Galectins in Equine Placental Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use and Tissue Collection
2.2. RNA Isolation
2.3. RNA Sequencing
2.4. Statistics
3. Results
3.1. Galectin Expression in Ascending Placentitis
3.2. Galectin Expression in Focal Mucoid Placentitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinovich, G.A.; Toscano, M.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Baum, L.G. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology 2002, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Ilarregui, J.M.; Tometten, M.; Garcia, M.; Orsal, A.S.; Cordo-Russo, R.; Toscano, M.A.; Bianco, G.A.; Kobelt, P.; Handjiski, B.; et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007, 13, 1450–1457. [Google Scholar] [CrossRef]
- Kolundžić, N.; Bojić-Trbojević, Ž.; Kovačević, T.; Stefanoska, I.; Kadoya, T.; Vićovac, L. Galectin-1 Is Part of Human Trophoblast Invasion Machinery—A Functional Study In Vitro. PLoS ONE 2011, 6, e28514. [Google Scholar] [CrossRef]
- Kolundžić, N.; Bojić-Trbojević, Ž.; Radojčić, L.; Petronijević, M.; Vićovac, L. Galectin-8 is expressed by villous and extravillous trophoblast of the human placenta. Placenta 2011, 32, 909–911. [Google Scholar] [CrossRef]
- Hirota, Y.; Burnum, K.E.; Acar, N.; Rabinovich, G.A.; Daikoku, T.; Dey, S.K. Galectin-1 Markedly Reduces the Incidence of Resorptions in Mice Missing Immunophilin FKBP52. Endocrinology 2012, 153, 2486–2493. [Google Scholar] [CrossRef]
- Alese, O.M.; Moodley, J.; Naicker, T. The role of Galectin-1 in HIV associated preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 138–144. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern. Fetal Neonatal Med. 2019, 34, 2965–2970. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Wang, L.-L.; Liu, H.; Muyayalo, K.P.; Huang, X.-B.; Mor, G.; Liao, A.-H. Galectin-9 Alleviates LPS-Induced Preeclampsia-Like Impairment in Rats via Switching Decidual Macrophage Polarization to M2 Subtype. Front. Immunol. 2018, 9, 3142. [Google Scholar] [CrossRef]
- Than, N.G.; Kim, S.S.; Abbas, A.; Han, Y.M.; Hotra, J.; Tarca, A.L.; Erez, O.; Wildman, D.E.; Kusanovic, J.P.; Pineles, B.; et al. Chorioamnionitis and Increased Galectin-1 Expression in Pprom—An Anti-Inflammatory Response in the Fetal Membranes? Am. J. Reprod. Immunol. 2008, 60, 298–311. [Google Scholar] [CrossRef]
- Stefanoska, I.; Tadić, J.; Vilotic, A.; Krivokuca, M.J.; Abu Rabi, T.; Vićovac, L. Histological chorioamnionitis in preterm prelabor rupture of the membranes is associated with increased expression of galectin-3 by amniotic epithelium. J. Matern. Fetal Neonatal Med. 2017, 30, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Turhan, U.; Sezer, S.; Kaya, S.; Dağ, I.; Tayyar, A. Maternal serum galectin-1 and galectin-3 levels in pregnancies complicated with preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2020, 33, 861–868. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, M.; Zhou, Y.; Li, F.; Yang, M.; Fan, Y.; Xie, Y.; Beejadhursing, R.; Feng, L.; Deng, D. Impaired Gal-9 Dysregulates the Pbmc-Induced Th1/Th2 Imbalance in Abortion-Prone Matings. J. Immunol. Res. 2018, 2018, 9517842. [Google Scholar] [CrossRef]
- Wu, M.; Liu, P.; Cheng, L. Galectin-1 reduction and changes in T regulatory cells may play crucial roles in patients with unexplained recurrent spontaneous abortion. Int. J. Clin. Exp. Pathol. 2015, 8, 1973–1978. [Google Scholar]
- Tirado-González, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef]
- Yalcin, I.; Taskin, S.; Pabuccu, E.G.; Soylemez, F. The Value of Placental Protein 13, Beta-Human Chorionic Gonadotropin and Progesterone in the Prediction of Miscarriages in Threatened Miscarriage Patients. J. Obstet. Gynaecol. 2015, 35, 283–286. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Ali, H.E.-S.; Troedsson, M.H.T. Galectinology of Equine Pregnancy. Animals 2023, 13, 129. [Google Scholar] [CrossRef]
- Giles, R.C.; Donahue, J.M.; Hong, C.B.; Tuttle, P.A.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Tramontin, R.R.; Smith, B.; Swerczek, T.W. Causes of abortion, stillbirth, and perinatal death in horses: 3527 cases (1986–1991). J. Am. Vet. Med. Assoc. 1993, 203, 1170–1175. [Google Scholar]
- Hong, C.B.; Donahue, J.M.; Giles, R.C., Jr.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Equine Abortion and Stillbirth in Central Kentucky during 1988 and 1989 Foaling Seasons. J. Veter- Diagn. Investig. 1993, 5, 560–566. [Google Scholar] [CrossRef]
- Ali, H.E.; Ball, B.A.; Fedorka, C.E.; Scoggin, K.E.; Schnobrich, M.R.; Erol, E.; Ruby, R.E.; Loynachan, A.T.; Smith, J.L.; Carter, C.N.; et al. Nocardioform Placentitis: A Continuing Question. In Proceedings of the American Association of Equine Practitioners, Nashville, TN, USA, 4–8 December 2021. [Google Scholar]
- Ali, H.E.-S.; Loux, S.C.; Kennedy, L.; Scoggin, K.E.; Dini, P.; Fedorka, C.E.; Kalbfleisch, T.S.; Esteller-Vico, A.; Horohov, D.W.; Erol, E.; et al. Transcriptomic analysis of equine chorioallantois reveals immune networks and molecular mechanisms involved in nocardioform placentitis. Vet. Res. 2021, 52, 103. [Google Scholar] [CrossRef]
- Canisso, I.F.; Ball, B.A.; Erol, E.; Claes, A.; Scoggin, K.E.; McDowell, K.J.; Williams, N.M.; Dorton, A.R.; Wolfsdorf, K.E.; Squires, E.L.; et al. Attempts to induce nocardioform placentitis (Crossiela equi) experimentally in mares. Equine Vet. J. 2015, 47, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, C.; Troedsson, M.; Gillis, C.; King, V.; Bodena, A. Ultrasonographic evaluation of the equine placenta by transrectal and transabdominal approach in the normal pregnant mare. Theriogenology 1997, 47, 559–573. [Google Scholar] [CrossRef]
- Leblanc, M. Ascending Placentitis in the Mare: An Update. Reprod. Domest. Anim. 2010, 45, 28–34. [Google Scholar] [CrossRef]
- El-Sheikh Ali, H.; Dini, P.; Scoggin, K.; Loux, S.; Fedorka, C.; Boakari, Y.; Norris, J.; Esteller-Vico, A.; Kalbfleisch, T.; Ball, B. Transcriptomic Analysis of Equine Placenta Reveals Key Regulators and Pathways Involved in Ascending Placentitis. Biol. Reprod. 2021, 104, 638–656. [Google Scholar] [CrossRef]
- Dobin, A.; Gingeras, T.R. Mapping Rna-Seq Reads with Star. Curr. Protoc. Bioinform. 2015, 51, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Ali, H.E.-S.; Ruby, R.E.; Scoggin, K.E.; Ball, B.A. Transcriptomic Analysis of the Chorioallantois in Equine Premature Placental Separation. Equine Vet. J. 2022. online before print. [Google Scholar] [CrossRef]
- Hong, C.B.; Donahue, J.M.; Giles, R.C.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Etiology and Pathology of Equine Placentitis. J. Vet. Diagn. Investig. 1993, 5, 56–63. [Google Scholar] [CrossRef]
- Iqbal, A.J.; Sampaio, A.L.; Maione, F.; Greco, K.V.; Niki, T.; Hirashima, M.; Perretti, M.; Cooper, D. Endogenous Galectin-1 and Acute Inflammation: Emerging Notion of a Galectin-9 Pro-Resolving Effect. Am. J. Pathol. 2011, 178, 1201–1209. [Google Scholar] [CrossRef]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, J.D.D.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3–binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef]
- Ali, H.E.-S.; Boakari, Y.L.; Loux, S.C.; Dini, P.; Scoggin, K.E.; Esteller-Vico, A.A.; Kalbfleisch, T.; Ball, B.A. Transcriptomic analysis reveals the key regulators and molecular mechanisms underlying myometrial activation during equine placentitis. Biol. Reprod. 2020, 102, 1306–1325. [Google Scholar] [CrossRef]
- Kavanaugh, D.; Kane, M.; Joshi, L.; Hickey, R.M. Detection of Galectin-3 Interaction with Commensal Bacteria. Appl. Environ. Microbiol. 2013, 79, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; He, M.; Li, J.; Zhou, Y.; Dang, J.; Li, F.; Yang, M.; Deng, D. Upregulation of the Tim-3/Gal-9 Pathway and Correlation with the Development of Preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.F.; Zuo, W.; Zhou, Y.; Hao, H.Y.; Dang, J.; Jiang, M.; He, M.Z.; Deng, D.R. Up-Regulated Expression of Tim-3/Gal-9 at Maternal-Fetal Interface in Pregnant Woman with Recurrent Spontaneous Abortion. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 586–590. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, D.; Hong, X.; Tao, Y.; Wang, S.; Xu, Y.; Piao, H.; Yin, W.; Yu, M.; et al. Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci. Signal. 2017, 10, eaah4323. [Google Scholar] [CrossRef] [PubMed]
- Burger, O.; Pick, E.; Zwickel, J.; Klayman, M.; Meiri, H.; Slotky, R.; Mandel, S.; Rabinovitch, L.; Paltieli, Y.; Admon, A.; et al. Placental protein 13 (PP-13): Effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta 2004, 25, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.M.; Nugnes, L.G.; Colombo, L.L.; Troncoso, M.F.; Fernandez, M.M.; Malchiodi, E.L.; Frahm, I.; Croci, D.O.; Compagno, D.; Rabinovich, G.A. Modulation of Endothelial Cell Migration and Angiogenesis: A Novel Function for the “Tandem-Repeat” Lectin Galectin-8. FASEB J. 2011, 25, 242–254. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, Y.W.; Kuo, C.F.; Lu, S.L.; Liu, F.T.; Anderson, R.; Lin, C.F.; Liu, Y.L.; Wang, W.Y.; Chen, Y.D.; et al. Galectin-3 Inhibits Galectin-8/Parkin-Mediated Ubiquitination of Group a Streptococcus. mBio 2017, 8, e00899-17. [Google Scholar] [CrossRef]
- Wan, L.; Lin, H.-J.; Huang, C.-C.; Chen, Y.-C.; Hsu, Y.-A.; Lin, C.-H.; Lin, H.-C.; Chang, C.-Y.; Huang, S.-H.; Lin, J.-M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744. [Google Scholar] [CrossRef]
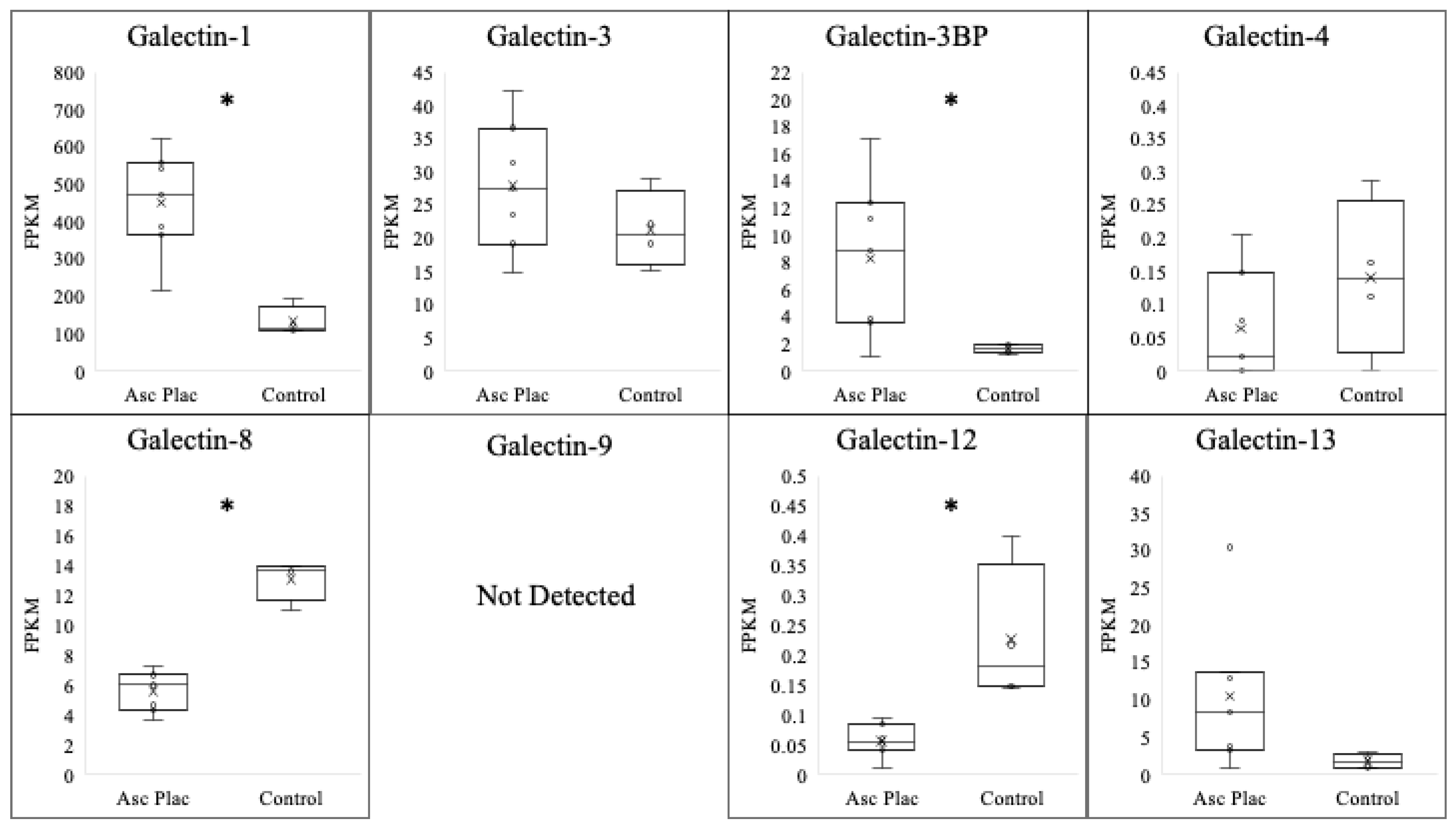
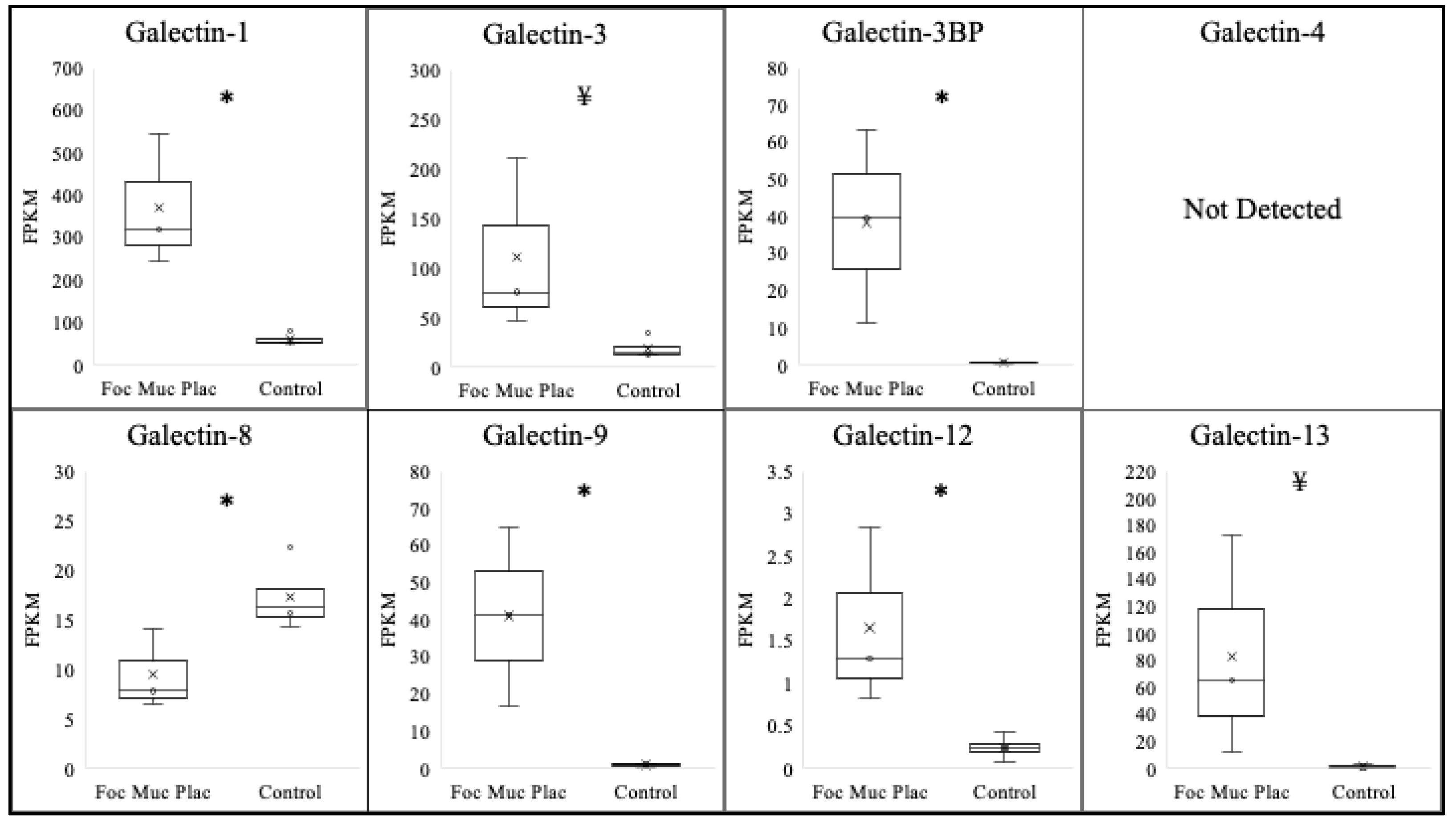
| Galectin | Normal Postpartum Chorioallantois (FPKM) | Ascending Placentitis Postpartum Chorioallantois (FPKM) | log2 |
|---|---|---|---|
| Galectin-1 | 52.3 ± 19.9 | 451.8 ± 52.3 * | 3.10 |
| Galectin-3 | 21.4 ± 2.9 | 27.9 ± 3.7 | N/A |
| Galectin-3BP | 1.6 ± 0.2 | 8.3 ± 2.2 * | 2.38 |
| Galectin-4 | 0.1 ± 0.1 | 0.1 ± 0.1 | N/A |
| Galectin-8 | 13.1 ± 0.7 | 5.6 ± 0.5 * | −1.20 |
| Galectin-9 | ND | ND | N/A |
| Galectin-12 | 0.2 ± 0.06 | 0.1 ± 0.01 * | −1 |
| Galectin-13 | 1.7 ± 0.5 | 10.5 ± 3.8 | N/A |
| Galectin | Normal Postpartum Chorioallantois (FPKM) | Focal Mucoid Placentitis Postpartum Chorioallantois (FPKM) | log2 |
|---|---|---|---|
| Galectin-1 | 61.3 ± 6.5 | 370.6 ± 90.3 * | 2.61 |
| Galectin-3 | 19.0 ± 5.5 | 110.0 ± 50.9 ¥ | 2.51 |
| Galectin-3BP | 0.7 ± 0.1 | 38.3 ± 15.0 * | 5.77 |
| Galectin-4 | ND | ND | N/A |
| Galectin-8 | 17.3 ± 1.7 * | 9.5 ± 2.3 | −0.85 |
| Galectin-9 | 1.0± 0.2 | 41.1± 12.0 * | 5.35 |
| Galectin-12 | 0.2± 0.07 | 1.7± 0.6 * | 3.09 |
| Galectin-13 | 1.3± 0.6 | 83.1± 47.1¥ | 6.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorka, C.E.; Ali, H.E.-S.; Troedsson, M.H.T. Galectins in Equine Placental Disease. Vet. Sci. 2023, 10, 218. https://doi.org/10.3390/vetsci10030218
Fedorka CE, Ali HE-S, Troedsson MHT. Galectins in Equine Placental Disease. Veterinary Sciences. 2023; 10(3):218. https://doi.org/10.3390/vetsci10030218
Chicago/Turabian StyleFedorka, Carleigh E., Hossam El-Sheikh Ali, and Mats H. T. Troedsson. 2023. "Galectins in Equine Placental Disease" Veterinary Sciences 10, no. 3: 218. https://doi.org/10.3390/vetsci10030218
APA StyleFedorka, C. E., Ali, H. E.-S., & Troedsson, M. H. T. (2023). Galectins in Equine Placental Disease. Veterinary Sciences, 10(3), 218. https://doi.org/10.3390/vetsci10030218








