Maternal and Fetal PI3K-p110α Deficiency Induces Sex-Specific Changes in Conceptus Growth and Placental Mitochondrial Bioenergetic Reserve in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Placental Lz Mitochondrial Respirometry
3. Statistical Analysis
4. Results
4.1. PI3K-p110α Deficiency Affects Maternal Body Composition
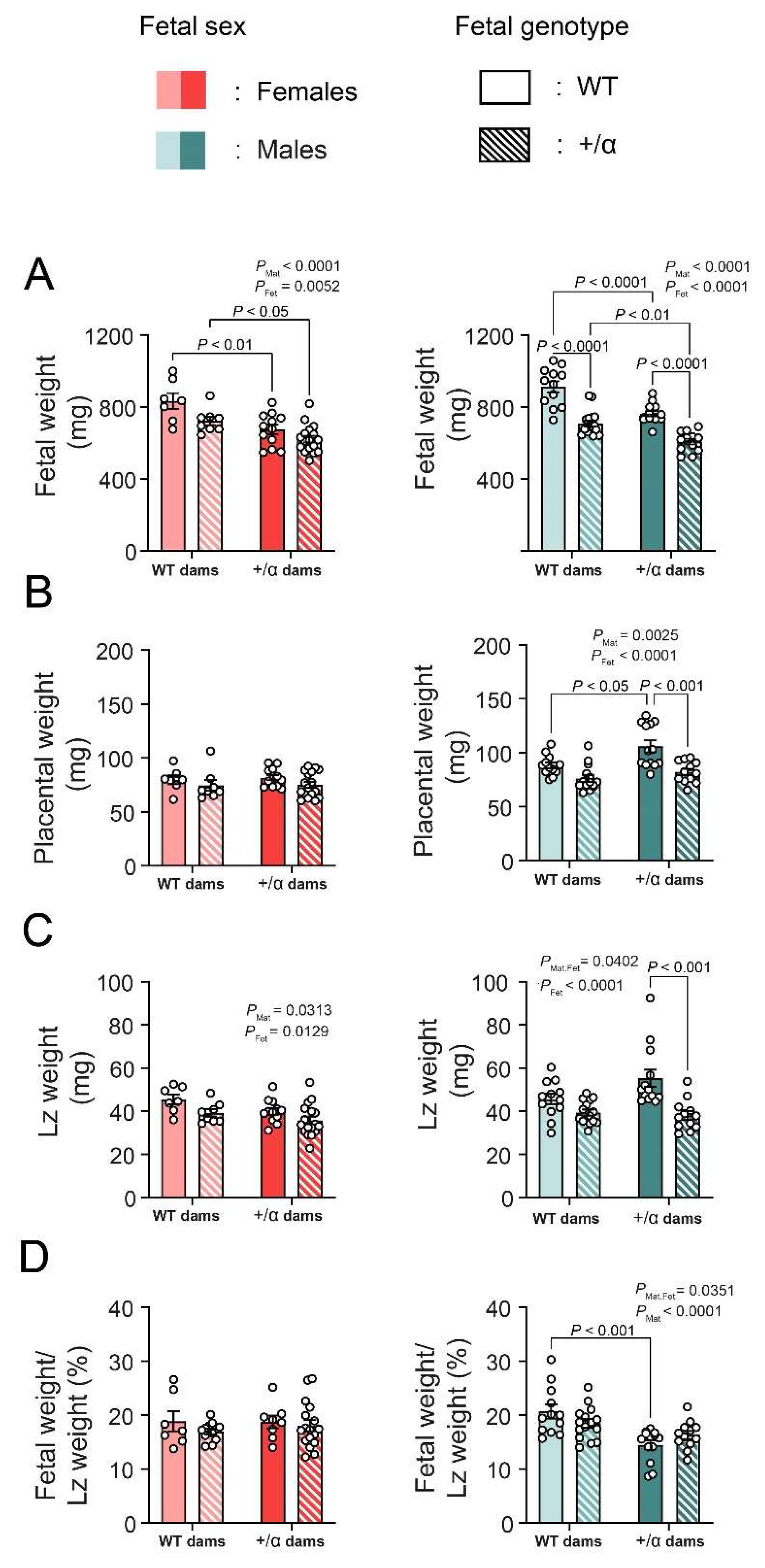
4.2. Fetal and Maternal PI3K-p110α Deficiency Induces Sex-Specific Changes in Feto-Placental Growth
4.3. Fetal and Maternal PI3K-p110α Deficiency Has Only a Minor Effect on Lz Mitochondrial Respiratory Capacity and Does So in a Sex-Specific Manner
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pantham, P.; Rosario, F.J.; Weintraub, S.T.; Nathanielsz, P.W.; Powell, T.L.; Li, C.; Jansson, T. Down-Regulation of Placental Transport of Amino Acids Precedes the Development of Intrauterine Growth Restriction in Maternal Nutrient Restricted Baboons. Biol. Reprod. 2016, 95, 98. [Google Scholar] [CrossRef]
- Glazier, J.D.; Cetin, I.; Perugino, G.; Ronzoni, S.; Grey, A.M.; Mahendran, D.; Marconi, A.M.; Pardi, G.; Sibley, C.P. Association between the Activity of the System A Amino Acid Transporter in the Microvillous Plasma Membrane of the Human Placenta and Severity of Fetal Compromise in Intrauterine Growth Restriction. Pediatr. Res. 1997, 42, 514–519. [Google Scholar] [CrossRef]
- Watson, E.D.; Cross, J.C.; Shen, H.; Cavallero, S.; Estrada, K.D.; Sandovici, I.; Kumar, S.R.; Makita, T.; Lien, C.; Constancia, M.; et al. Development of Structures and Transport Functions in the Mouse Placenta Development of Structures and Transport. Physiology 2015, 20, 180–193. [Google Scholar] [CrossRef]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal Growth Restriction: Current Knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Expert Reviews Pathophysiology of Placental-Derived Fetal Growth Restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Sferruzzi-perri, A.N.; Camm, E.J. The Programming Power of the Placenta. Front. Physiol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Coan, P.M.; Vaughan, O.R.; Sekita, Y.; Finn, S.L.; Burton, G.J.; Constancia, M.; Fowden, A.L. Adaptations in Placental Phenotype Support Fetal Growth during Undernutrition of Pregnant Mice. J. Physiol. 2010, 588, 527–538. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Vaughan, O.R.; Haro, M.; Cooper, W.N.; Musial, B.; Charalambous, M.; Pestana, D.; Ayyar, S.; Ferguson-Smith, A.C.; Burton, G.J.; et al. An Obesogenic Diet during Mouse Pregnancy Modifies Maternal Nutrient Partitioning and the Fetal Growth Trajectory. FASEB J. 2013, 27, 3928–3937. [Google Scholar] [CrossRef]
- Higgins, J.S.; Vaughan, O.R.; Fernandez de Liger, E.; Fowden, A.L.; Sferruzzi-Perri, A.N. Placental Phenotype and Resource Allocation to Fetal Growth Are Modified by the Timing and Degree of Hypoxia during Mouse Pregnancy. J. Physiol. 2016, 594, 1341–1356. [Google Scholar] [CrossRef]
- Ganguly, A.; McKnight, R.A.; Raychaudhuri, S.; Shin, B.C.; Ma, Z.; Moley, K.; Devaskar, S.U. Glucose Transporter Isoform-3 Mutations Cause Early Pregnancy Loss and Fetal Growth Restriction. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1241–E1255. [Google Scholar] [CrossRef] [Green Version]
- Constância, M.; Angiolini, E.; Sandovici, I.; Smith, P.; Smith, R.; Kelsey, G.; Dean, W.; Ferguson-smith, A.; Sibley, C.P.; Reik, W.; et al. Adaptation of Nutrient Supply to Fetal Demand in the Mouse Involves Interaction between the Igf2 Gene and Placental Transporter Systems. Proc. Natl. Acad. Sci. USA 2005, 102, 19219–19224. [Google Scholar] [CrossRef]
- Wyrwoll, C.S.; Seckl, J.R.; Holmes, M.C. Altered Placental Function of 11β-Hydroxysteroid Dehydrogenase 2 Knockout Mice. Endocrinology 2009, 150, 1287–1293. [Google Scholar] [CrossRef]
- López-Tello, J.; Pérez-García, V.; Khaira, J.; Kusinski, L.C.; Cooper, W.N.; Andreani, A.; Grant, I.; de Liger, E.F.; Lam, B.Y.H.; Hemberger, M.; et al. Fetal and Trophoblast PI3K P110α Have Distinct Roles in Regulating Resource Supply to the Growing Fetus in Mice. eLife 2019, 8, e45282. [Google Scholar] [CrossRef]
- Siragher, E.; Sferruzzi-Perri, A.N. Placental Hypoxia: What Have We Learnt from Small Animal Models? Placenta 2021, 113, 29–47. [Google Scholar] [CrossRef]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-Induced Maternal Obesity Impacts Feto-Placental Growth and Induces Sex-Specific Alterations in Placental Morphology, Mitochondrial Bioenergetics, Dynamics, Lipid Metabolism and Oxidative Stress in Mice. Acta Physiol. Oxf. Engl. 2022, 234, e13795. [Google Scholar] [CrossRef]
- Cuffe, J.S.M.; Walton, S.L.; Singh, R.R.; Spiers, J.G.; Wilkinson, L.; Little, M.H.; Moritz, K.M. Mid- to Late Term Hypoxia in the Mouse Alters Placental Morphology, Glucocorticoid Regulatory Pathways and Nutrient Transporters in a Sex-Specific Manner. J. Physiol. 2014, 14, 3127–3141. [Google Scholar] [CrossRef]
- Cuffe, J.S.M.; Dickinson, H.; Simmons, D.G.; Moritz, K.M. Sex Specific Changes in Placental Growth and MAPK Following Short Term Maternal Dexamethasone Exposure in the Mouse. Placenta 2011, 32, 981–989. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; López-Tello, J.; Fowden, A.L.; Constancia, M. Maternal and Fetal Genomes Interplay through Phosphoinositol 3-Kinase(PI3K)-P110α Signaling to Modify Placental Resource Allocation. Proc. Natl. Acad. Sci. USA 2016, 113, 11255–11260. [Google Scholar] [CrossRef]
- Aye, I.L.M.H.; Aiken, C.E.; Charnock-Jones, D.S.; Smith, G.C.S. Placental Energy Metabolism in Health and Disease—Significance of Development and Implications for Preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S928–S944. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Salazar-Petres, E.; Webb, L.; Fowden, A.L.; Sferruzzi-Perri, A.N. Ablation of PI3K-P110alpha Impairs Maternal Metabolic Adaptations to Pregnancy. Front. Cell Dev. Biol. 2022, 10, 928210. [Google Scholar] [CrossRef]
- Salazar-Petres, E.; Carvalho, D.P.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. Placental Structure, Function and Mitochondrial Phenotype Relate to Fetal Size in Each Fetal Sex in Mice. Biol. Reprod. 2022, 106, 1292–1311. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Kriplani, N.; Hermida, M.A.; Brown, E.R.; Leslie, N.R. Class I PI 3-Kinases: Function and Evolution. Adv. Biol. Regul. 2015, 59, 53–64. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Vaughan, O.R.; Coan, P.M.; Suciu, M.C.; Darbyshire, R.; Constancia, M.; Burton, G.J.; Fowden, A.L. Placental-Specific Igf2 Deficiency Alters Developmental Adaptations to Undernutrition in Mice. Endocrinology 2011, 152, 3202–3212. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Sferruzzi-Perri, A.N.; Coan, P.M.; Fowden, A.L. Adaptations in Placental Phenotype Depend on Route and Timing of Maternal Dexamethasone Administration in Mice. Biol. Reprod. 2013, 89, 80. [Google Scholar] [CrossRef]
- Foukas, L.C.; Claret, M.; Pearce, W.; Okkenhaug, K.; Meek, S.; Peskett, E.; Sancho, S.; Smith, A.J.H.; Withers, D.J.; Vanhaesebroeck, B. Critical Role for the P110α Phosphoinositide-3-OH Kinase in Growth and Metabolic Regulation. Nature 2006, 441, 366–370. [Google Scholar] [CrossRef]
- Nelson, V.L.B.; Ballou, L.M.; Lin, R.Z. Energy Balancing by Fat Pik3ca. Adipocyte 2015, 4, 70–74. [Google Scholar] [CrossRef]
- Li, M.E.; Lauritzen, H.P.M.M.; O’Neill, B.T.; Wang, C.H.; Cai, W.; Brandao, B.B.; Sakaguchi, M.; Tao, R.; Hirshman, M.F.; Softic, S.; et al. Role of P110a Subunit of PI3-Kinase in Skeletal Muscle Mitochondrial Homeostasis and Metabolism. Nat. Commun. 2019, 10, 3412. [Google Scholar] [CrossRef]
- Napso, T.; Hung, Y.P.; Davidge, S.T.; Care, A.S.; Sferruzzi-Perri, A.N. Advanced Maternal Age Compromises Fetal Growth and Induces Sex-Specific Changes in Placental Phenotype in Rats. Sci. Rep. 2019, 9, 16916. [Google Scholar] [CrossRef]
- Aykroyd, B.R.L.; Tunster, S.J.; Sferruzzi-Perri, A.N. Igf2 Deletion Alters Mouse Placenta Endocrine Capacity in a Sexually Dimorphic Manner. J. Endocrinol. 2020, 246, 93–108. [Google Scholar] [CrossRef]
- Barke, T.L.; Money, K.M.; Du, L.; Serezani, A.; Gannon, M.; Mirnics, K.; Aronoff, D.M. Sex Modifies Placental Gene Expression in Response to Metabolic and Inflammatory Stress. Placenta 2019, 78, 1–9. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.I.; Simmons, D.G.; Dickinson, H.; Moritz, K.M. Review: Sexual Dimorphism in the Formation, Function and Adaptation of the Placenta. Placenta 2017, 54, 10–16. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef]
- Desler, C.; Hansen, T.L.; Frederiksen, J.B.; Marcker, M.L.; Singh, K.K.; Juel Rasmussen, L. Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? J. Aging Res. 2012, 2012, 192503. [Google Scholar] [CrossRef]
- Hastie, R.; Lappas, M. The Effect of Pre-Existing Maternal Obesity and Diabetes on Placental Mitochondrial Content and Electron Transport Chain Activity. Placenta 2014, 35, 673–683. [Google Scholar] [CrossRef]
- Song, H.; Telugu, B.P.; Thompson, L.P. Sexual Dimorphism of Mitochondrial Function in the Hypoxic Guinea Pig Placenta†. Biol. Reprod. 2019, 100, 208–216. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Guo, C.; Myatt, L.; Maloyan, A. Sexual Dimorphism in MiR-210 Expression and Mitochondrial Dysfunction in the Placenta with Maternal Obesity. Int. J. Obes. 2015, 39, 1274–1281. [Google Scholar] [CrossRef]
- Wang, Y.; Bucher, M.; Myatt, L. Use of Glucose, Glutamine and Fatty Acids for Trophoblast Respiration in Lean, Obese and Gestational Diabetic Women. J. Clin. Endocrinol. Metab. 2019, 104, 4178–4187. [Google Scholar] [CrossRef]
- Foukas, L.C.; Bilanges, B.; Bettedi, L.; Pearce, W.; Ali, K.; Sancho, S.; Withers, D.J.; Vanhaesebroeck, B. Long-Term P110 a PI3K Inactivation Exerts a Beneficial Effect on Metabolism. EMBO Mol. Med. 2013, 5, 563–571. [Google Scholar] [CrossRef]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J.; Street, M.E.; Viani, I.; Ziveri, M.A.; et al. Impairment of Insulin Receptor Signal Transduction in Placentas of Intra-Uterine Growth-Restricted Newborns and Its Relationship with Fetal Growth. Eur. J. Endocrinol. 2011, 164, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of Placental Translation Inhibition and Endoplasmic Reticulum Stress in the Etiology of Human Intrauterine Growth Restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Musial, B.; Vaughan, O.R.; Fernandez-twinn, D.S.; Voshol, P.; Ozanne, S.E.; Fowden, A.L.; Sferruzzi-perri, A.N. A Western-Style Obesogenic Diet Alters Maternal Metabolic Physiology with Consequences for Fetal Nutrient Acquisition in Mice Key Points. J. Physiol. 2017, 595, 4875–4892. [Google Scholar] [CrossRef] [PubMed]
- Musial, B.; Fernandez-Twinn, D.S.; Duque-Guimaraes, D.; Carr, S.K.; Fowden, A.L.; Ozanne, S.E.; Sferruzzi-Perri, A.N. Exercise Alters the Molecular Pathways of Insulin Signaling and Lipid Handling in Maternal Tissues of Obese Pregnant Mice. Physiol. Rep. 2019, 7, e14202. [Google Scholar] [CrossRef] [PubMed]
- Määttä, J.; Sissala, N.; Dimova, E.Y.; Serpi, R.; Moore, L.G.; Koivunen, P. Hypoxia Causes Reductions in Birth Weight by Altering Maternal Glucose and Lipid Metabolism. Sci. Rep. 2018, 8, 13583. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.-W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, E.; Kirschenman, R.; Spaans, F.; Holody, C.D.; Phillips, T.E.J.; Case, C.P.; Murphy, M.P.; Lemieux, H.; Davidge, S.T. Nanoparticle-Encapsulated Antioxidant Improves Placental Mitochondrial Function in a Sexually Dimorphic Manner in a Rat Model of Prenatal Hypoxia. FASEB J. 2021, 35, e21338. [Google Scholar] [CrossRef]

| Parameter Measured | Function | Reagents | Required Calculations |
|---|---|---|---|
| Complex I OXPHOS | O2 consumption linked to ATP synthesis via Complex I | Pyruvate (20 mM) and glutamate (10 mM) | Raw O2 consumption after glutamate addition |
| Complex II | O2 consumption linked to ATP synthesis via Complex II | Malonate (1 µM) | Difference between O2 consumption before and after adding malonate |
| Complex I + II OXPHOS | Complex I and II dependent oxidative phosphorylation | Succinate (10 mM) | Raw O2 consumption after succinate addition |
| Total ETS | Maximal uncoupled ETS-respiratory capacity | Trifluoromethoxy carbonyl-cyanide phenylhydrazone (FCCP, 3 doses of 0.5 mM) | Raw O2 consumption after 2 doses of FCCP addition |
| Complex IV | Complex IV activity | Sodium ascorbate (2 mM), N, N, N’, N’-tetramethyl-p-phenylenediamine (TMPD, 0.5 mM), and sodium azide (200 mM). | Correction for chemical background oxygen consumption in the presence of sodium azide after sodium ascorbate and TMPD addition |
| Reserve capacity | Mitochondrial capacity to produce extra ATP by OXPHOS | Succinate (10 mM) and FCCP (3 doses of 0.5 mM) | The difference between total ETS and CI + IIP values: Reserve = Total ETS—(CI + II)P |
| FAO | Fatty acid oxidation | ADP (5 mM) | Raw O2 consumption after ADP addition |
| WT Female × α/+ Male (n = 5) | α/+ Female × WT Male (n = 7) | p Value | |
|---|---|---|---|
| Starting weight (g) | 22.7 ± 0.40 | 20.2 ± 0.54 | 0.006 |
| Hysterectomy weight (g) | 24 ± 1.26 | 21.4 ± 0.67 | 0.08 |
| Absolute weights | |||
| Gonadal fat (mg) | 505 ± 31 | 318 ± 43.5 | 0.009 |
| Retroperitoneal fat (mg) | 78.3 ± 9.29 | 58.1 ± 9.50 | 0.17 |
| Renal fat (mg) | 111 ± 18.60 | 97.4 ± 19.40 | 0.64 |
| Mesenteric fat (mg) | 267 ± 28.7 | 136 ± 15.8 | 0.001 |
| Subcutaneous inguinal fat (mg) | 444 ± 26.7 | 380 ± 28.7 | 0.14 |
| Subcutaneous dorsal fat (mg) | 299 ± 36.5 | 236 ± 31.2 | 0.21 |
| Kidneys (mg) | 279 ± 7.97 | 246 ± 14.5 | 0.11 |
| Spleen (mg) | 70.8 ± 2.54 | 90.7 ± 15.1 | 0.30 |
| Ratios | |||
| Gonadal fat (%) | 2.12 ± 0.15 | 1.47 ± 0.17 | 0.025 |
| Retroperitoneal fat (%) | 0.32 ± 0.03 | 0.26 ± 0.03 | 0.28 |
| Renal fat (%) | 0.46 ± 0.08 | 0.44 ± 0.07 | 0.85 |
| Mesenteric fat (%) | 1.10 ± 0.07 | 0.62 ± 0.05 | 0.0005 |
| Subcutaneous inguinal fat (%) | 1.87 ± 0.12 | 1.76 ± 0.09 | 0.50 |
| Subcutaneous dorsal fat (%) | 1.25 ± 0.13 | 1.09 ± 0.11 | 0.38 |
| Kidneys (%) | 1.17 ± 0.04 | 1.15 ± 0.03 | 0.68 |
| Spleen (%) | 0.30 ± 0.02 | 0.42 ± 0.07 | 0.20 |
| WT Female × α/+ Male (n = 5) | α/+ Female × WT Male (n = 7) | p Value | |
|---|---|---|---|
| Litter size | 7.20 ± 0.99 | 7.27 ± 0.57 | 0.85 |
| % Females | 41.67 ± 0.35 | 55.77 ± 0.34 | 0.28 |
| % Males | 58.33 ± 0.36 | 44.23 ± 0.36 | 0.41 |
| % α/+ Females | 18.69 ± 6.58 | 28.57 ± 8.32 | 0.42 |
| % WT Females | 25.63 ± 9.96 | 24.29 ± 4.88 | 0.91 |
| % α/+ Males | 22.74 ± 7.14 | 17.43 ± 4.92 | 0.59 |
| % WT Males | 32.94 ± 2.93 | 29.71 ± 10.43 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Carvalho, D.; Salazar-Petres, E.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. Maternal and Fetal PI3K-p110α Deficiency Induces Sex-Specific Changes in Conceptus Growth and Placental Mitochondrial Bioenergetic Reserve in Mice. Vet. Sci. 2022, 9, 501. https://doi.org/10.3390/vetsci9090501
Pereira-Carvalho D, Salazar-Petres E, Lopez-Tello J, Sferruzzi-Perri AN. Maternal and Fetal PI3K-p110α Deficiency Induces Sex-Specific Changes in Conceptus Growth and Placental Mitochondrial Bioenergetic Reserve in Mice. Veterinary Sciences. 2022; 9(9):501. https://doi.org/10.3390/vetsci9090501
Chicago/Turabian StylePereira-Carvalho, Daniela, Esteban Salazar-Petres, Jorge Lopez-Tello, and Amanda N. Sferruzzi-Perri. 2022. "Maternal and Fetal PI3K-p110α Deficiency Induces Sex-Specific Changes in Conceptus Growth and Placental Mitochondrial Bioenergetic Reserve in Mice" Veterinary Sciences 9, no. 9: 501. https://doi.org/10.3390/vetsci9090501
APA StylePereira-Carvalho, D., Salazar-Petres, E., Lopez-Tello, J., & Sferruzzi-Perri, A. N. (2022). Maternal and Fetal PI3K-p110α Deficiency Induces Sex-Specific Changes in Conceptus Growth and Placental Mitochondrial Bioenergetic Reserve in Mice. Veterinary Sciences, 9(9), 501. https://doi.org/10.3390/vetsci9090501







