Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
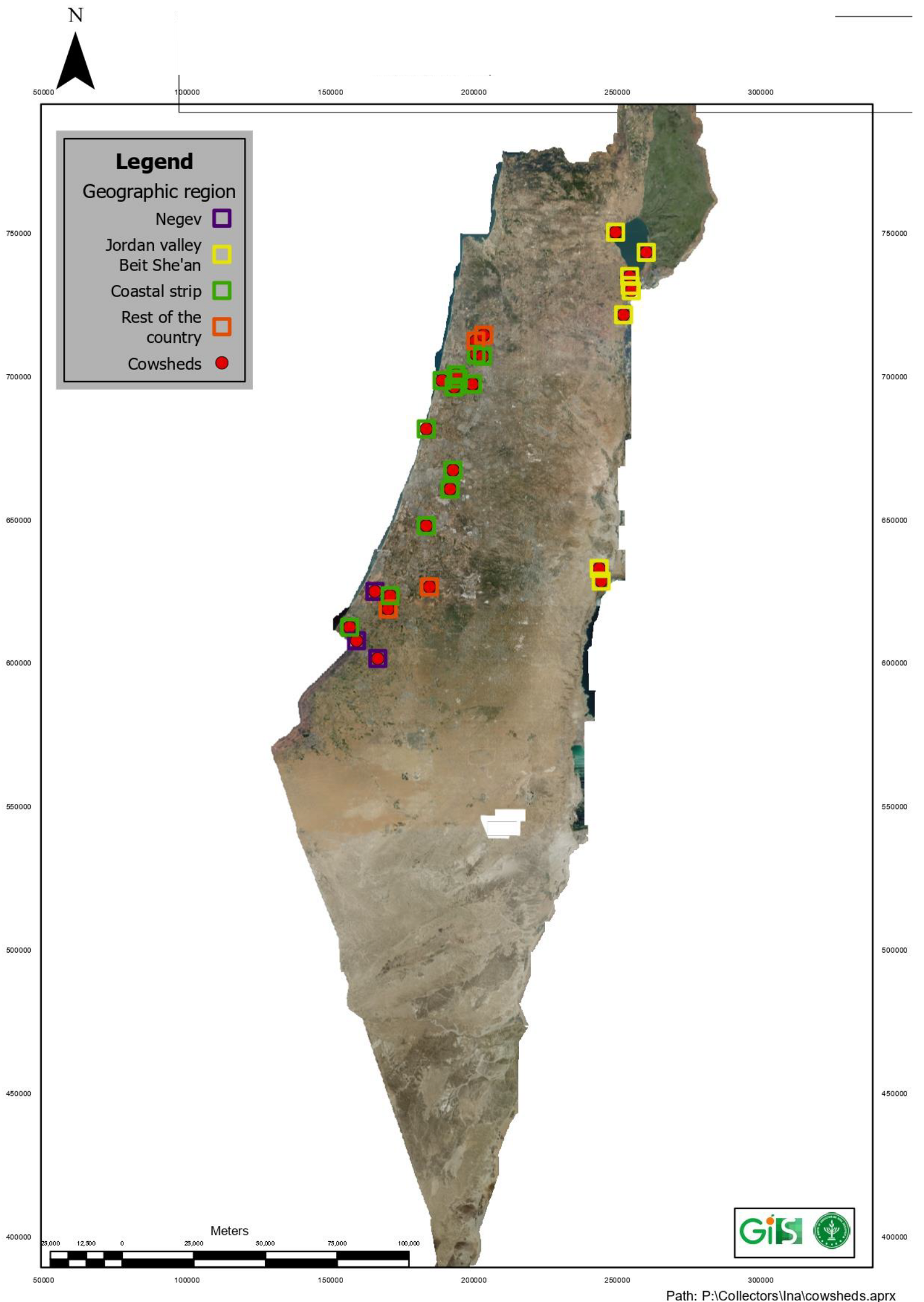
2.1. Infected Herds and Outbreak Distribution Countrywide
2.2. Economic Effect in Subsets of Highly Infected Dairy Farms
2.3. Economic Evaluations
3. Results
3.1. The 2021 BEF Outbreak
3.2. Economic Losses in Affected Herds
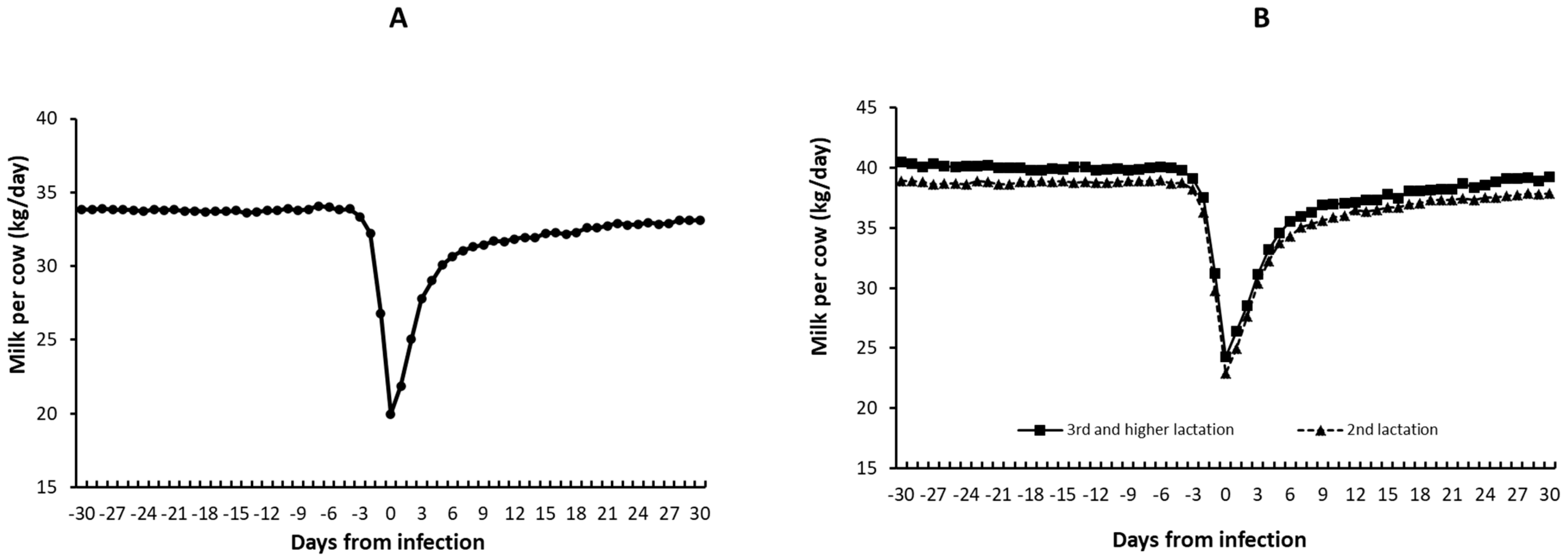
3.2.1. Milk Production
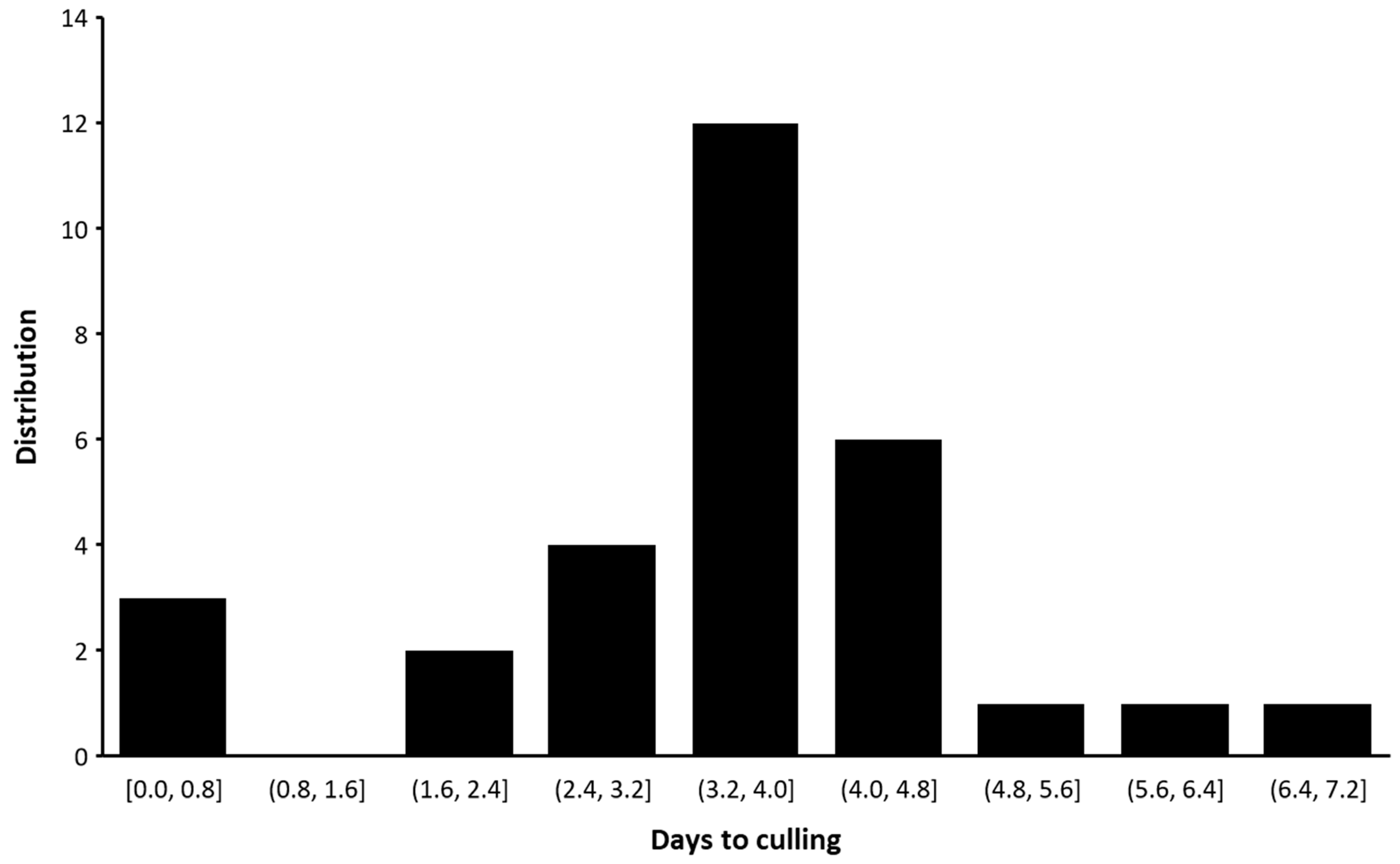
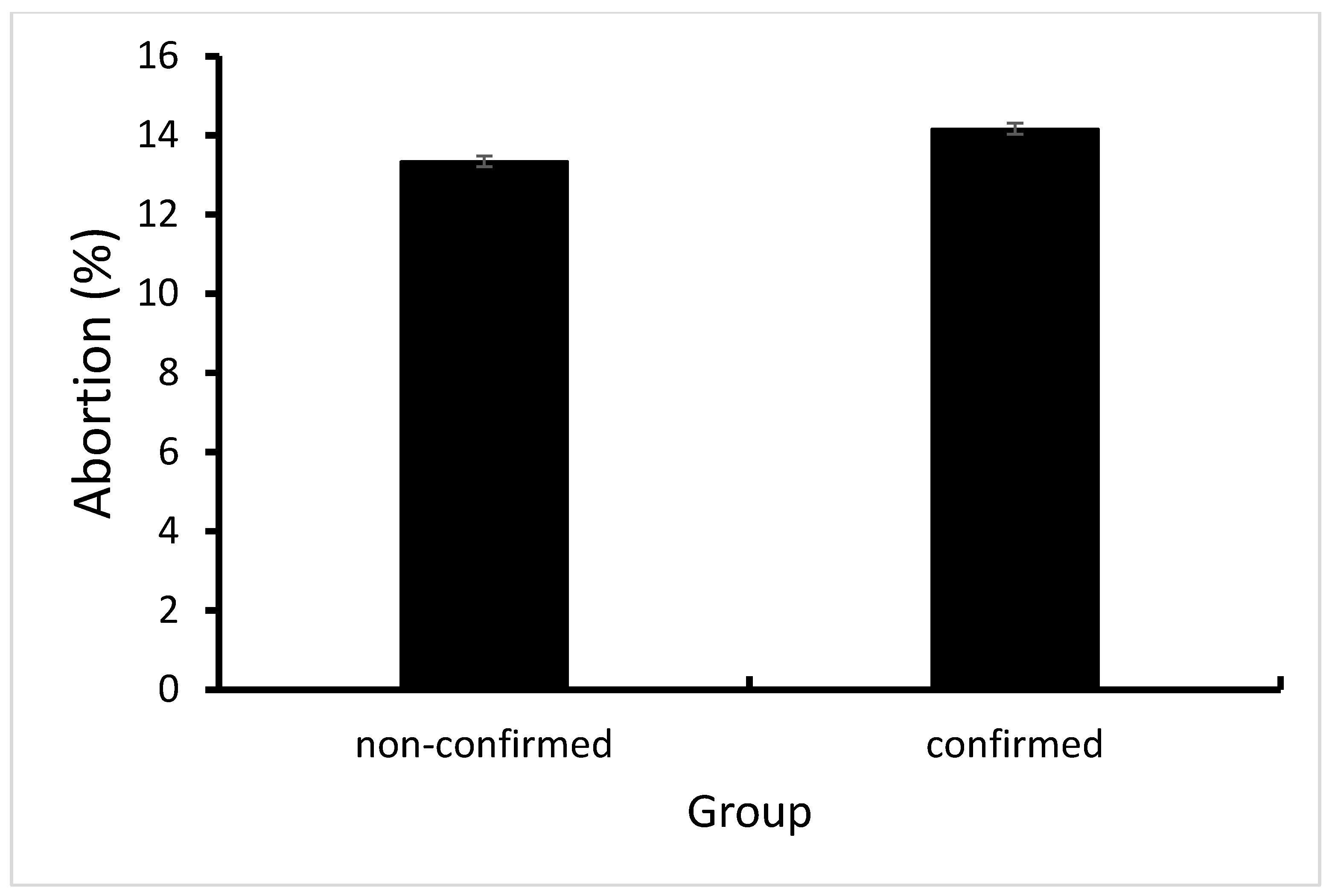
3.2.2. Culling Rates and Abortion Rates
4. Discussion
4.1. Characteristics of the 2021 BEF Outbreak
4.2. Reduction in Milk Production
4.3. Culling Rates
4.4. Abortion Rates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Della-Porta, A.J.; Brown, F. The physico-chemical characterization of bovine ephemeral fever virus as a member of the family Rhabdoviridae. J. Gen. Virol. 1979, 44, 99–112. [Google Scholar] [CrossRef]
- Erster, O.; Stram, R.; Menasherow, S.; Rubistein-Giuni, M.; Sharir, B.; Kchinich, E.; Stram, Y. High-resolution melting (HRM) for genotyping bovine ephemeral fever virus (BEFV). Virus Res. 2017, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J. Bovine ephemeral fever in Australia and the world. World Rhabdoviruses 2005, 292, 57–80. [Google Scholar]
- Stokes, J.E.; Darpel, K.E.; Gubbins, S.; Carpenter, S.; Marco, M.d.M.F.d.; Hernández-Triana, L.M.; Fooks, A.R.; Johnson, N.; Sanders, C. Investigation of bovine ephemeral fever virus transmission by putative dipteran vectors under experimental conditions. Parasites Vectors 2020, 13, 597. [Google Scholar] [CrossRef]
- Chizov-Ginzburg, A.; Stram, Y.; Rot, A.; Taub-Umansky, L.; Izhaki, O.; Behar, A. Stretching the wings further- susceptibility of Culex pipiens Linnaeus to bovine ephemeral fever virus infection under experimental conditions. Acta Trop. 2023, 246, 106995. [Google Scholar] [CrossRef]
- Venter, G.J.; Hamblin, C.; Paweska, J.T. Determination of the oral susceptibility of South African livestock-associated biting midges, Culicoides species, to bovine ephemeral fever virus. Med. Vet. Entomol. 2003, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- St. George, T.D. Bovine ephemeral fever virus. In Virus Infections of Ruminants: Virus Infections of Vertebrates Series; Dinter, Z., Morein, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 3, pp. 405–415. [Google Scholar]
- Nandi, S.; Negi, B.S. Bovine ephemeral fever: A review. Comp. Immunol. Microbiol. Infect. Dis. 1999, 22, 81–91. [Google Scholar] [CrossRef]
- Kirkland, P.D. Akabane and bovine ephemeral fever virus infections. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Yeruham, I. Bovine ephemeral fever in beef cattle herds in the Jordan Valley, Israel. Vet. Rec. 2003, 152, 86–88. [Google Scholar] [CrossRef]
- Yeruham, I.; Van-Ham, M.; Bar, D.; Yadin, H.; Tiomkin, D. Bovine Ephemeral Fever in dairy cattle herdseconomic aspects of 1999 outbreak in the Jordan Valley. Vet. Rec. 2003, 153, 180–182. [Google Scholar] [CrossRef]
- Lancelot, R.; Béral, M.; Rakotoharinome, V.M.; Andriamandimby, S.-F.; Héraud, J.-M.; Coste, C.; Apolloni, A.; Squarzoni-Diaw, C.; de La Rocque, S.; Formenty, P.B.H.; et al. Drivers of Rift Valley fever epidemics in Madagascar. Proc. Natl. Acad. Sci. USA 2017, 114, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Yasur-Landau, D.; Leszkowicz-Mazuz, M. Vector-Borne Diseases in Ruminants. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Èntomol. 2000, 45, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of temperate and tropical Culicoides midges: Knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu. Rev. Èntomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Walker, P.J.; Klement, E. Epidemiology and control of bovine ephemeral fever. Vet. Res. 2015, 46, 124. [Google Scholar] [CrossRef]
- Fofana, A.; Toma, L.; Moran, D.; Gunn, G.J.; Gubbins, S.; Szmaragd, C.; Stott, A.W. An ex-ante economic appraisal of Bluetongue virus incursions and control strategies. J. Agric. Sci. 2015, 154, 118–135. [Google Scholar] [CrossRef]
- Martinelle, L.; Dal Pozzo, F.; Gauthier, B.; Kirschvink, N.; Saegerman, C. Field Veterinary Survey on Clinical and Economic Impact of S chmallenberg Virus in B elgium. Transbound. Emerg. Dis. 2014, 61, 285–288. [Google Scholar] [CrossRef]
- Behar, A.; Friedgut, O.; Rotenberg, D.; Zalesky, O.; Izhaki, O.; Yulzary, A.; Rot, A.; Wolkomirsky, R.; Zamir, L.; Hmd, F. Insights on Transmission, Spread, and Possible Endemization of Selected Arboviruses in Israel—Interim Results from Five-Year Surveillance. Vet. Sci. 2022, 9, 65. [Google Scholar] [CrossRef]
- Yeruham, I.; Braverman, Y.; Yadin, H.; Chai, D.; Van Ham, M.; Tiomkin, D.; Frank, D. Epidemiological investigations of outbreaks of bovine ephemeral fever in Israel. Vet. Rec. 2002, 151, 117–121. [Google Scholar] [CrossRef]
- Yeruham, I.; Van Ham, M.; Stram, Y.; Friedgut, O.; Yadin, H.; Mumcuoglu, K.Y.; Braverman, Y. Epidemiological Investigation of Bovine Ephemeral Fever Outbreaks in Israel. Vet. Med. Int. 2010, 2010, 290541. [Google Scholar] [CrossRef]
- Aziz-Boaron, O.; Gleser, D.; Yadin, H.; Gelman, B.; Kedmi, M.; Galon, N.; Klement, E. The protective effectiveness of an inactivated bovine ephemeral fever virus vaccine. Vet. Microbiol. 2014, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stram, Y.; Kuznetzova, L.; Levin, A.; Yadin, H.; Rubinstein-Giuni, M. A real-time RT-quantative(q)PCR for the detection of bovine ephemeral fever virus. J. Virol. Methods 2005, 130, 1–6. [Google Scholar] [CrossRef] [PubMed]
- St. George, T.D. Bovine ephemeral fever. In Infectious Diseases of Livestock, 2nd ed.; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 2004; pp. 1183–1193. [Google Scholar]
- Flamenbaum, I.; Galon, N. Management of heat stress to improve fertility in dairy cows in Israel. J. Reprod. Dev. 2010, 56, S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Beer, M. Re-circulation of Schmallenberg virus, Germany. Transbound. Emerg. Dis. 2019, 67, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Murota, K.; Hayama, Y. Endemic and Emerging Arboviruses in Domestic Ruminants in East Asia. Front. Vet. Sci. 2020, 7, 168. [Google Scholar] [CrossRef]
- Morgenstern, M.; Sok, J.; Klement, E. Perception of low social pressure and lack of capacity reduces vaccination compliance–The case of lumpy skin disease. Transbound. Emerg. Dis. 2022, 69, E2779–E2788. [Google Scholar] [CrossRef]
- Gleser, D.; Spinner, K.; Klement, E. Effectiveness of the strain 919 bovine ephemeral fever virus vaccine in the face of a real-world outbreak: A field study in Israeli dairy herds. Vaccine 2023, 41, 5126–5133. [Google Scholar] [CrossRef]

| Farm | Average Number of Cows on the Farm during the BEF Outbreak | Number of Cows that Were Clinically Diagnosed with BEF during the Outbreak | (% Morbidity) | Number of Cows Culled within Ten Days of Diagnosis | Culling Rates (%) of BEF-Diagnosed Cows | Average Number of Days from Clinical Diagnosis to Culling |
|---|---|---|---|---|---|---|
| 1 | 687 | 69 | 10.0% | 11 | 15.9% | 3.8 |
| 2 | 329 | 44 | 13.4% | 2 | 4.5% | 3.0 |
| 3 | 735 | 99 | 13.5% | 3 | 3.0% | 4.7 |
| 4 | 354 | 49 | 13.8% | 0 | 0.0% | N/A |
| 5 | 422 | 60 | 14.2% | 8 | 13.3% | 3.6 |
| 6 | 353 | 55 | 15.6% | 3 | 5.5% | 4.3 |
| 7 | 295 | 51 | 17.3% | 0 | 0.0% | N/A |
| 8 | 412 | 78 | 18.9% | 6 | 7.7% | 3.3 |
| 9 | 302 | 77 | 25.5% | 8 | 10.4% | 2.3 |
| 10 | 321 | 87 | 27.1% | 4 | 4.6% | 4.0 |
| 11 | 308 | 85 | 27.6% | 2 | 2.4% | 3.0 |
| 12 | 322 | 89 | 27.6% | 8 | 9.0% | 4.0 |
| 13 | 411 | 123 | 29.9% | 7 | 5.7% | 4.0 |
| 14 | 678 | 221 | 32.6% | 13 | 5.9% | 4.8 |
| 15 | 406 | 133 | 32.8% | 5 | 3.8% | 2.2 |
| 16 | 176 | 64 | 36.4% | 1 | 1.6% | 3.0 |
| 17 | 407 | 148 | 36.4% | 3 | 2.0% | 3.3 |
| 18 | 320 | 121 | 37.8% | 3 | 2.5% | 4.0 |
| 19 | 364 | 144 | 39.6% | 8 | 5.6% | 3.1 |
| 20 | 586 | 245 | 41.8% | 11 | 4.5% | 4.1 |
| 21 | 950 | 444 | 46.7% | 22 | 5.0% | 3.5 |
| 22 | 356 | 168 | 47.2% | 2 | 1.2% | 7.0 |
| 23 | 340 | 180 | 52.9% | 3 | 1.7% | 3.7 |
| 24 | 415 | 223 | 53.7% | 3 | 1.3% | 5.7 |
| 25 | 100 | 59 | 59.0% | 3 | 5.1% | 0.7 |
| 26 | 626 | 401 | 64.1% | 20 | 5.0% | 3.4 |
| 27 | 943 | 627 | 66.5% | 52 | 8.3% | 3.3 |
| 28 | 334 | 266 | 79.6% | 11 | 4.1% | 4.2 |
| 29 | 909 | 746 | 82.1% | 25 | 3.4% | 4.6 |
| 30 | 237 | 215 | 90.7% | 4 | 1.9% | 5.5 |
| Lactation Number | Losses during Infection (9 Days) (USD per Cow) | Losses in the Month after Infection (USD per Cow) |
|---|---|---|
| 1st | 90 | 300 |
| 2nd | 100 | 334 |
| 3rd and higher | 93 | 311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavon, Y.; Ezra, E.; Friedgut, O.; Behar, A. Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds. Vet. Sci. 2023, 10, 645. https://doi.org/10.3390/vetsci10110645
Lavon Y, Ezra E, Friedgut O, Behar A. Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds. Veterinary Sciences. 2023; 10(11):645. https://doi.org/10.3390/vetsci10110645
Chicago/Turabian StyleLavon, Yaniv, Ephraim Ezra, Orly Friedgut, and Adi Behar. 2023. "Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds" Veterinary Sciences 10, no. 11: 645. https://doi.org/10.3390/vetsci10110645
APA StyleLavon, Y., Ezra, E., Friedgut, O., & Behar, A. (2023). Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds. Veterinary Sciences, 10(11), 645. https://doi.org/10.3390/vetsci10110645





