Morphological Characteristics of Genital Organ-Associated Lymphoid Tissue in the Vaginal Vestibule of Goats and Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparations from Healthy Goats and Pigs
2.2. Vaginal Smear Observation in Goats
2.3. Determination of Vaginal pH and Temperature in Goats
2.4. Whole-Mount Observation
2.5. Histological Analysis
2.6. Immunohistochemistry (IHC) and Immunofluorescence (IF)
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analyses
3. Results
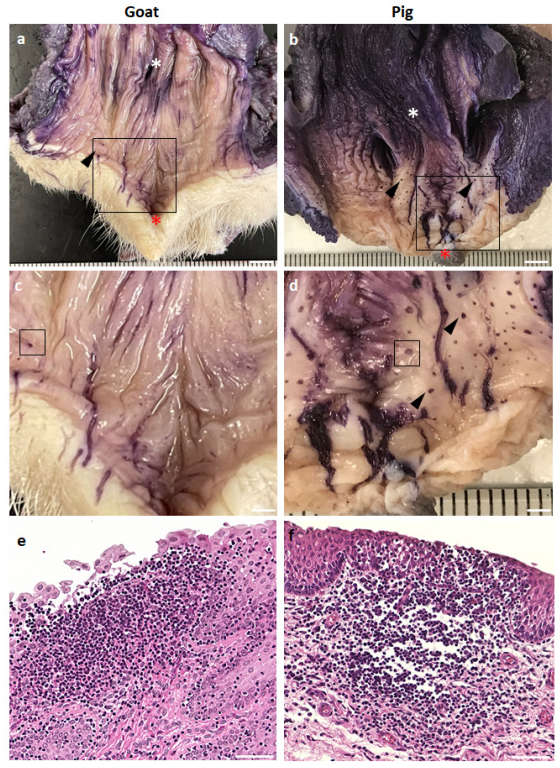
3.1. GOALTs Found in the VV of Goats and Pigs
3.2. Histological Description of GOALTs in the VV of Goats and Pigs
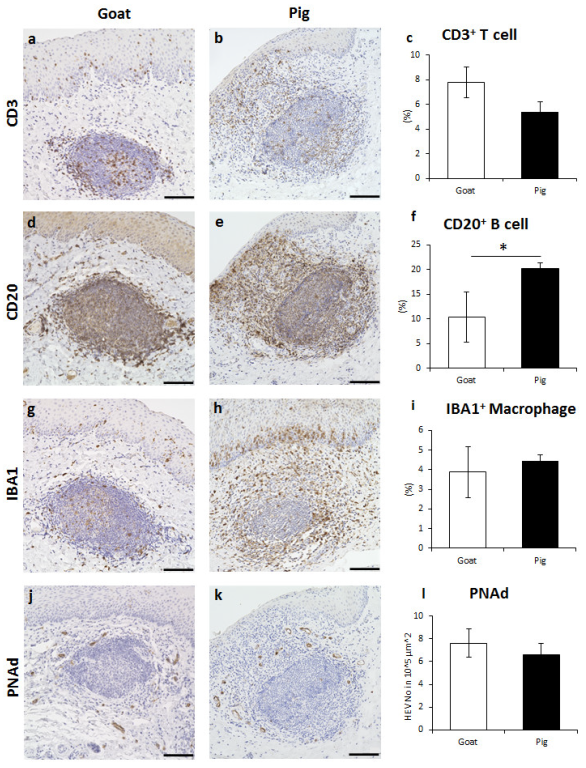
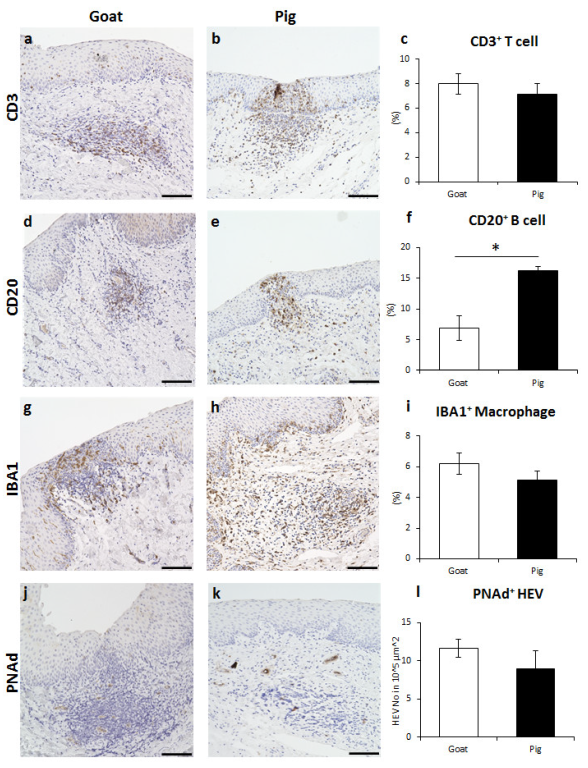
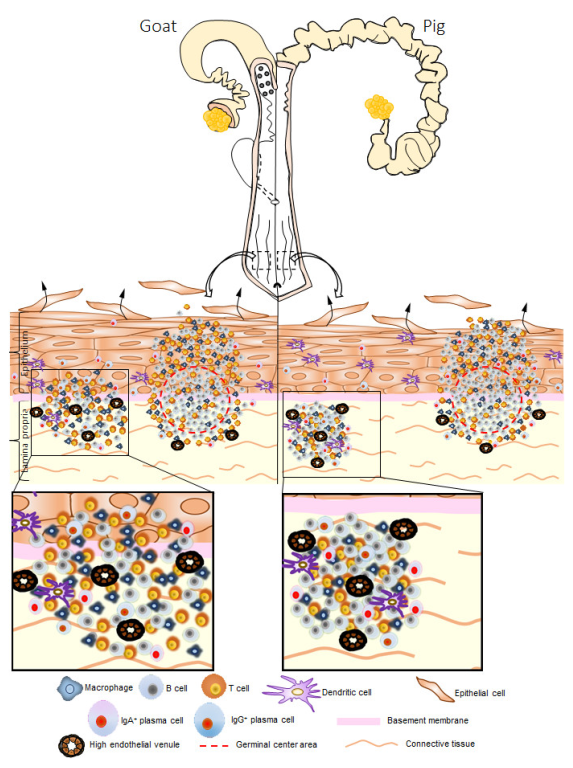
3.3. Immune Cell Characteristics Composing GOALTs in the VV of Goats and Pigs
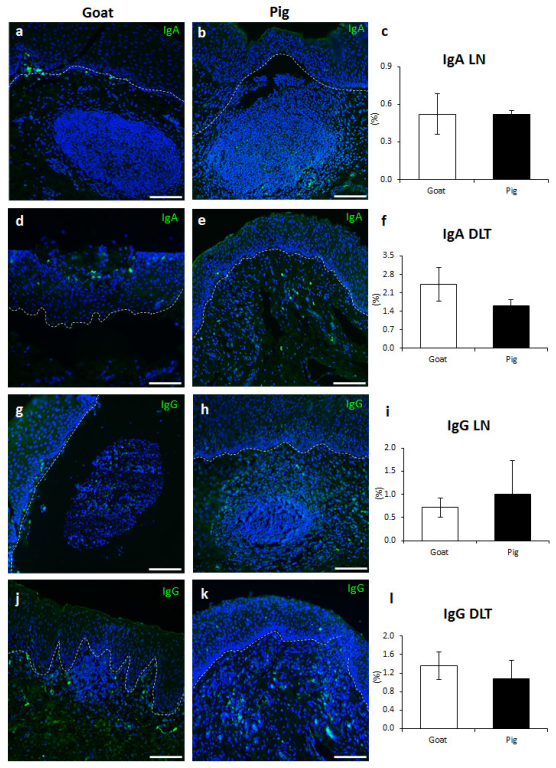
3.4. Immunoglobulin Producing Cells and Antigen Presenting Cells in the VV of Goats and Pigs
3.5. Surface Structures of GOALTs in the VV of Goats and Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandtzaeg, P.; Pabst, R. Let’s Go Mucosal: Communication on Slippery Ground. Trends Immunol. 2004, 25, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Koornstra, P.J.; Hameleers, D.M.H.; Biewenga, J.; Spit, B.J.; Duijvestijn, A.M.; Vriesman, V.B.; Peter, C.J.; Sminia, T. The Role of Nasopharyngeal Lymphoid Tissue. Immunol. Today 1992, 12, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of Inducible Bronchus Associated Lymphoid Tissue (IBALT) in Respiratory Immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.; Jorissen, M.; Ceuppens, J. The Waldeyer’s Ring. Acta Otorhinolaryngol. Belg. 2000, 54, 237–241. [Google Scholar]
- Forchielli, M.L.; Walker, W.A. The Role of Gut-Associated Lymphoid Tissues and Mucosal Defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef]
- Chuluunbaatar, T.; Ichii, O.; Nakamura, T.; Irie, T.; Namba, T.; Islam, M.R.; Otani, Y.; Masum, M.A.; Okamatsu-Ogura, Y.; Elewa, Y.H.A.; et al. Unique Running Pattern and Mucosal Morphology Found in the Colon of Cotton Rats. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Jørgensen, P.B.; Eriksen, L.L.; Fenton, T.M.; Bailey, M.; Agace, W.W.; Mörbe, U.M. The Porcine Large Intestine Contains Developmentally Distinct Submucosal Lymphoid Clusters and Mucosal Isolated Lymphoid Follicles. Dev. Comp. Immunol. 2022, 131, 104375. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chu, R.M.; Liu, R.S.; Weng, C.N. Morphologic Studies of Intrapulmonary Airway Mucosa-Associated Lymphoid Tissues in Swine. Vet. Immunol. Immunopathol. 1990, 25, 13–22. [Google Scholar] [CrossRef]
- Gahlot, P.K.; Kumar, P. Gross Localization and Distribution of Gut Associated Lymphoid Tissue in Goat (Capra hircus). Haryana Vet. 2018, 57, 159–160. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 1st ed.; Saunders: Philadelphia, AR, USA, 1987; ISBN 0721613322. [Google Scholar]
- Barone, R. Anatomia Comparata Dei Mammiferi Domestici; Edagricole: Bologna, Italy, 1993. [Google Scholar]
- Sisson, S.; Grossman, J.D.; Getty, R. Sisson and Grossman’s The Anatomy of the Domestic Animals; Saunders: Philadelphia, AR, USA, 1975; ISBN 0721641024. [Google Scholar]
- Hume, I.D.; Morgan, K.R.; Kenagy, G.J. Digesta Retention and Digestive Performance in Sciurid and Microtine Rodents: Effects of Hindgut Morphology and Body Size. Physiol. Zool. 1993, 66, 396–411. [Google Scholar] [CrossRef]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The Porcine Innate Immune System: An Update. Dev. Comp. Immunol. 2014, 45, 321. [Google Scholar] [CrossRef]
- Russell, M.W.; Mestecky, J. Humoral Immune Responses to Microbial Infections in the Genital Tract. Microbes Infect. 2002, 4, 667–677. [Google Scholar] [CrossRef]
- Ochiel, D.O.; Fahey, J.V.; Ghosh, M.; Haddad, S.N.; Wira, C.R. Innate Immunity in the Female Reproductive Tract: Role of Sex Hormones in Regulating Uterine Epithelial Cell Protection Against Pathogens. Curr. Womens Health Rev. 2008, 4, 102. [Google Scholar] [CrossRef]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and Adaptive Immunity at Mucosal Surfaces of the Female Reproductive Tract: Stratification and Integration of Immune Protection against the Transmission of Sexually Transmitted Infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef]
- Blazquez, N.B.; Batten, E.H.; Long, S.E.; Perry, G.C. Amount and Distribution of Vestibular-Associated Lymphoid Tissue in Calves and Adult Cows. Res. Vet. Sci. 1987, 43, 239–242. [Google Scholar] [CrossRef]
- Lehner, T.; Panagiotidi, C.; Bergmeier, L.A.; Tao, L.; Brookes, R.; Gearing, A.; Adams, S. Genital-Associated Lymphoid Tissue in Female Non-Human Primates. Adv. Exp. Med. Biol. 1995, 371, 357–365. [Google Scholar] [CrossRef]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A Review of the Human vs. Porcine Female Genital Tract and Associated Immune System in the Perspective of Using Minipigs as a Model of Human Genital Chlamydia Infection. Vet. Res. 2015, 46, 116. [Google Scholar] [CrossRef]
- Chuluunbaatar, T.; Ichii, O.; Masum, M.A.; Namba, T.; Islam, M.R.; Otani, Y.; Elewa, Y.H.A.; Kon, Y. Genital Organ-Associated Lymphoid Tissues Arranged in a Ring in the Mucosa of Cow Vaginal Vestibules. Res. Vet. Sci. 2022, 145, 147–158. [Google Scholar] [CrossRef]
- Silva, F.M.O.; Guimarães, J.P.; Vergara-Parente, J.E.; Carvalho, V.L.; Carolina, A.; Meirelles, O.; Marmontel, M.; Oliveira, B.S.S.P.; Santos, S.M.; Becegato, E.Z.; et al. Morphology of Mucosa-Associated Lymphoid Tissue in Odontocetes. Microsc. Res. Tech. 2016, 79, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sui, Y.; Kato, S.; Hogg, A.E.; Steel, J.C.; Morris, J.C.; Berzofsky, J.A. Vaginal Type-II Mucosa Is an Inductive Site for Primary CD8+ T-Cell Mucosal Immunity. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Otsuki, Y.; Maeda, Y.; Magari, S.; Sugimoto, O. Lymphatics and Lymphoid Tissue of the Fallopian Tube: Immunoelectronmicroscopic Study. Anat. Rec. 1989, 225, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Tommola, P.; Bützow, R.; Unkila-Kallio, L.; Paavonen, J.; Meri, S. Activation of Vestibule-Associated Lymphoid Tissue in Localized Provoked Vulvodynia. Am. J. Obstet. Gynecol. 2015, 212, 476.e1–476.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Way, S.S.; Chen, K. Immunology of the Uterine and Vaginal Mucosae. Trends Immunol. 2018, 39, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, C.; Breugelmans, S.; Simoens, P.; Van Den Broeck, W. The Tonsils Revisited: Review of the Anatomical Localization and Histological Characteristics of the Tonsils of Domestic and Laboratory Animals. Clin. Dev. Immunol. 2011, 2011. [Google Scholar] [CrossRef]
- Yang, H.; Parkhouse, R.M.E. Phenotypic Classification of Porcine Lymphocyte Subpopulations in Blood and Lymphoid Tissues. Immunology 1996, 89, 76–83. [Google Scholar] [CrossRef]
- Oláh, I.; Takács, L.; Törö, I. Formation of Lymphoepithelial Tissue in the Sheep’s Palatine Tonsil. Acta Otolaryngol. Suppl. 1988, 454, 7–17. [Google Scholar] [CrossRef]
- Haley, J.P. The Lymphoid System: A Review of Species Differences. J. Toxicol. Pathol. 2017, 30, 111–123. [Google Scholar] [CrossRef]
- Lorenzen, E.; Agerholm, J.S.; Grossi, A.B.; Bojesen, A.M.; Skytte, C.; Erneholm, K.; Follmann, F.; Jungersen, G. Characterization of Cytological Changes, IgA, IgG and IL-8 Levels and PH Value in the Vagina of Prepubertal and Sexually Mature Ellegaard Göttingen Minipigs during an Estrous Cycle. Dev. Comp. Immunol. 2016, 59, 57–62. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, E.; Arshad, U.; Riaz, A.; Akhtar, M.S.; Ahmad, T.; Khan, J.A.; Mohsin, I.; Shi, Z.; Sattar, A. Effects of Immunization against Inhibin α-Subunit on Ovarian Structures, Pregnancy Rate, Embryonic and Fetal Losses, and Prolificacy Rate in Goats Where Estrus Was Induced during the Non-Breeding Season. Anim. Reprod. Sci. 2021, 224, 106654. [Google Scholar] [CrossRef]
- Rothkotter, H.J. Lymphocyte Subsets in Jejunal and Ileal Peyer’s Patches of Normal and Gnotobiotic Minipigs. Immunology 1989, 67, 103–108. [Google Scholar]
- Reynolds, J.D.; Pabst, R. The Emigration of Lymphocytes from Peyer’s Patches in Sheep. Eur. J. Immunol. 1984, 14, 7–13. [Google Scholar] [CrossRef]
- Wojciech, P.; Ross, M.H. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, 8th ed.; Wolters Kluwer: Philadelphia, AR, USA, 2020; ISBN 9781496383426. [Google Scholar]
- Masum, M.A.; Ichii, O.; Elewa, Y.H.A.; Otani, Y.; Namba, T.; Kon, Y. Vasculature-Associated Lymphoid Tissue: A Unique Tertiary Lymphoid Tissue Correlates With Renal Lesions in Lupus Nephritis Mouse Model. Front. Immunol. 2020, 11, e595672. [Google Scholar] [CrossRef]
- Miller, C.J.; McChesney, M.; Moore, P.F. Langerhans Cells, Macrophages and Lymphocyte Subsets in the Cervix and Vagina of Rhesus Macaques. Lab. Investig. 1992, 67, 628–634. [Google Scholar]
- Hussain, L.A.; Lehner, T. Comparative Investigation of Langerhans’ Cells and Potential Receptors for HIV in Oral, Genitourinary and Rectal Epithelia. Immunology 1995, 85, 475–484. [Google Scholar]
- Romani, N.; Clausen, B.E.; Stoitzner, P. Langerhans Cells and More: Langerin-Expressing Dendritic Cell Subsets in the Skin. Immunol. Rev. 2010, 234, 120–141. [Google Scholar] [CrossRef]
- Iijima, N.; Linehan, M.M.; Saeland, S.; Iwasaki, A. Vaginal Epithelial Dendritic Cells Renew from Bone Marrow Precursors. Proc. Natl. Acad. Sci. USA 2007, 104, 19061–19066. [Google Scholar] [CrossRef]
- Caucheteux, S.; Piguet, V. Vaginal Epidermal Dendritic Cells: Defense against HIV-1 or a Safe Haven? J. Clin. Invest. 2018, 128, 3228–3230. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ohwada, N.; Aoki, T.; Fujimoto, T. Langerhans Cells in the Human Oviduct Mucosa. Ital. J. Anat. Embryol. 1998, 103, 253–258. [Google Scholar]
- Duluc, D.; Gannevat, J.; Anguiano, E.; Zurawski, S.; Carley, M.; Boreham, M.; Stecher, J.; Dullaers, M.; Banchereau, J.; Oh, S. Functional Diversity of Human Vaginal APC Subsets in Directing T-Cell Responses. Mucosal Immunol. 2013, 6, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Fagraeus, A. Plasma Cellular Reaction and Its Relation to the Formation of Antibodies in Vitro. Nature 1947, 159, 499. [Google Scholar] [CrossRef] [PubMed]
- Manz, R.A.; Thiel, A.; Radbruch, A. Lifetime of Plasma Cells in the Bone Marrow. Nature 1997, 388, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Wang, W.H.; Jia, S. The Distribution of Siga and Igg Antibody-Secreting Cells in the Small Intestine of Bactrian Camels (Camelus Bactrianus) of Different Ages. PLoS ONE 2016, 11, e0156635. [Google Scholar] [CrossRef]
- Wira, C.R.; Rossoll, R.M.; Kaushic, C. Antigen-Presenting Cells in the Female Reproductive Tract: Influence of Estradiol on Antigen Presentation by Vaginal Cells. Endocrinology 2000, 141, 2877–2885. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuluunbaatar, T.; Ichii, O.; Masum, M.A.; Namba, T.; Kon, Y. Morphological Characteristics of Genital Organ-Associated Lymphoid Tissue in the Vaginal Vestibule of Goats and Pigs. Vet. Sci. 2023, 10, 51. https://doi.org/10.3390/vetsci10010051
Chuluunbaatar T, Ichii O, Masum MA, Namba T, Kon Y. Morphological Characteristics of Genital Organ-Associated Lymphoid Tissue in the Vaginal Vestibule of Goats and Pigs. Veterinary Sciences. 2023; 10(1):51. https://doi.org/10.3390/vetsci10010051
Chicago/Turabian StyleChuluunbaatar, Tsolmon, Osamu Ichii, Md. Abdul Masum, Takashi Namba, and Yasuhiro Kon. 2023. "Morphological Characteristics of Genital Organ-Associated Lymphoid Tissue in the Vaginal Vestibule of Goats and Pigs" Veterinary Sciences 10, no. 1: 51. https://doi.org/10.3390/vetsci10010051
APA StyleChuluunbaatar, T., Ichii, O., Masum, M. A., Namba, T., & Kon, Y. (2023). Morphological Characteristics of Genital Organ-Associated Lymphoid Tissue in the Vaginal Vestibule of Goats and Pigs. Veterinary Sciences, 10(1), 51. https://doi.org/10.3390/vetsci10010051





