aurich, C. Equine Endometritis: A Review of Challenges and New Approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T. Breeding-Induced Endometritis in Mares. Vet. Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Troedsson, M.H. Equine Breeding-Induced Endometritis: A Review. J. Equine Vet. Sci. 2013, 33, 673–682. [Google Scholar] [CrossRef]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of Three Diagnostic Methods to Identify Subclinical Endometritis in Mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef]
- Ball, B.A.; Shin, S.J.; Patten, V.H.; Lein, D.H.; Woods, G.L. Use of a low-volume uterine flush for micropiologic and cytologic examination of the mare’s endometrium. Theriogenology 1988, 29, 1269–1283. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a Low-Volume Uterine Flush for Diagnosing Endometritis in Chronically Infertile Mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef]
- Santos, T.M.; Bicalho, R.C. Diversity and Succession of Bacterial Communities in the Uterine Fluid of Postpartum Metritic, Endometritic and Healthy Dairy Cows. PLoS ONE 2012, 7, e53048. [Google Scholar] [CrossRef]
- Green, K.A.; Zarek, S.M.; Catherino, W.H. Gynecologic Health and Disease in Relation to the Microbiome of the Female Reproductive Tract. Fertil. Steril. 2015, 104, 1351–1357. [Google Scholar] [CrossRef]
- Moreno, I.; Franasiak, J.M. Endometrial Microbiota—New Player in Town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Chapwanya, A. A Review of the Diversity of the Genital Tract Microbiome and Implications for Fertility of Cattle. Animals 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the Mammalian Female Reproductive Tract Microbiome in Pregnancy Outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Hambidge, M.; Firmanes, B.; Shabandri, A.M.; Wilsher, S. Bacteria Isolated From Equine Uteri in The United Arab Emirates: A Retrospective Study. J. Equine Vet. Sci. 2022, 115, 104029. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of Uterine Microbiota in Postpartum Dairy Cows with Clinical or Subclinical Endometritis. Sci. Rep. 2020, 10, 12353. [Google Scholar] [CrossRef]
- Holyoak, G.R. The Equine Endometrial Microbiome: A Brief Review. Am. J. Biomed. Sci. Res. 2021, 11, 532–534. [Google Scholar] [CrossRef]
- van Heule, M.; Monteiro, H.F.; Bazzazan, A.; Scoggin, K.; Rolston, M.; El-Sheikh Ali, H.; Weimer, B.C.; Ball, B.; Daels, P.; Dini, P. Characterization of the Equine Placental Microbial Population in Healthy Pregnancies. Theriogenology 2023, 206, 60–70. [Google Scholar] [CrossRef]
- Beckers, K.F.; Sones, J.L. Maternal Microbiome and the Hypertensive Disorder of Pregnancy, Preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1–H10. [Google Scholar] [CrossRef]
- Fox, G.E.; Stackebrandt, E.; Hespell, R.B.; Gibson, J.; Maniloff, J.; Dyer, T.A.; Wolfe, R.S.; Balch, W.E.; Tanner, R.S.; Magrum, L.J.; et al. The Phylogeny of Prokaryotes. Science 1980, 209, 457–463. [Google Scholar] [CrossRef]
- Kolbert, C.P.; Persing, D.H. Ribosomal DNA Sequencing as a Tool for Identification of Bacterial Pathogens. Curr. Opin. Microbiol. 1999, 2, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.S.; Love, B.C.; DeSilva, U.; Rezabek, G.B.; Meijer, W.G.; Carrington, S.D.; Holyoak, G.R. Detectable Differences in the Endometrial Microbiome between Normal and Susceptible Mares Using Metagenomic Profiling and Conventional Bacterial Culture. Clin. Theriogenol. 2011, 3, 376. [Google Scholar]
- Quinn, P.J.; Carter, M.E.; Markey, B.; Carter, G.R. Bacterial Pathogens: Microscopy, Culture and Identification. Clin. Vet. Microbiol. 1994, 20, 60. [Google Scholar]
- Ferris, R.A.; Bohn, A.; McCue, P.M. Equine Endometrial Cytology: Collection Techniques and Interpretation. Equine Vet. Educ. 2015, 27, 316–322. [Google Scholar] [CrossRef]
- Katila, T. Evaluation of Diagnostic Methods in Equine Endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Haugaard, M.M.; Petersen, M.R.; Nielsen, J.M.; Pedersen, H.G.; Bojesen, A.M. Streptococcus Equi Subsp. Zooepidemicus Isolates from Equine Infectious Endometritis Belong to a Distinct Genetic Group. Vet. Res. 2013, 44, 26. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- McKnight, D.T.; Huerlimann, R.; Bower, D.S.; Schwarzkopf, L.; Alford, R.A.; Zenger, K.R. MicroDecon: A Highly Accurate Read-Subtraction Tool for the Post-Sequencing Removal of Contamination in Metabarcoding Studies. Environ. DNA 2019, 1, 14–25. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An Easy-to-Use, Open-Source, Reproducible, and Community-Based Pipeline for Amplicon Data Analysis in Microbiome Research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 6 September 2023).
- Holyoak, G.R.; Premathilake, H.U.; Lyman, C.C.; Sones, J.L.; Gunn, A.; Wieneke, X.; DeSilva, U. The Healthy Equine Uterus Harbors a Distinct Core Microbiome plus a Rich and Diverse Microbiome That Varies with Geographical Location. Sci. Rep. 2022, 12, 14790. [Google Scholar] [CrossRef]
- Petersen, M.R.; Skive, B.; Christoffersen, M.; Lu, K.; Nielsen, J.M.; Troedsson, M.H.T.; Bojesen, A.M. Activation of Persistent Streptococcus Equi Subspecies Zooepidemicus in Mares with Subclinical Endometritis. Vet. Microbiol. 2015, 179, 119–125. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Endometritis. In Equine Reproduction; John Wiley & Sons: Chichester, UK, 2011; p. 2608. [Google Scholar]
- Punzón-Jiménez, P.; Labarta, E. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Barba, M.; Martínez-Boví, R.; Quereda, J.J.; Mocé, M.L.; Plaza-Dávila, M.; Jiménez-Trigos, E.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal Microbiota Is Stable throughout the Estrous Cycle in Arabian Mares. Animals 2020, 10, 2020. [Google Scholar] [CrossRef]


| Sample Method 1 | Sample Method 2 | p-Value |
|---|---|---|
| Endometrial biopsy | Endometrial swab | 0.016 |
| Endometrial biopsy | LVL | 0.001 |
| Endometrial swab | LVL | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heil, B.A.; van Heule, M.; Thompson, S.K.; Kearns, T.A.; Oberhaus, E.L.; King, G.; Daels, P.; Dini, P.; Sones, J.L. Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus. Vet. Sci. 2023, 10, 644. https://doi.org/10.3390/vetsci10110644
Heil BA, van Heule M, Thompson SK, Kearns TA, Oberhaus EL, King G, Daels P, Dini P, Sones JL. Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus. Veterinary Sciences. 2023; 10(11):644. https://doi.org/10.3390/vetsci10110644
Chicago/Turabian StyleHeil, B. A., M. van Heule, S. K. Thompson, T. A. Kearns, E. L. Oberhaus, G. King, P. Daels, P. Dini, and J. L. Sones. 2023. "Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus" Veterinary Sciences 10, no. 11: 644. https://doi.org/10.3390/vetsci10110644
APA StyleHeil, B. A., van Heule, M., Thompson, S. K., Kearns, T. A., Oberhaus, E. L., King, G., Daels, P., Dini, P., & Sones, J. L. (2023). Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus. Veterinary Sciences, 10(11), 644. https://doi.org/10.3390/vetsci10110644








