Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Serum Biochemical Index Evaluation
2.3. Antioxidant Evaluation
2.4. Histopathological Evaluation
2.5. Total RNA Isolation and Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
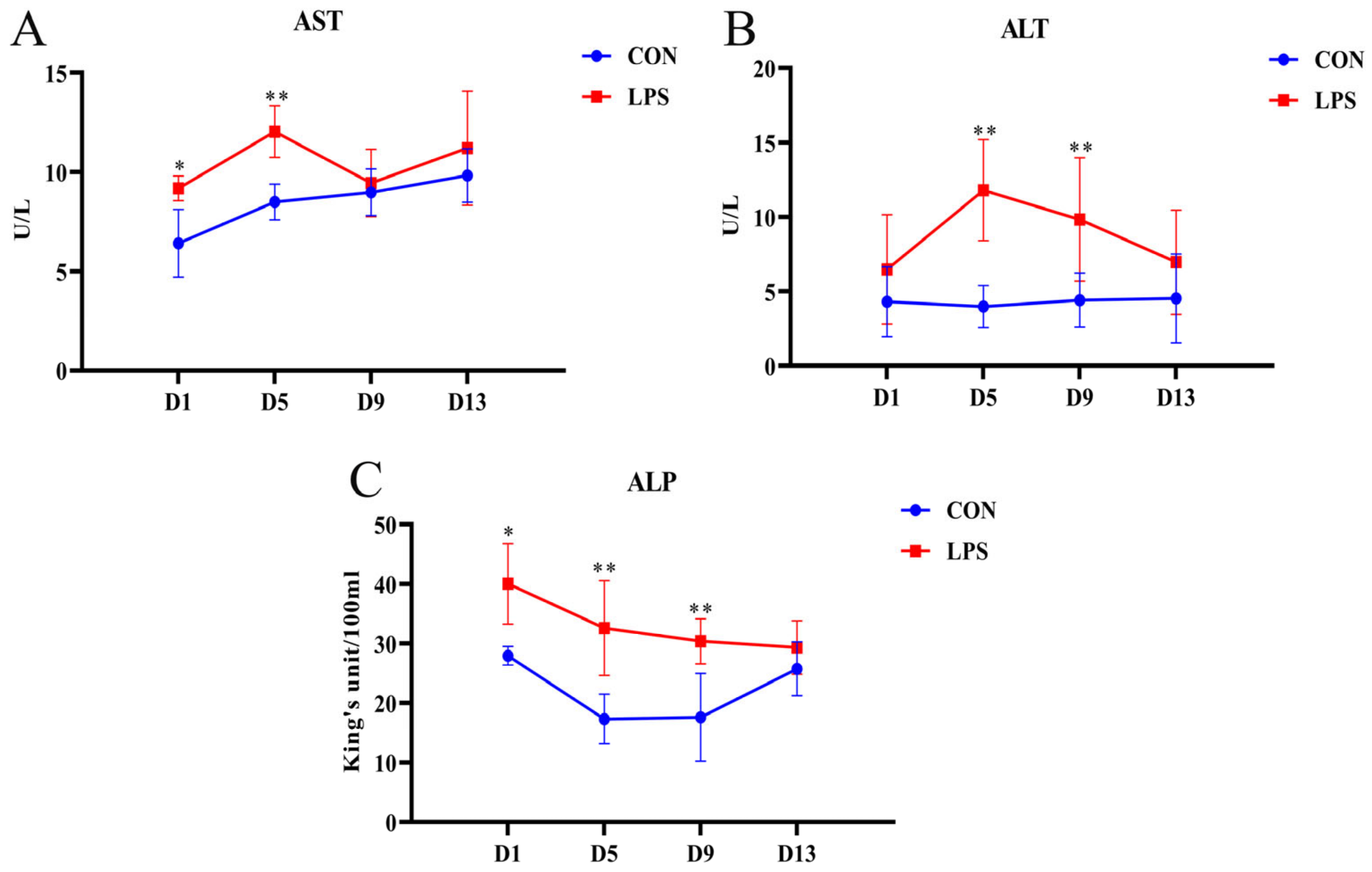
3.1. Changes in Serum Biochemical Indexes
3.2. Antioxidant Capacity
3.2.1. Changes in Serum Antioxidant Parameters
3.2.2. Changes in the Antioxidant Parameters of Livers
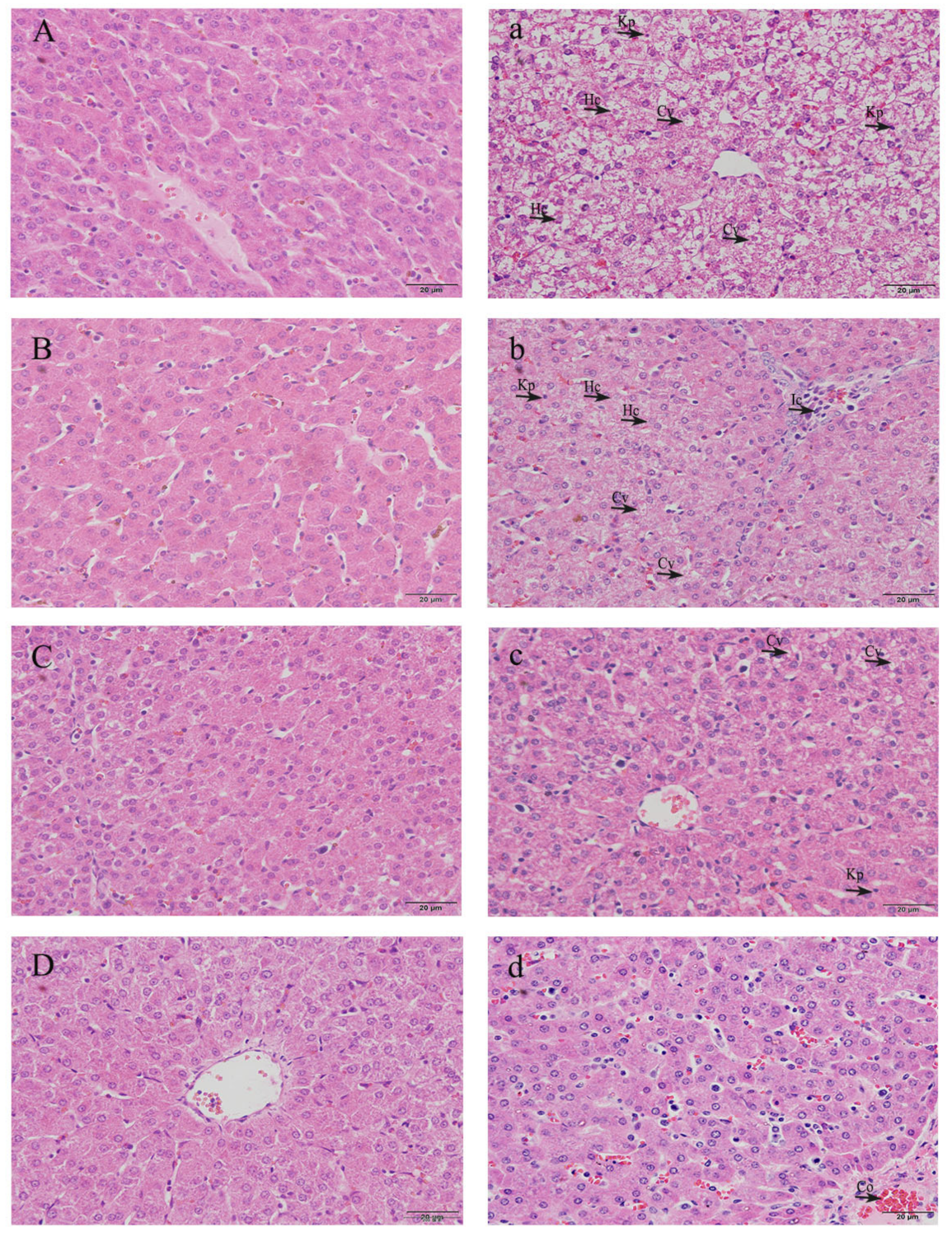
3.3. Histopathological Observation of Livers
3.4. Gene mRNA Expression in Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhainaut, J.F.; Marin, N.; Mignon, A.; Vinsonneau, C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit. Care Med. 2001, 29, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, W.; Zhang, W.; Yuan, Y.; Zhu, X.; Wang, Q.; Wei, Y.; Yang, D.; Yang, C.; Chen, Y.; et al. Histone methyltransferase G9a protects against acute liver injury through GSTP1. Cell Death Differ. 2020, 27, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Carteni, M.; Nardone, G. Gut-liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Bracarense, A.; Pinton, P. Deoxynivalenol induces apoptosis and inflammation in the liver: Analysis using precision-cut liver slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.Y.; Kim, M.K.; Lee, S.H.; Hwang, J.S.; Park, K.G.; Jang, B.K. Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver-guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Kaur, G.; Tirkey, N.; Chopra, K. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology 2006, 226, 152–160. [Google Scholar] [CrossRef]
- Elazab, M.F.A.; Nasr, N.E.; Ahmed, M.S.; Alrashdi, B.M.; Dahran, N.; Alblihed, M.A.; Elmahallawy, E.K. The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes. Vet. Sci. 2022, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury. J. Gastroenterol. Hepatol. 2000, 15, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Jiang, X.; Chen, L.; Hong, L.; Zhuo, Y.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2020, 98, skaa067. [Google Scholar] [CrossRef]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart. Circ. Physiol. 2001, 280, H509–H521. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Backhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, K.; Cao, T.; Zhang, J.; Lian, M.; Huang, D.; Wei, C.; Gu, Z.; Feng, X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 2018, 70, 1023–1035. [Google Scholar] [CrossRef]
- Sabroe, I.; Parker, L.C.; Dower, S.K.; Whyte, M.K. The role of TLR activation in inflammation. J. Pathol. 2008, 214, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, N.; Saitoh, S.; Matsumoto, F.; Akashi-Takamura, S.; Miyake, K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem. Biophys. Res. Commun. 2008, 368, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Motshwene, P.G.; Moncrieffe, M.C.; Grossmann, J.G.; Kao, C.; Ayaluru, M.; Sandercock, A.M.; Robinson, C.V.; Latz, E.; Gay, N.J. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 2009, 284, 25404–25411. [Google Scholar] [CrossRef]
- Kim, I.D.; Ha, B.J. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch. Pharm. Res. 2010, 33, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Garcia-Conesa, M.T.; Tomas-Barberan, F.; Espin, J.C. Lack of effect of oral administration of resveratrol in LPS-induced systemic inflammation. Eur. J. Nutr. 2011, 50, 673–680. [Google Scholar] [CrossRef]
- Carroll, J.A.; Touchette, K.J.; Matteri, R.L.; Dyer, C.J.; Allee, G.L. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: II. Effects on the hypothalamic-pituitary-adrenal axis of weaned pigs. J. Anim. Sci. 2002, 80, 502–509. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294. [Google Scholar] [CrossRef]
- Tsai, T.H.; Tam, K.; Chen, S.F.; Liou, J.Y.; Tsai, Y.C.; Lee, Y.M.; Huang, T.Y.; Shyue, S.K. Deletion of caveolin-1 attenuates LPS/GalN-induced acute liver injury in mice. J. Cell Mol. Med. 2018, 22, 5573–5582. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Che, Z.; Zhu, H.; Meng, G.; Hou, Y.; Ding, B.; Yin, Y.; Chen, F. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immun. 2012, 18, 804–814. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Zhu, H.; Wang, Y.; Hou, Y.; Liu, Y. Dietary fish oil supplementation alters liver gene expressions to protect against LPS-induced liver injury in weanling piglets. Innate Immun. 2019, 25, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nykonenko, A.; Vávra, P.; Zonča, P. Anatomic Peculiarities of Pig and Human Liver. Exp. Clin. Transplant. 2017, 15, 21–26. [Google Scholar] [PubMed]
- Simon, G.A.; Maibach, H.I. The pig as an experimental animal model of percutaneous permeation in man: Qualitative and quantitative observations--an overview. Skin Pharmacol. Appl. Skin Physiol. 2000, 13, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Nyblom, H.; Berggren, U.; Balldin, J.; Olsson, R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004, 39, 336–339. [Google Scholar] [CrossRef]
- Mitra, S.K.; Venkataranganna, M.V.; Sundaram, R.; Gopumadhavan, S. Protective effect of HD-03, a herbal formulation, against various hepatotoxic agents in rats. J. Ethnopharmacol. 1998, 63, 181–186. [Google Scholar] [CrossRef]
- Khan, H.U.; Aamir, K.; Jusuf, P.R.; Sethi, G.; Sisinthy, S.P.; Ghildyal, R.; Arya, A. Lauric acid ameliorates lipopolysaccharide (LPS)-induced liver inflammation by mediating TLR4/MyD88 pathway in Sprague Dawley (SD) rats. Life Sci. 2021, 265, 118750. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.; Zhang, K.; Zhong, M.; Cao, H.; Wei, R.; Jin, L.; Gao, Y. Study on the protective effect and mechanism of Dicliptera chinensis (L.) Juss (Acanthaceae) polysaccharide on immune liver injury induced by LPS. Biomed. Pharmacother. 2021, 134, 111159. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, J.; Li, X.; Wang, Y.; Wang, D.; Xiao, K.; Zhu, H.; Wang, X.; Hu, C.A.; Zhang, G.; et al. Necroptosis Underlies Hepatic Damage in a Piglet Model of Lipopolysaccharide-Induced Sepsis. Front. Immunol. 2021, 12, 633830. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun. Signal. 2019, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, O.; Reichhold, S.; Nics, L.; Hoelzl, C.; Valentini, J.; Stadlmayr, B.; Knasmüller, S.; Wagner, K.-H. Antioxidant responses to an acute ultra-endurance exercise: Impact on DNA stability and indications for an increased need for nutritive antioxidants in the early recovery phase. Br. J. Nutr. 2010, 104, 1129–1138. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, T.; Yi, D.; Wang, L.; Li, P.; Zhang, J.; Hou, Y.; Wu, G. Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model. Int. J. Mol. Sci. 2017, 18, 2535. [Google Scholar] [CrossRef]
- Saita, E.; Kondo, K.; Momiyama, Y. Anti-Inflammatory Diet for Atherosclerosis and Coronary Artery Disease: Antioxidant Foods. Clin. Med. Insights Cardiol. 2015, 8, 61–65. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharm. Immunot. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar] [CrossRef]
- Nandi, D.; Mishra, M.K.; Basu, A.; Bishayi, B. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology 2010, 215, 443–451. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Dunzendorfer, S.; Lee, H.K.; Soldau, K.; Tobias, P.S. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J. Immunol. 2004, 173, 1166–1170. [Google Scholar] [CrossRef]
- Jerala, R. Structural biology of the LPS recognition. Int. J. Med. Microbiol. 2007, 297, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; O'Neill, L.A. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; He, Q.; Li, S.; Liu, T.; Zhang, J. Hydrogen-Rich Water Mitigates LPS-Induced Chronic Intestinal Inflammatory Response in Rats via Nrf-2 and NF-κB Signaling Pathways. Vet. Sci. 2022, 9, 621. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Mietto, B.S.; Kroner, A.; Girolami, E.I.; Santos-Nogueira, E.; Zhang, J.; David, S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2015, 35, 16431–16442. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bai, J.; Gao, C.; Liang, Y.; Zhao, B.; Bian, Y. Chronic unpredictable stress abrogates the endotoxin tolerance induced by repeated peripheral LPS challenge via the TLR4 signaling pathway. Neurosci. Lett. 2017, 645, 7–13. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adrie, C.; Fitting, C.; Adib-Conquy, M. Endotoxin tolerance: Is there a clinical relevance? J. Endotoxin Res. 2003, 9, 101–107. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence (5′→3′) | Product Size (bp) | GenBank No. |
|---|---|---|---|
| GAPDH | F: CCTGGAGAAACCTGCAAAATA R: AACCTGGTCCTCAGTGTAGCC | 100 | NM_001206359.1 |
| TLR4 | F: GACGAAGACTGGGTGAGGAATGAAC R: CCTGGATGATGTTAGCAGCGATGG | 124 | NM_001113039.2 |
| MyD88 | F: CGTCTGGTCCATTGCTAGAACTC R: TTCTGATGGGCACCTGGAGAGAG | 141 | NM_001099923.1 |
| NF-κB | F: CTGAGGCTATAACTCGCTTGGTGAC R: CATGTCCGCAATGGAGGAGAAGTC | 131 | NM_001114281.1 |
| IL-6 | F: ATAAGGGAAATGTCGAGGCTGTGC R: GGGTGGTGGCTTTGTCTGGATTC | 93 | NM_001252429.1 |
| TNF-α | F: TCTATTTTGGGATCATTGCCC R: CCAGCCCCTCATTCTCTTTCT | 127 | NM_214022.1 |
| IL-10 | F: GCATCCACTTCCCAACCA R: GCAACAAGTCGCCCATCT | 108 | NM_214041.1 |
| Items | Group | D1 | D5 | D9 | D13 |
|---|---|---|---|---|---|
| T-AOC (mM) | CON | 0.22 ± 0.020 | 0.21 ± 0.008 | 0.19 ± 0.019 | 0.19 ± 0.039 |
| LPS | 0.16 ± 0.020 ## | 0.16 ± 0.025 # | 0.21 ± 0.037 | 0.19 ± 0.035 | |
| SOD (U/mL) | CON | 28.01 ± 2.48 | 27.73 ± 1.19 | 27.40 ± 0.89 | 29.29 ± 0.67 |
| LPS | 27.65 ± 2.56 | 24.73 ± 1.59 # | 29.92 ± 1.24 | 31.86 ± 0.79 | |
| GSH-Px (U/mL) | CON | 270.80 ± 30.46 | 317.20 ± 28.96 | 260.60 ± 34.96 | 241.48 ± 22.81 |
| LPS | 295.86 ± 34.13 | 245.63 ± 21.37 ## | 258.68 ± 15.93 | 216.05 ± 28.98 | |
| CAT (U/mL) | CON | 6.52 ± 0.98 | 6.86 ± 1.88 | 6.24 ± 2.24 | 7.19 ± 2.21 |
| LPS | 8.58 ± 0.49 | 6.91 ± 1.33 | 6.73 ± 1.86 | 5.97 ± 0.75 | |
| MDA (nmol/mL) | CON | 2.60 ± 0.24 | 2.77 ± 0.72 | 2.65 ± 0.17 | 2.49 ± 0.26 |
| LPS | 2.31 ± 0.22 | 2.38 ± 0.30 | 3.16 ± 0.74 | 3.21 ± 0.83 * |
| Items | Group | D1 | D5 | D9 | D13 |
|---|---|---|---|---|---|
| T-AOC (mmol/gprot) | CON | 0.110 ± 0.021 | 0.093 ± 0.018 | 0.061 ± 0.004 | 0.093 ± 0.035 |
| LPS | 0.086 ± 0.003 | 0.065 ± 0.008 # | 0.063 ± 0.005 | 0.075 ± 0.012 | |
| SOD (U/mgprot) | CON | 95.43 ± 14.18 | 101.21 ± 11.72 | 102.35 ± 13.52 | 102.52 ± 15.03 |
| LPS | 116.62 ± 2.74 * | 119.57 ± 4.03 * | 97.40 ± 7.63 | 95.88 ± 7.12 | |
| GSH-Px (U/mgprot) | CON | 39.67 ± 7.86 | 45.24 ± 7.11 | 44.47 ± 4.17 | 33.29 ± 6.20 |
| LPS | 41.41 ± 3.71 | 33.63 ± 0.44 # | 51.24 ± 3.68 | 32.76 ± 8.74 | |
| CAT (U/mgprot) | CON | 26.21 ± 5.06 | 26.88 ± 7.38 | 33.84 ± 1.25 | 28.34 ± 12.40 |
| LPS | 39.54 ± 5.89 ** | 38.20 ± 6.99 * | 36.53 ± 3.32 | 20.14 ± 2.00 | |
| MDA (nmol/mgprot) | CON | 0.86 ± 0.09 | 0.48 ± 0.24 | 0.60 ± 0.09 | 0.72 ± 0.16 |
| LPS | 0.62 ± 0.15 | 0.55 ± 0.07 | 0.55 ± 0.17 | 0.65 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Hu, X.; Zhong, S.; Yu, W.; Wang, J.; Zhu, W.; Yang, T.; Zhao, G.; Jiang, Y.; Li, Y. Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets. Vet. Sci. 2023, 10, 22. https://doi.org/10.3390/vetsci10010022
Zhou Y, Hu X, Zhong S, Yu W, Wang J, Zhu W, Yang T, Zhao G, Jiang Y, Li Y. Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets. Veterinary Sciences. 2023; 10(1):22. https://doi.org/10.3390/vetsci10010022
Chicago/Turabian StyleZhou, Yunxiao, Xiaofen Hu, Shengwei Zhong, Wanting Yu, Jue Wang, Wenlu Zhu, Tingyu Yang, Guotong Zhao, Yijie Jiang, and Yong Li. 2023. "Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets" Veterinary Sciences 10, no. 1: 22. https://doi.org/10.3390/vetsci10010022
APA StyleZhou, Y., Hu, X., Zhong, S., Yu, W., Wang, J., Zhu, W., Yang, T., Zhao, G., Jiang, Y., & Li, Y. (2023). Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets. Veterinary Sciences, 10(1), 22. https://doi.org/10.3390/vetsci10010022





