Importance of Water Transport in Mammalian Female Reproductive Tract
Abstract
Simple Summary
Abstract
1. Aquaporins and Reproduction
2. Molecular Structure, Function, and Location of Aquaporins (AQPs)
3. AQPs in the Female Reproductive Tract
3.1. AQPs in the Ovary
3.2. AQPs in the Vagina and Cervix
3.3. AQPs in the Uterus
3.4. AQPs in the Placenta
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Di, X.D.; Ma, T.H. Maternal-Fetal Fluid Balance and Aquaporins: From Molecule to Physiology. Acta Pharmacol. Sin. 2011, 32, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Saboori, A.M.; Asimos, A.; Smith, B.L. Purification and Partial Characterization of the M(r) 30,000 Integral Membrane Protein Associated with The Erythrocyte Rh(D) Antigen. J. Biol. Chem. 1987, 262, 17497–17503. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Agre, P. Erythrocyte M(r) 28,000 Transmembrane Protein Exists as a Multisubunit Oligomer Similar to Channel Proteins. J. Biol. Chem. 1991, 266, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Agre, P. Isolation of the CDNA for Erythrocyte Integral Membrane Protein of 28 Kilodaltons: Member of an Ancient Channel Family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The Archetypal Molecular Water Channel. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef]
- Moon, C.; Preston, G.M.; Griffin, C.A.; Jabs, E.W.; Agre, P. The Human Aquaporin-CHIP Gene. Structure, Organization, and Chromosomal Localization. J. Biol. Chem. 1993, 268, 15772–15778. [Google Scholar] [CrossRef]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of Aquaporins in Female and Male Reproductive Systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, Purification, and Partial Characterization of a Novel M(r) 28,000 Integral Membrane Protein from Erythrocytes and Renal Tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [CrossRef]
- Yasui, M.; Kwon, T.H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An Intracellular Vesicle Water Channel Protein in Renal Epithelia. Proc. Natl. Acad. Sci. USA 1999, 96, 5808–5813. [Google Scholar] [CrossRef]
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and Selective Ammonia Transport by Aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; Van Hoek, A.N.; Hediger, M.A. Molecular Characterization of a Broad Selectivity Neutral Solute Channel. J. Biol. Chem. 1998, 273, 24737–24743. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Aquaporin Water Channels. Biosci. Rep. 2004, 24, 127–163. [Google Scholar] [CrossRef]
- Ishibashi, K. New Members of Mammalian Aquaporins: AQP10-AQP12. Handb. Exp. Pharmacol. 2009, 190, 251–262. [Google Scholar]
- Gomes, D.; Agasse, A.; Thiébaud, P.; Delrot, S.; Gerós, H.; Chaumont, F. Aquaporins Are Multifunctional Water and Solute Transporters Highly Divergent in Living Organisms. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1213–1228. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and Functional Expression of a New Water Channel Abundantly Expressed in the Testis Permeable to Water, Glycerol, and Urea. J. Biol. Chem. 1997, 272, 20782–20786. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Connolly, D.L.; Tyson, K.L.; Cary, N.R.B.; Osbourn, J.K.; Agre, P.; Weissberg, P.L. Aquaporin-1 Is Expressed by Vascular Smooth Muscle Cells and Mediates Rapid Water Transport across Vascular Cell Membranes. J. Vasc. Res. 1999, 36, 353–362. [Google Scholar] [CrossRef]
- Nielsen, S.; King, L.S.; Christensen, B.M.; Acre, P. Aquaporins in Complex Tissues. II. Subcellular Distribution in Respiratory and Glandular Tissues of Rat. Am. J. Physiol. Cell Physiol. 1997, 273, C1549–C1561. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Koide, S.S. The Water Channel Gene in Human Uterus. Biochem. Mol. Biol. Int. 1994, 32, 371–377. [Google Scholar]
- Umenishi, F. Quantitative Analysis of Aquaporin MRNA Expression in Rat Tissues by RNase Protection Assay. DNA Cell Biol. 1996, 15, 475–480. [Google Scholar] [CrossRef]
- King, L.S.; Nielsen, S.; Agre, P. Aquaporins in Complex Tissues. I. Developmental Patterns in Respiratory and Glandular Tissues of Rat. Am. J. Physiol. Cell Physiol. 1997, 273, C1541–C1548. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.R. Specialized Membrane Domains for Water Transport in Glial Cells: High- Resolution Immunogold Cytochemistry of Aquaporin-4 in Rat Brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.; Koukoulas, I.; Jeyaseelan, K.; Armugam, A.; Earnest, L.; Baird, R.; Dawson, N.; Ferraro, T.; Wintour, E.M. Ontogeny of Aquaporins 1 and 3 in Ovine Placenta and Fetal Membranes. Placenta 2000, 21, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin Water Channels—From Atomic Structure to Clinical Medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef]
- Newman, J. Physics of the Life Sciences; Springer: New York, NY, USA, 2008; pp. 1–30. [Google Scholar]
- Li, X.J.; Yu, H.M.; Koide, S.S. Regulation of Water Channel Gene (AQP-CHIP) Expression by Estradiol and Anordiol in Rat Uterus. Acta Pharm. Sin. 1997, 32, 586–592. [Google Scholar]
- Frigeri, A.; Nicchia, G.P.; Verbavatz, J.M.; Valenti, G.; Svelto, M. Expression of Aquaporin-4 in Fast-Twitch Fibers of Mammalian Skeletal Muscle. J. Clin. Investig. 1998, 102, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Page, E.; Winterfield, J.; Goings, G.; Bastawrous, A.; Upshaw-Earley, J.; Doyle, D. Water Channel Proteins in Rat Cardiac Myocyte Caveolae: Osmolarity-Dependent Reversible Internalization. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H1988–H2000. [Google Scholar] [CrossRef]
- Beitz, E.; Kumagami, H.; Krippeit-Drews, P.; Ruppersberg, J.P.; Schultz, J.E. Expression Pattern of Aquaporin Water Channels in the Inner Ear of the Rat. The Molecular Basis for a Water Regulation System in the Endolymphatic Sac. Hear. Res. 1999, 132, 76–84. [Google Scholar] [CrossRef]
- Deen, P.M.T.; Knoers, N.V.A.M. Vasopressin Type-2 Receptor and Aquaporin-2 Water Channel Mutants in Nephrogenic Diabetes Insipidus. Am. J. Med. Sci. 1998, 316, 300–309. [Google Scholar] [CrossRef]
- King, L.S.; Yasui, M.; Agre, P. Aquaporins in Health and Disease. Mol. Med. 2000, 6, 60–65. [Google Scholar] [CrossRef]
- Nielsen, S.; Frøkiær, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the Kidney: From Molecules to Medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Aralla, M.; Borromeo, V.; Groppetti, D.; Secchi, C.; Cremonesi, F.; Arrighi, S. A Collaboration of Aquaporins Handles Water Transport in Relation to the Estrous Cycle in the Bitch Uterus. Theriogenology 2009, 72, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Frackowiak, L.; Skowronska, A. The Expression of Aquaporin 1 and 5 in Uterine Leiomyomata in Premenopausal Women: A Preliminary Study. Reprod. Biol. 2012, 12, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Troedsson, M.; Rutllant, J. Expression of Aquaporin Water Channels in Equine Endometrium Is Differentially Regulated during the Oestrous Cycle and Early Pregnancy. Reprod. Domest. Anim. 2013, 48, 529–537. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, J.; Jiang, J.; Jiang, R.; He, Y.; Lin, H. Effects of Estrogen Deprivation on Expression of Aquaporins in Rat Vagina. Menopause 2015, 22, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Koukoulas, I.; Ross, M.C.; Wang, S.; Wintour, E.M. Quantitative Comparison of Placental Expression of Three Aquaporin Genes. Placenta 2004, 25, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Aralla, M.; Mobasheri, A.; Groppetti, D.; Cremonesi, F.; Arrighi, S. Expression of Aquaporin Water Channels in Canine Fetal Adnexa in Respect to the Regulation of Amniotic Fluid Production and Absorption. Placenta 2012, 33, 502–510. [Google Scholar] [CrossRef]
- Thoroddsen, A.; Dahm-Kähler, P.; Lind, A.K.; Weijdegard, B.; Lindenthal, B.; Müller, J.; Brännström, M. The Water Permeability Channels Aquaporins 1-4 Are Differentially Expressed in Granulosa and Theca Cells of the Preovulatory Follicle during Precise Stages of Human Ovulation. J. Clin. Endocrinol. Metab. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Kim, S.O.; Oh, K.J.; Lee, H.S.; Ahn, K.; Kim, S.W.; Park, K. Expression of Aquaporin Water Channels in the Vagina in Premenopausal Women. J. Sex. Med. 2011, 8, 1925–1930. [Google Scholar] [CrossRef]
- Shi, Y.H.; Chen, R.; Talafu, T.; Nijiati, R.; Lalai, S. Significance and Expression of Aquaporin 1, 3, 8 in Cervical Carcinoma in Xinjiang Uygur Women of China. Asian Pac. J. Cancer Prev. 2012, 13, 1971–1975. [Google Scholar] [CrossRef]
- Prat, C.; Blanchon, L.; Borel, V.; Gallot, D.; Herbet, A.; Bouvier, D.; Marceau, G.; Sapin, V. Ontogeny of Aquaporins in Human Fetal Membranes1. Biol. Reprod. 2012, 86, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.J.Q.J.; Jiang, S.S.; Zhu, X.Q.J.Q.J.; Zou, S.W.; Wang, Y.H.; Hu, Y.C. Expression of Aquaporin 1 and Aquaporin 3 in Fetal Membranes and Placenta in Human Term Pregnancies with Oligohydramnios. Placenta 2009, 30, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.E.; Ricke, E.A.; Yang, B.A.; Verkman, A.S.; Taylor, R.N. Expression and Localization of Aquaporin 1 and 3 in Human Fetal Membranes. Am. J. Obstet. Gynecol. 2002, 187, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Gormaz, M.; Arduini, A.; Gosens, K.; Martinez, A.; Perales, A.; Escrig, R.; Tormos, E.; Roselló, M.; Orellana, C.; et al. Expression of Aquaporins Early in Human Pregnancy. Early Hum. Dev. 2012, 88, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 Water Channels in Human Tissue Microarrays. J. Mol. Histol. 2005, 36, 1. [Google Scholar] [CrossRef]
- Kim, S.O.; Lee, H.S.; Ahn, K.; Park, K. Effect of Estrogen Deprivation on the Expression of Aquaporins and Nitric Oxide Synthases in Rat Vagina. J. Sex. Med. 2009, 6, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.J.; Warnest, G.M.; Carati, C.J.; Verco, C.J. Aquaporin-1 Expression in Visceral Smooth Muscle Cells of Female Rat Reproductive Tract. J. Smooth Muscle Res. 2000, 36, 155–167. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Murphy, C.R. Redistribution of Aquaporins 1 and 5 in the Rat Uterus Is Dependent on Progesterone: A Study with Light and Electron Microscopy. Reproduction 2006, 131, 369–378. [Google Scholar] [CrossRef]
- Eddy, C.A.; Pauerstein, C.J. Anatomy and Physiology of the Fallopian Tube. Clin. Obstet. Gynecol. 1980, 23, 1177–1193. [Google Scholar] [CrossRef]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef]
- Beall, M.H.; Wang, S.; Yang, B.; Chaudhri, N.; Amidi, F.; Ross, M.G. Placental and Membrane Aquaporin Water Channels: Correlation with Amniotic Fluid Volume and Composition. Placenta 2007, 28, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yasui, M. Cellular and Subcellular Localization of Aquaporins 1, 3, 8, and 9 in Amniotic Membranes during Pregnancy in Mice. Cell Tissue Res. 2010, 342, 307–316. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Yeste, M.; Vendrell, M.; Rigau, T.; Rodríguez-Gil, J.E.; del Alamo, M.M.R. Uterine and Placental Specific Localization of AQP2 and AQP8 Is Related with Changes of Serum Progesterone Levels in Pregnant Queens. Theriogenology 2020, 142, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of Aquaporin 1, 5, and 9 in the Female Pig Reproductive System. J. Histochem. Cytochem. 2009, 57, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, L.; Lai, A. Expression and Significance of Aquaporin-2 and Serum Hormones in Placenta of Patients with Preeclampsia. J. Obstet. Gynaecol. 2018, 38, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Damiano, A.; Zotta, E.; Goldstein, J.; Reisin, I.; Ibarra, C. Water Channel Proteins AQP3 and AQP9 Are Present in Syncytiotrophoblast of Human Term Placenta. Placenta 2001, 22, 776–781. [Google Scholar] [CrossRef]
- Anderson, J.; Brown, N.; Mahendroo, M.S.; Reese, J. Utilization of Different Aquaporin Water Channels in the Mouse Cervix during Pregnancy and Parturition and in Models of Preterm and Delayed Cervical Ripening. Endocrinology 2006, 147, 130–140. [Google Scholar] [CrossRef]
- Soh, Y.M.; Tiwari, A.; Mahendroo, M.; Conrad, K.P.; Parry, L.J. Relaxin Regulates Hyaluronan Synthesis and Aquaporins in the Cervix of Late Pregnant Mice. Endocrinology 2012, 153, 6054–6064. [Google Scholar] [CrossRef]
- Johns, A.; Buchanan, J.D.; Coons, L.W. Effect of Ovulation on the Ionic and Water Content of Rabbit Oviduct. Biol. Reprod. 1982, 26, 367–377. [Google Scholar] [CrossRef]
- De Falco, M.; Cobellis, L.; Torella, M.; Acone, G.; Varano, L.; Sellitti, A.; Ragucci, A.; Coppola, G.; Cassandro, R.; Laforgia, V.; et al. Down-Regulation of Aquaporin 4 in Human Placenta throughout Pregnancy. In Vivo 2007, 21, 813–818. [Google Scholar]
- Brañes, M.C.; Morales, B.; Ríos, M.; Villalón, M.J. Regulation of the Immunoexpression of Aquaporin 9 by Ovarian Hormones in the Rat Oviductal Epithelium. Am. J. Physiol. Cell Physiol. 2005, 288, C1048–C1057. [Google Scholar] [CrossRef]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M. Water Permeability of an Ovarian Antral Follicle Is Predominantly Transcellular and Mediated by Aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef]
- Shioji, M.; Fukuda, H.; Kanzaki, T.; Wasada, K.; Kanagawa, T.; Shimoya, K.; Mu, J.; Sugimoto, Y.; Murata, Y. Reduction of Aquaporin-8 on Fetal Membranes under Oligohydramnios in Mice Lacking Prostaglandin F2α Receptor. J. Obstet. Gynaecol. Res. 2006, 32, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, B.; Verkman, A.S. Cloning of a Novel Water and Urea-Permeable Aquaporin from Mouse Expressed Strongly in Colon, Placenta, Liver, and Heart. Biochem. Biophys. Res. Commun. 1997, 240, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kallichanda, N.; Song, W.; Ramirez, B.A.; Ross, M.G. Expression of Aquaporin-8 in Human Placenta and Chorioamniotic Membranes: Evidence of Molecular Mechanism for Intramembranous Amniotic Fluid Resorption. Am. J. Obstet. Gynecol. 2001, 185, 1226–1231. [Google Scholar] [CrossRef]
- Skowronska, A.; Mlotkowska, P.; Nielsen, S.; Skowronski, M.T. Difference in Expression between AQP1 and AQP5 in Porcine Endometrium and Myometrium in Response to Steroid Hormones, Oxytocin, Arachidonic Acid, Forskolin and CAMP during the Mid-Luteal Phase of the Estrous Cycle and Luteolysis. Reprod. Biol. Endocrinol. 2015, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, H.J.; Kim, S.W.; Jung, S., II; Kim, S.O.; Lee, H.S.; Lee, M.N.; Ahn, K. Expression of Aquaporin Water Channels in Rat Vagina: Potential Role in Vaginal Lubrication. J. Sex. Med. 2008, 5, 77–82. [Google Scholar] [CrossRef] [PubMed]
- He, R.H.; Sheng, J.Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.L.; Zhou, C.Y.; Sheng, X.; Huang, H.F. Aquaporin-2 Expression in Human Endometrium Correlates with Serum Ovarian Steroid Hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef]
- Finlay, T.H.; Katz, J.; Kirsch, L.; Levitz, M.; Nathoo, S.A.; Seiler, S. Estrogen-Stimulated Uptake of Plasminogen by the Mouse Uterus. Endocrinology 1983, 112, 856–861. [Google Scholar] [CrossRef]
- Maier, D.B.; Kuslis, S.T. Human Uterine Luminal Fluid Volumes and Prolactin Levels in Normal Menstrual Cycles. Am. J. Obstet. Gynecol. 1988, 159, 434–439. [Google Scholar] [CrossRef]
- Cullinan-Bove, K.; Koos, R.D. Vascular Endothelial Growth Factor/Vascular Permeability Factor Expression in the Rat Uterus: Rapid Stimulation by Estrogen Correlates with Estrogen-Induced Increases in Uterine Capillary Permeability and Growth. Endocrinology 1993, 133, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Asahina, T.; Kobayashi, T.; Goto, J.; Terao, T. Studies on the Mechanism of Edematous Changes at the Endometrial Stroma for Implantation. Semin. Thromb. Hemost. 2001, 27, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.; Glazier, J.; D’Souza, S. Placental Transporter Activity and Expression in Relation to Fetal Growth. Exp. Physiol. 1997, 82, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.E.; Ricke, E.A.; Torres, E.A.; Taylor, R.N.; Gilbert, W.; Henderson, S.; Steinke, R. A Novel Model of Polyhydramnios: Amniotic Fluid Volume Is Increased in Aquaporin 1 Knockout Mice. Am. J. Obstet. Gynecol. 2005, 192, 2041–2044. [Google Scholar] [CrossRef]
- Mann, S.E.; Dvorak, N.; Gilbert, H.; Taylor, R.N. Steady-State Levels of Aquaporin 1 MRNA Expression Are Increased in Idiopathic Polyhydramnios. Am. J. Obstet. Gynecol. 2006, 194, 884–887. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Z.; Wintour, E.M. Aquaporins and Fetal Fluid Balance. Placenta 2008, 29, 840–847. [Google Scholar] [CrossRef]
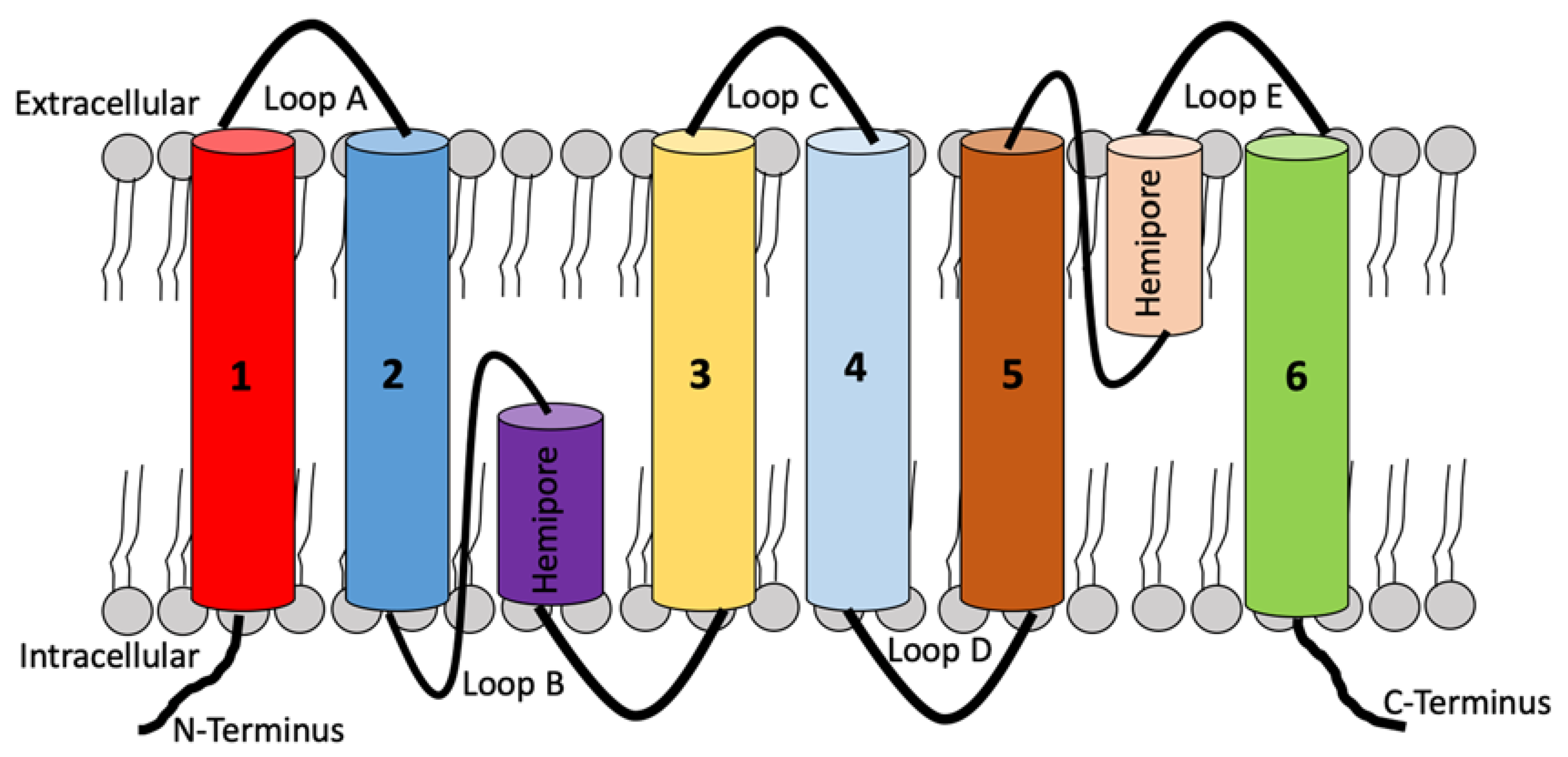
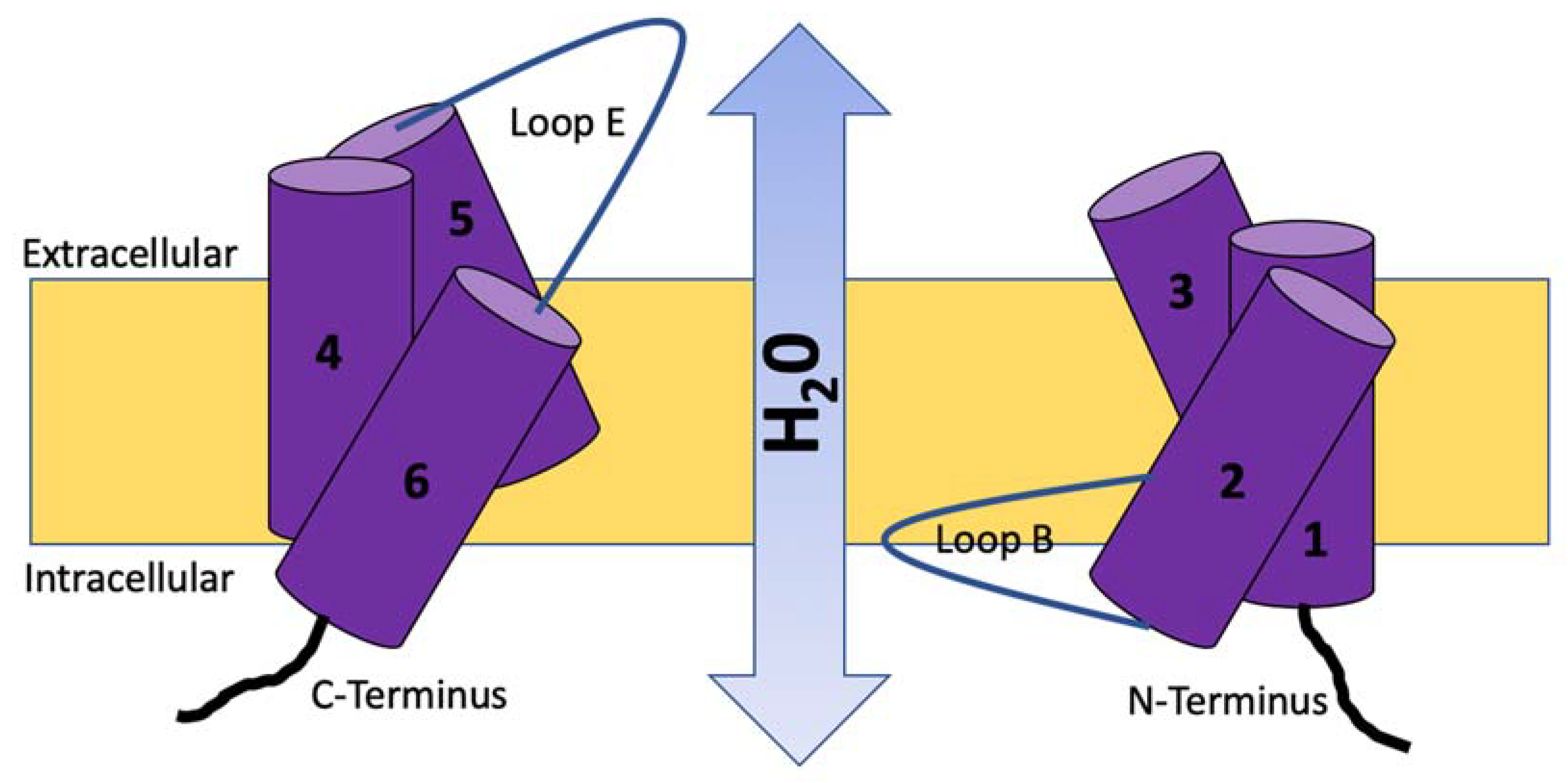
| Aquaporin | Species | Localization | Analytic Method | References |
|---|---|---|---|---|
| AQP0 | Horse | Endometrial luminal, glandular epithelial and stromal cells | RT-PCR, WB, IHC | [35] |
| Rat | Cytoplasm of vaginal epithelia | WB, IHC | [36] | |
| AQP1 | Sheep | Endothelium of capillaries under the trophoblast layer of the chorion and maternal capillary endothelium of cotyledons | RT-PCR, WB, IHC | [23,37] |
| Dog | Vascular endothelia, glandular epithelium of the endometrium, smooth muscle cells of myometrium | WB, IHC | [33,38] | |
| Horse | Endometrium | RT-PCR | [35] | |
| Human | Amniotic and chorionic epithelia and villi. Placenta. Uterus. Large, rounded cells of the theca, granulosa cell layer. Capillaries and venules of vagina and cervix. | RT-PCR, WB, IHC | [39,40,41,42,43,44,45,46] | |
| Rat | Cytoplasm and plasma membrane of myometrium. Capillaries and venules of vaginal lamina propia | WB, IHC | [47,48,49,50] | |
| Mouse | Myometrium. Vessel walls of placental labyrinth and yolk sac | RT-PCR, WB, IHC | [51,52,53] | |
| Cat | Endometrial endothelia, glandular epithelium and myometrium | WB, IHC | [54] | |
| Pig | Ovarian, oviductal and uterine capillary epithelium | IHC | [55] | |
| AQP2 | Dog | Apical membrane of glandular epithelium of the uterus, smooth muscle cells of myometrium and blood vessel musculature | WB, IHC | [33] |
| Horse | Endometrial luminal, glandular epithelial and stromal cells. Cytoplasm of vaginal epithelium | RT-PCR, WB, IHC | [35,40] | |
| Human | Uterus. Large rounded cells of the theca, granulosa cell layer. Fetal membrane, trophoblastic cells and syncytiotrophoblsts | RT-PCR, IHC | [39,45,46,56] | |
| Rat | Superficial layer of vaginal epithelium | WB, IHC | [47] | |
| Mouse | Epithelial endometrial cells and myometrium. Fetal membranes and placenta | RT-PCR, WB, IHC | [51,52] | |
| Cat | Endometrial cells of luminal and glandular epithelia. Syncytiotrophoblasts and cytotrophoblasts | WB, IHC | [54] | |
| AQP3 | Sheep | Apical membrane of trophoblastic cell layer of villous chorion and fetal trophoblastic cell layer within chorionic villi | RT-PCR, WB, IHC | [23,37] |
| Horse | Endometrium | RT-PCR | [35] | |
| Human | Amiotic and chorionic epithelia and villi. Placenta. Uterus. Granulosa and theca cell layers. Plasma membrane of vaginal epithelium. Squamous cervical epithelia | RT-PCR, WB, IHC | [39,40,41,42,43,44,45,57] | |
| Rat | Plasma membrane and cytoplasm of vaginal epithelia | WB, IHC | [36] | |
| Mouse | Basal cell layers of cervical epithelium. Epithelial cells of endometrium and myometrium. Trophoblastic cells | RT-PCR, Northern hybridation, in situ hybridation, WB, IHC | [51,52,53,58,59] | |
| Cat | Endometrial cells of luminal and glandular epithelia. Syncytiotrophoblasts and cytotrophoblasts | WB, IHC | [54] | |
| Dog | Apical endometrial cell membrane of glandular epithelium. Placental allantochorion. Yolk sac | IHC | [60] | |
| AQP4 | Horse | Endometrium | RT-PCR | [35] |
| Human | Placenta, chorionic villi and uterus. Granulosa and theca cell layers | RT-PCR, IHC | [39,45,61] | |
| Mouse | Apical cell layers and mucus-secreting cervical cell surface. Fetal membranes and placental | RT-PCR, Northern hybridation, in situ hybridation, IHC | [52,58] | |
| AQP5 | Dog | Apical membrane of glandular and luminal epithelium of the uterus. Cervical luminal epithelia. Allantochorion cells | WB, IHC | [33,38] |
| Horse | Endometrial luminal, glandular epithelial and stromal cells | RT-PCR, WB, IHC | [35] | |
| Rat | Plasma membrane and cytoplasm of vaginal epithelia. Endometrial luminal epithelial cells. Cytoplasm of oviductal epithelial cells | RT-PCR, WB, IHC | [36,49,50,54,62] | |
| Mouse | Basal and apical cervical epithelium cells. Fetal membranes and placenta | RT-PCR, Northern hybridation, in situ hybridation, IHC | [52,58,59] | |
| Pig | Flattened follicle cells of primordial follicles and granulosa cells of developing follicles. Muscle layers and luminal epithelial cells of the oviduct and uterus | IHC | [55] | |
| Human | Placenta, chorionic villi and uterus | RT-PCR | [45] | |
| AQP6 | Rat | Plasma membrane and cytoplasm of vaginal epithelia | WB, IHC | [36] |
| Human | Cytoplasm of vaginal epithelium | WB, IHC | [40] | |
| Mouse | Fetal membranes and placenta | RT-PCR, IHC | [52] | |
| AQP7 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Granulosa cells | ELISA, WB, IHC | [40,63] | |
| Mouse | Fetal membranes and placenta | RT-PCR, IHC | [52] | |
| Human | Placenta and uterus. Cytoplasm of vaginal epithelium | RT-PCR, WB, IHC | [45] | |
| AQP8 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Cytoplasm of oviductal epithelial cells. Granulosa cells | RT-PCR, ELISA, WB, IHC | [62,63] | |
| Mouse | Basal and apical cervical epithelium cells. Stromal endometrial cells and myometrium. Basal component of fetal membranes. Placenta | RT-PCR, Northern hybridation, in situ hybridation, WB, IHC | [51,52,53,58,64,65] | |
| Human | Amiotic and chorionic epithelia and villi. Uterus. Squamous cervical epithelia | RT-PCR, WB, IHC | [41,42,45,66] | |
| Cat | Endometrial cells of luminal and glandular epithelia. Syncytiotrophoblasts and cytotrophoblasts | WB, IHC | [54] | |
| Pig | Granulosa cells of developing follicles, oviductal myometrium and glandular and luminal epithelium of the uterus | IHC | [55] | |
| Dog | Placental spongy zone and lining cells of amnion and allantoic sac | IHC | [38] | |
| Sheep | Trophoblasts | RT-PCR | [37] | |
| AQP9 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Apical membrane of oviductal epithelial cells. Granulosa cells | RT-PCR, ELISA, WB, IHC | [62,63] | |
| Human | Amiotic and chorionic epithelia and villi. Uterus | RT-PCR, WB, IHC | [42,45,57] | |
| Mouse | Fetal membranes and placenta | RT-PCR, IHC | [52,53] | |
| Dog | Syncytiotrophoblasts | IHC | [38] | |
| AQP10 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Vagina | WB | [36] | |
| AQP11 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Capillaries and venules | WB, IHC | [36] | |
| Human | Amiotic and chorionic epithelia and villi. Uterus | RT-PCR, WB, IHC | [42,45,52] | |
| AQP12 | Horse | Endometrium | RT-PCR | [35] |
| Rat | Vagina | WB | [36] | |
| Mouse | Fetal membranes and placenta | RT-PCR, IHC | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferré-Dolcet, L.; Rivera del Alamo, M.M. Importance of Water Transport in Mammalian Female Reproductive Tract. Vet. Sci. 2023, 10, 50. https://doi.org/10.3390/vetsci10010050
Ferré-Dolcet L, Rivera del Alamo MM. Importance of Water Transport in Mammalian Female Reproductive Tract. Veterinary Sciences. 2023; 10(1):50. https://doi.org/10.3390/vetsci10010050
Chicago/Turabian StyleFerré-Dolcet, Lluis, and Maria Montserrat Rivera del Alamo. 2023. "Importance of Water Transport in Mammalian Female Reproductive Tract" Veterinary Sciences 10, no. 1: 50. https://doi.org/10.3390/vetsci10010050
APA StyleFerré-Dolcet, L., & Rivera del Alamo, M. M. (2023). Importance of Water Transport in Mammalian Female Reproductive Tract. Veterinary Sciences, 10(1), 50. https://doi.org/10.3390/vetsci10010050






