A Case of Congenital Pulmonary Vein Stenosis with Secondary Post-Capillary Pulmonary Hypertension and Left Sided Congestive Heart Failure in a Cat
Abstract
Simple Summary
Abstract
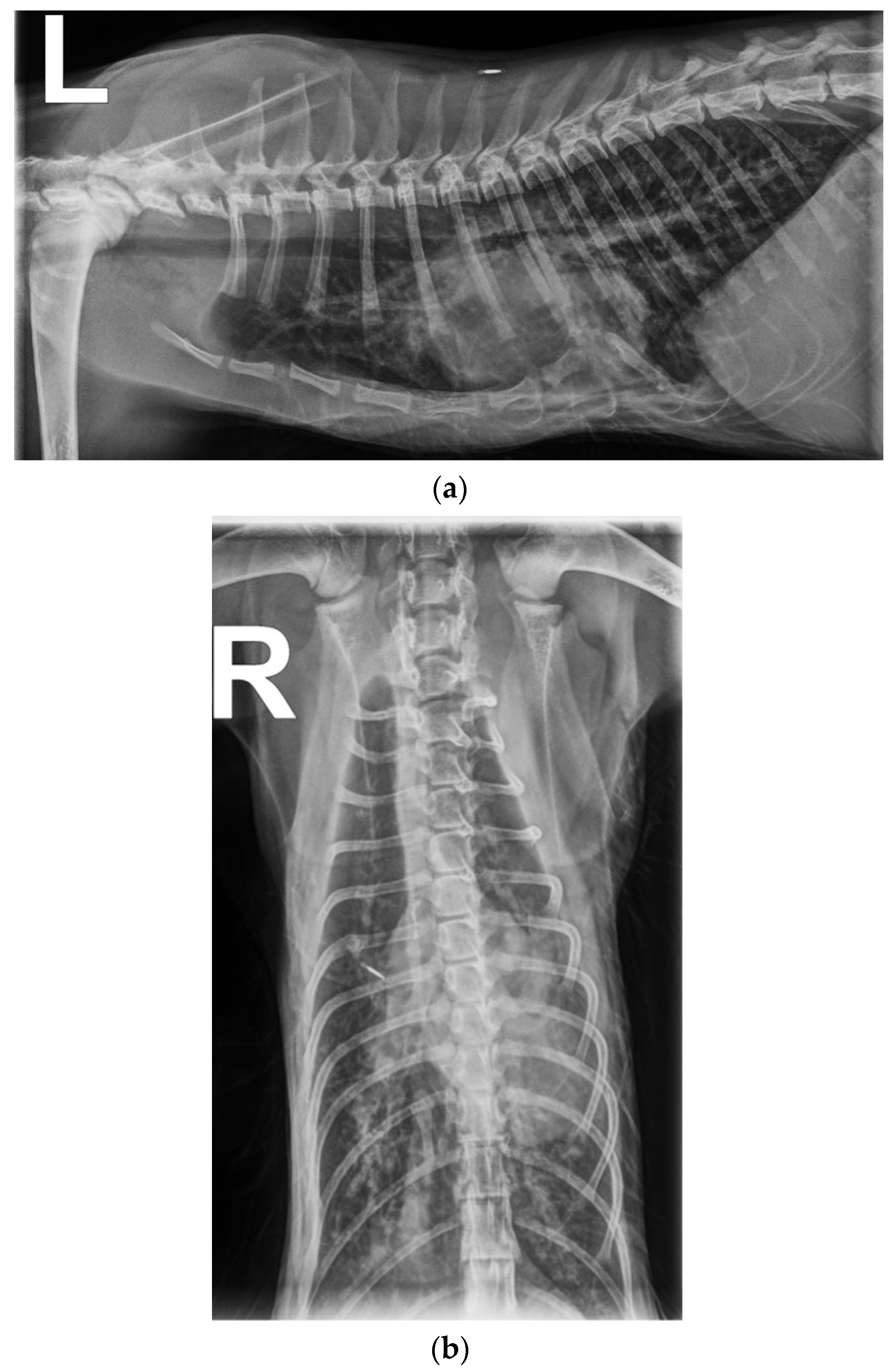
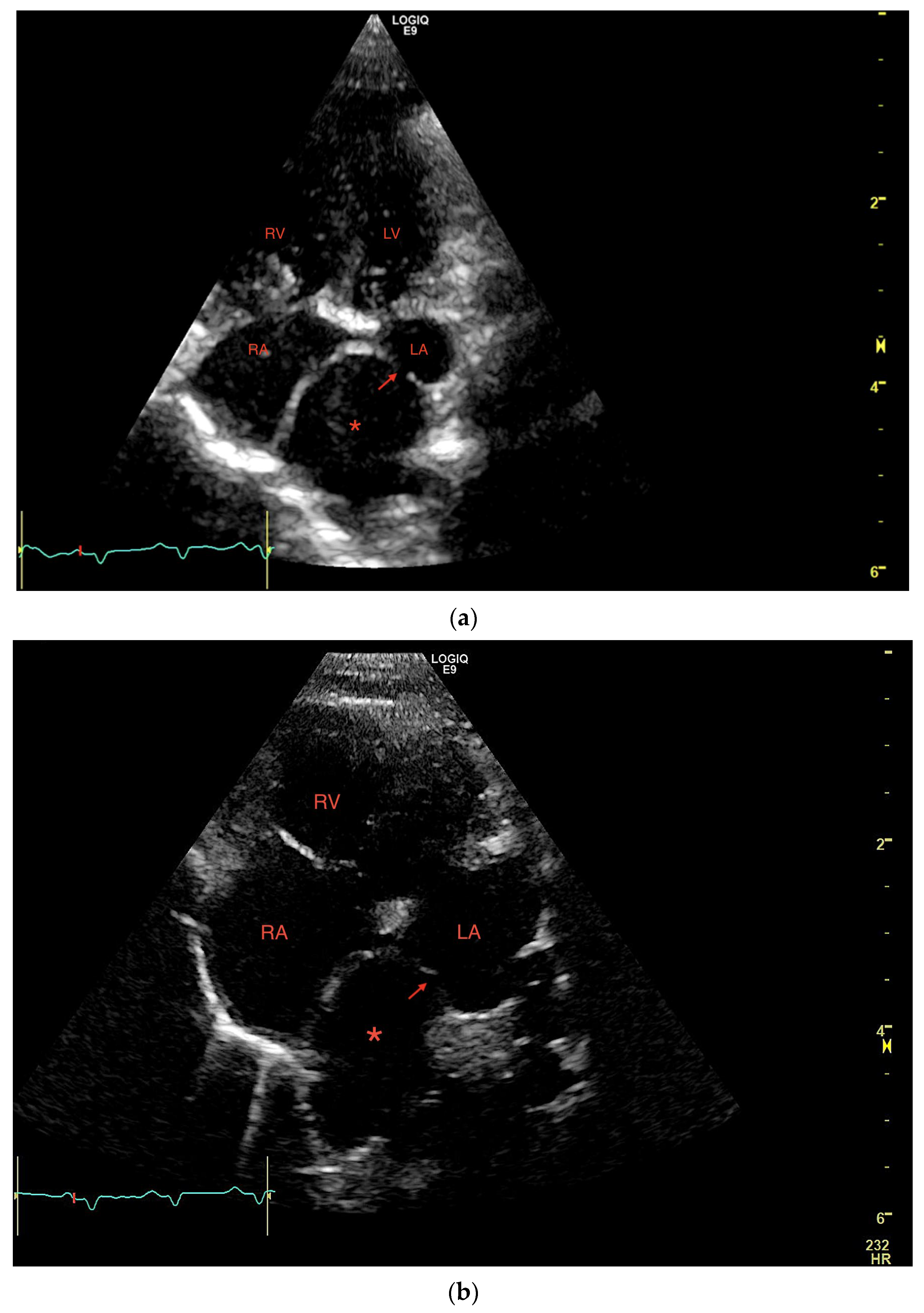
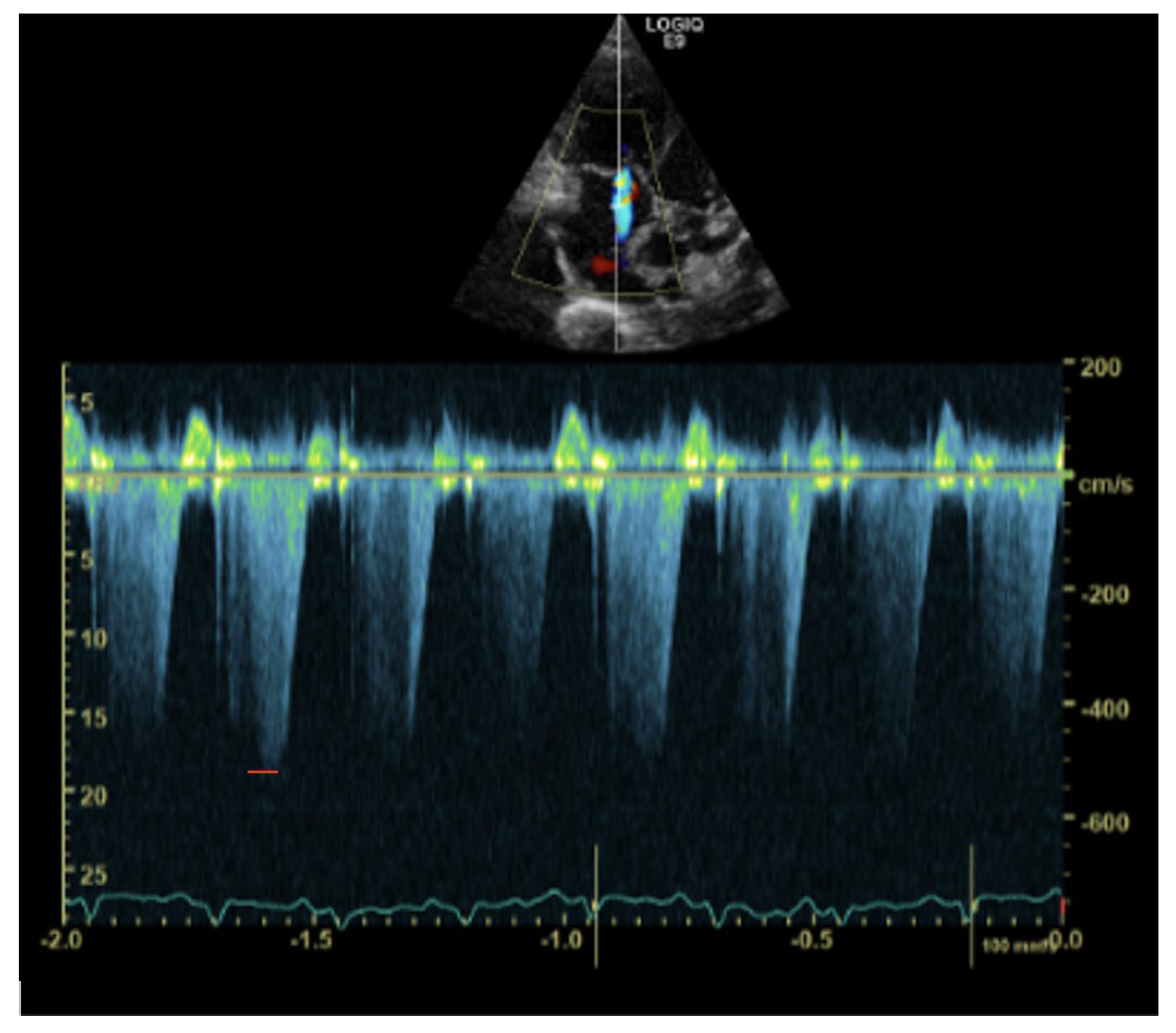
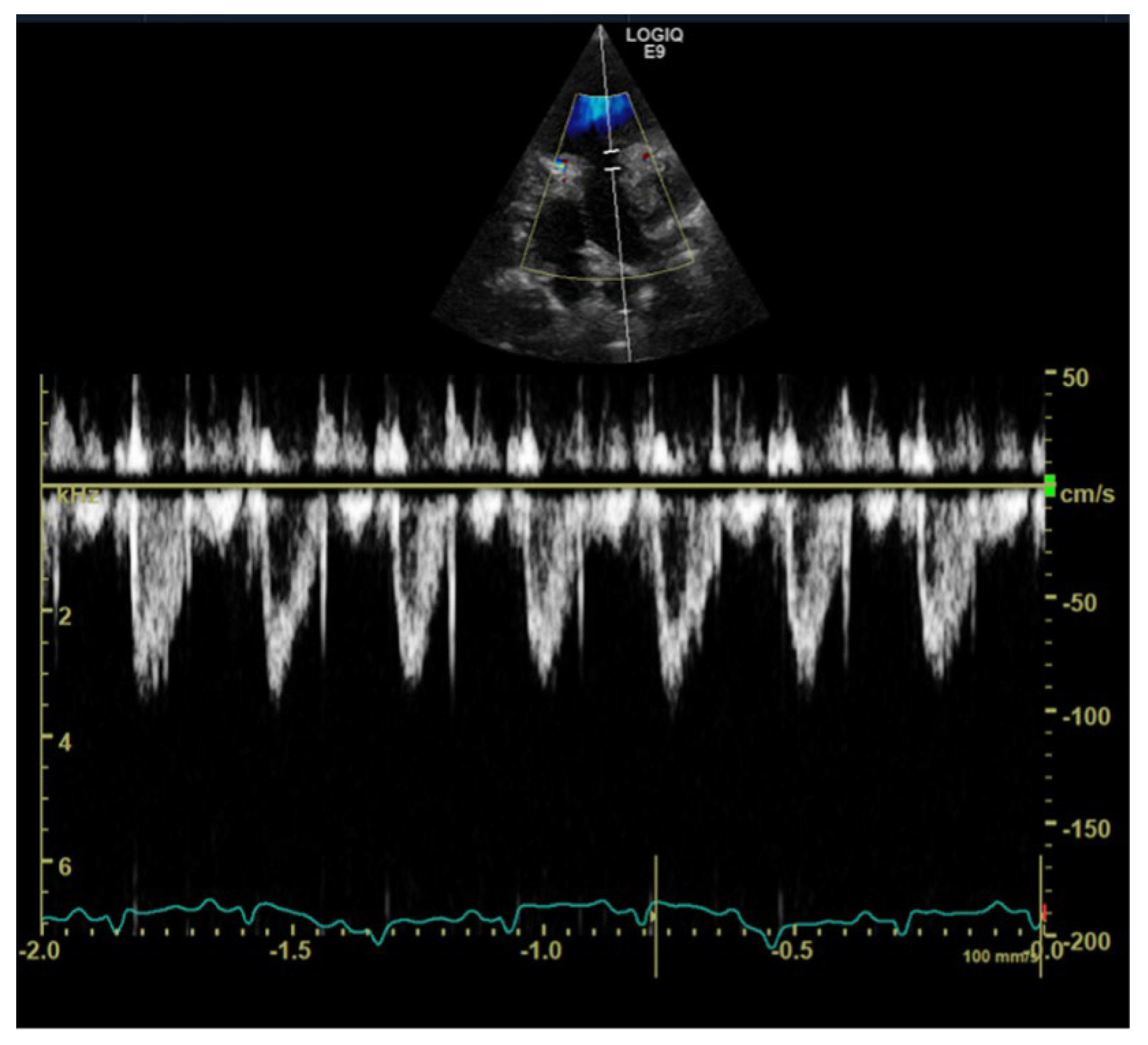
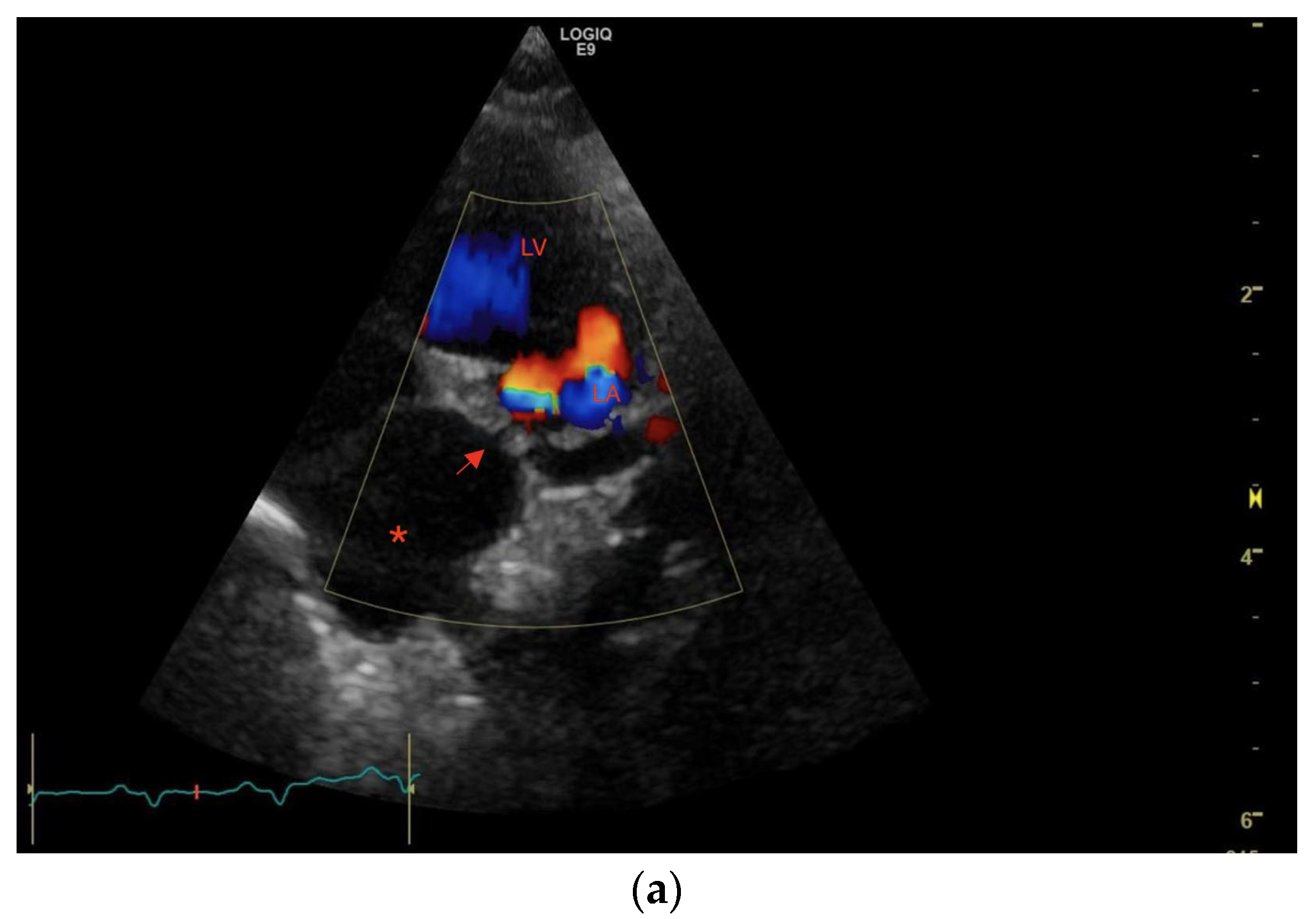
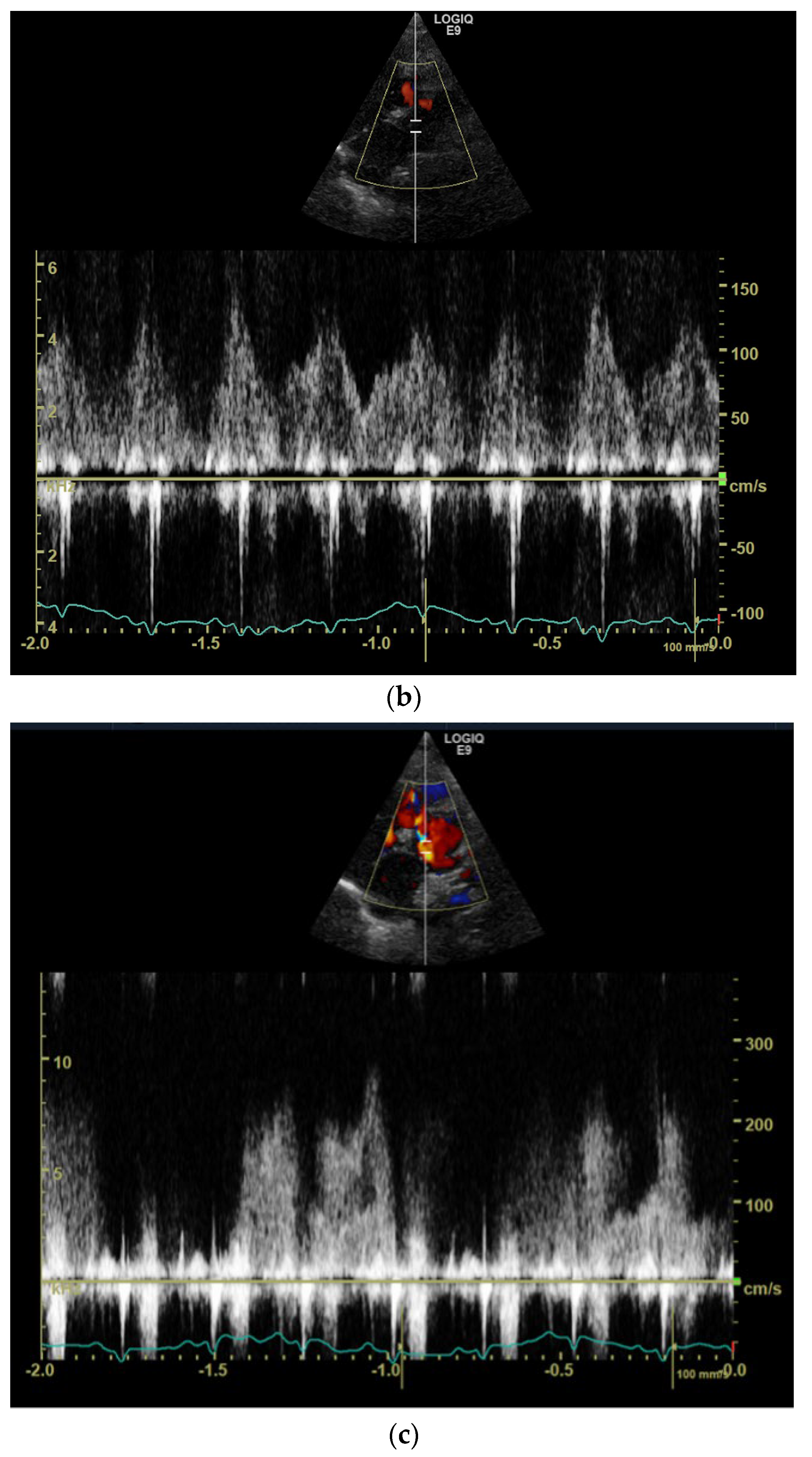
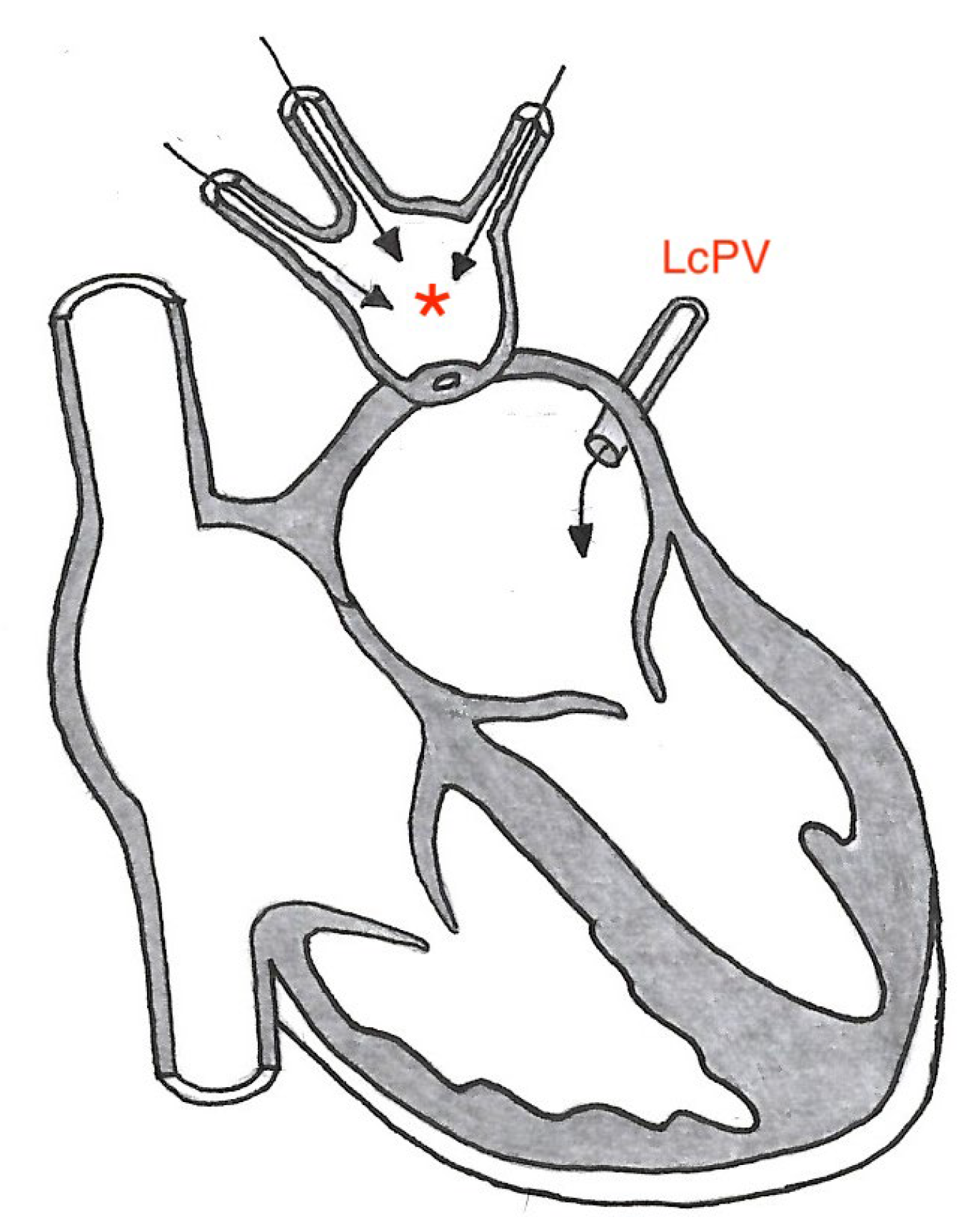
1. Case Description
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Vet, M.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Latson, L.A.; Prieto, L.R. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.J.; Doyle, T.; Ringel, R.E. Pulmonary vein stenosis. Hum. Pathol. 1995, 26, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.D.; Driscoll, D.J.; Shaddy, R.E.; Feltes, T.F. Moss & Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Saad, E.B.; Rossillo, A.; Saad, C.P.; Martin, D.O.; Bhargava, M.; Erciyes, D.; Bash, D.; Williams-Andrews, M.; Beheiry, S.; Marrouche, N.F. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: Functional characterization, evolution, and influence of the ablation strategy. Circulation 2003, 108, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.A.; Morel, K.J.C.; Viswanathan, M.N.; de Jesus Perez, V.A. Pulmonary Vein Stenosis and Pulmonary Hypertension Following a Catheter-Based Radiofrequency Ablation for Atrial Fibrillation: A Case Report. Am. J. Case Rep. 2020, 21, e924709. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Haines, D.E.; Verma, A.; Packer, D.; Kirchhof, N.; Barka, N.; Onal, B.; Fraasch, S.; Miklavčič, D.; Stewart, M.T. Reduction in pulmonary vein stenosis and collateral damage with pulsed field ablation compared with radiofrequency ablation in a canine model. Circ. Arrhythmia Electrophysiol. 2020, 13, e008337. [Google Scholar] [CrossRef]
- Abdulla, R.-i.; Blew, G.; Holterman, M. Cardiovascular embryology. Pediatr. Cardiol. 2004, 25, 191–200. [Google Scholar] [CrossRef]
- Van den Berg, G.; Moorman, A.F. Development of the pulmonary vein and the systemic venous sinus: An interactive 3D overview. PLoS ONE 2011, 6, e22055. [Google Scholar] [CrossRef]
- Sade, R.M.; Freed, M.D.; Matthews, E.C.; Castaneda, A.R. Stenosis of individual pulmonary veins: Review of the literature and report of a surgical case. J. Thorac. Cardiovasc. Surg. 1974, 67, 953–962. [Google Scholar] [CrossRef]
- Pazos-López, P.; García-Rodríguez, C.; Guitián-González, A.; Paredes-Galán, E.; Álvarez, M.Á.D.L.G.; Rodríguez-Álvarez, M.; Baz-Alonso, J.A.; Teijeira-Fernández, E.; Calvo-Iglesias, F.E.; Íñiguez-Romo, A. Pulmonary vein stenosis: Etiology, diagnosis and management. World J. Cardiol. 2016, 8, 81. [Google Scholar] [CrossRef]
- Jha, A.K.; Makhija, N. Cor triatriatum: A review. In Seminars in Cardiothoracic and Vascular Anesthesia; SAGE Publications: Los Angeles, CA, USA, 2017; pp. 178–185. [Google Scholar]
- Bharucha, T.; Spicer, D.E.; Mohun, T.J.; Black, D.; Henry, G.W.; Anderson, R.H. Cor triatriatum or divided atriums: Which approach provides the better understanding? Cardiol. Young 2015, 25, 193–207. [Google Scholar] [CrossRef]
- Sivakumar, K.; Satish, R.; Tailor, K.; Coelho, R. Transcatheter management of subtotal cor triatriatum sinister: A rare anomaly. Pediatr. Cardiol. 2008, 29, 812–815. [Google Scholar] [CrossRef]
- Chennadi, S.; Zulqarnain, S. A rare case of subtotal cor triatriatum sinistrum. Chest 2015, 148, 976A. [Google Scholar] [CrossRef]
- Ward, J.L.; Lisciandro, G.R.; Ware, W.A.; Viall, A.K.; Aona, B.D.; Kurtz, K.A.; Reina-Doreste, Y.; DeFrancesco, T.C. Evaluation of point-of-care thoracic ultrasound and NT-proBNP for the diagnosis of congestive heart failure in cats with respiratory distress. J. Vet. Intern. Med. 2018, 32, 1530–1540. [Google Scholar] [CrossRef]
- Smith, S.; Dukes-McEwan, J. Clinical signs and left atrial size in cats with cardiovascular disease in general practice. J. Small Anim. Pract. 2012, 53, 27–33. [Google Scholar] [CrossRef]
- Rostamian, A.; Narayan, S.M.; Thomson, L.; Fishbein, M.; Siegel, R.J. The incidence, diagnosis, and management of pulmonary vein stenosis as a complication of atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Vanderlaan, R.D.; Rome, J.; Hirsch, R.; Ivy, D.; Caldarone, C.A. Pulmonary vein stenosis: Treatment and challenges. J. Thorac. Cardiovasc. Surg. 2021, 161, 2169–2176. [Google Scholar] [CrossRef]
- Kato, H.; Fu, Y.Y.; Zhu, J.; Wang, L.; Aafaqi, S.; Rahkonen, O.; Slorach, C.; Traister, A.; Leung, C.H.; Chiasson, D. Pulmonary vein stenosis and the pathophysiology of “upstream” pulmonary veins. J. Thorac. Cardiovasc. Surg. 2014, 148, 245–253. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Rolph, K.E.; Cavanaugh, S.M. Feline pulmonary hypertension: Are we overlooking an important comorbidity? J. Feline Med. Surg. 2022, 24, e636–e646. [Google Scholar] [CrossRef] [PubMed]
- Pouchelon, J.L.; Chetboul, V.; Devauchelle, P.; Delisle, F.; Mai, W.; Vial, V. Diagnosis of pulmonary thromboembolism in a cat using echocardiography and pulmonary scintigraphy. J. Small Anim. Pract. 1997, 38, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Schober, K. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J. Vet. Cardiol. 2019, 23, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Lamb, C.; Boswood, A. Right-to-left shunting patent ductus arteriosus with pulmonary hypertension in a cat. J. Small Anim. Pract. 2003, 44, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.L.; Rozanski, E.A.; Tidwell, A.S.; Hoffman, A.M. Pulmonary thromboembolism in a heartworm-positive cat. J. Vet. Intern. Med. 2006, 20, 1037–1041. [Google Scholar] [CrossRef]
- Shamberger, R.C.; Welch, K.J.; Castaneda, A.R.; Keane, J.F.; Fyler, D.C. Anterior chest wall deformities and congenital heart disease. J. Thorac. Cardiovasc. Surg. 1988, 96, 427–432. [Google Scholar] [CrossRef]
- Boudrieau, R.; Fossum, T.; Hartsfield, S.; Hobson, H.; Rudy, R. Pectus excavatum in dogs and cats. Compend. Contin. Educ. Pract. Vet. 1990, 12, 341–355. [Google Scholar]
- Charlesworth, T. Pectus excavatum: Congenital thoracic deformity in cats. Practice 2017, 39, 73–78. [Google Scholar] [CrossRef]
- Cobben, J.M.; Oostra, R.-J.; van Dijk, F.S. Pectus excavatum and carinatum. Eur. J. Med. Genet. 2014, 57, 414–417. [Google Scholar] [CrossRef]
- Funabashi, N.; Koyama, G.; Miyauchi, H.; Fujimoto, Y.; Takaoka, H.; Horikoshi, T.; Matsumiya, G.; Kobayashi, Y. Right inferior pulmonary vein stenosis by pectus excavatum in a 35 year old female who underwent coronary artery bypass graft procedure due to kawasaki disease: Differentiation of the occurrence of partial pulmonary vein congestion. J. Am. Coll. Cardiol. 2018, 71, A2380. [Google Scholar] [CrossRef]
- Kindzelski, B.A.; Ghobrial, J.; Schlenk, R.; Pettersson, G.B.; Raymond, D.P. Multidisciplinary Approach to Isolated Pulmonary Vein Compression by an Enlarging Vertebral Osteophyte. Case Rep. 2022, 4, 145–149. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.W.; Orso, F.; Rossignol, P. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Suntharos, P.; Prieto, L.R. Treatment of congenital and acquired pulmonary vein stenosis. Curr. Cardiol. Rep. 2020, 22, 153. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriström, K.; Karlstam, E.; Nielsen, T.; Lagerqvist, A.; Dirven, M. A Case of Congenital Pulmonary Vein Stenosis with Secondary Post-Capillary Pulmonary Hypertension and Left Sided Congestive Heart Failure in a Cat. Vet. Sci. 2023, 10, 23. https://doi.org/10.3390/vetsci10010023
Kriström K, Karlstam E, Nielsen T, Lagerqvist A, Dirven M. A Case of Congenital Pulmonary Vein Stenosis with Secondary Post-Capillary Pulmonary Hypertension and Left Sided Congestive Heart Failure in a Cat. Veterinary Sciences. 2023; 10(1):23. https://doi.org/10.3390/vetsci10010023
Chicago/Turabian StyleKriström, Karin, Erika Karlstam, Tove Nielsen, Anne Lagerqvist, and Mark Dirven. 2023. "A Case of Congenital Pulmonary Vein Stenosis with Secondary Post-Capillary Pulmonary Hypertension and Left Sided Congestive Heart Failure in a Cat" Veterinary Sciences 10, no. 1: 23. https://doi.org/10.3390/vetsci10010023
APA StyleKriström, K., Karlstam, E., Nielsen, T., Lagerqvist, A., & Dirven, M. (2023). A Case of Congenital Pulmonary Vein Stenosis with Secondary Post-Capillary Pulmonary Hypertension and Left Sided Congestive Heart Failure in a Cat. Veterinary Sciences, 10(1), 23. https://doi.org/10.3390/vetsci10010023




