Local and Systemic Antibody Responses in Beef Calves Vaccinated with a Modified-Live Virus Bovine Respiratory Syncytial Virus (BRSV) Vaccine at Birth following BRSV Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Challenge with BRSV
2.3. Clinical Evaluation and Sample Collection
2.4. BRSV Neutralizing Antibodies in Serum
2.5. Determination of Anti-BRSV IgG-1 and IgA in Nasal Secretions
2.6. Real-Time Reverse Transcription PCR
2.7. Statistical Analysis
3. Results
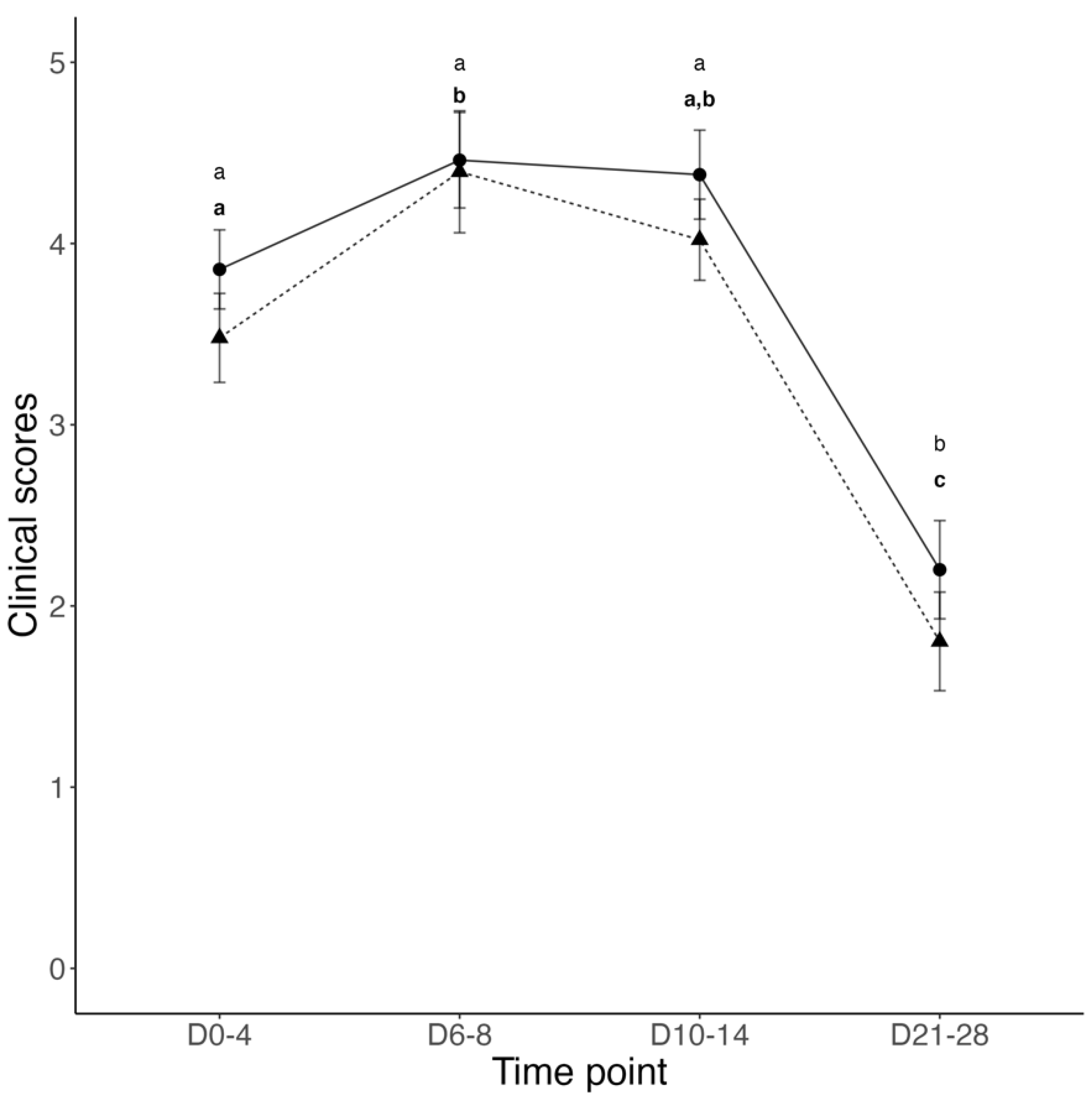
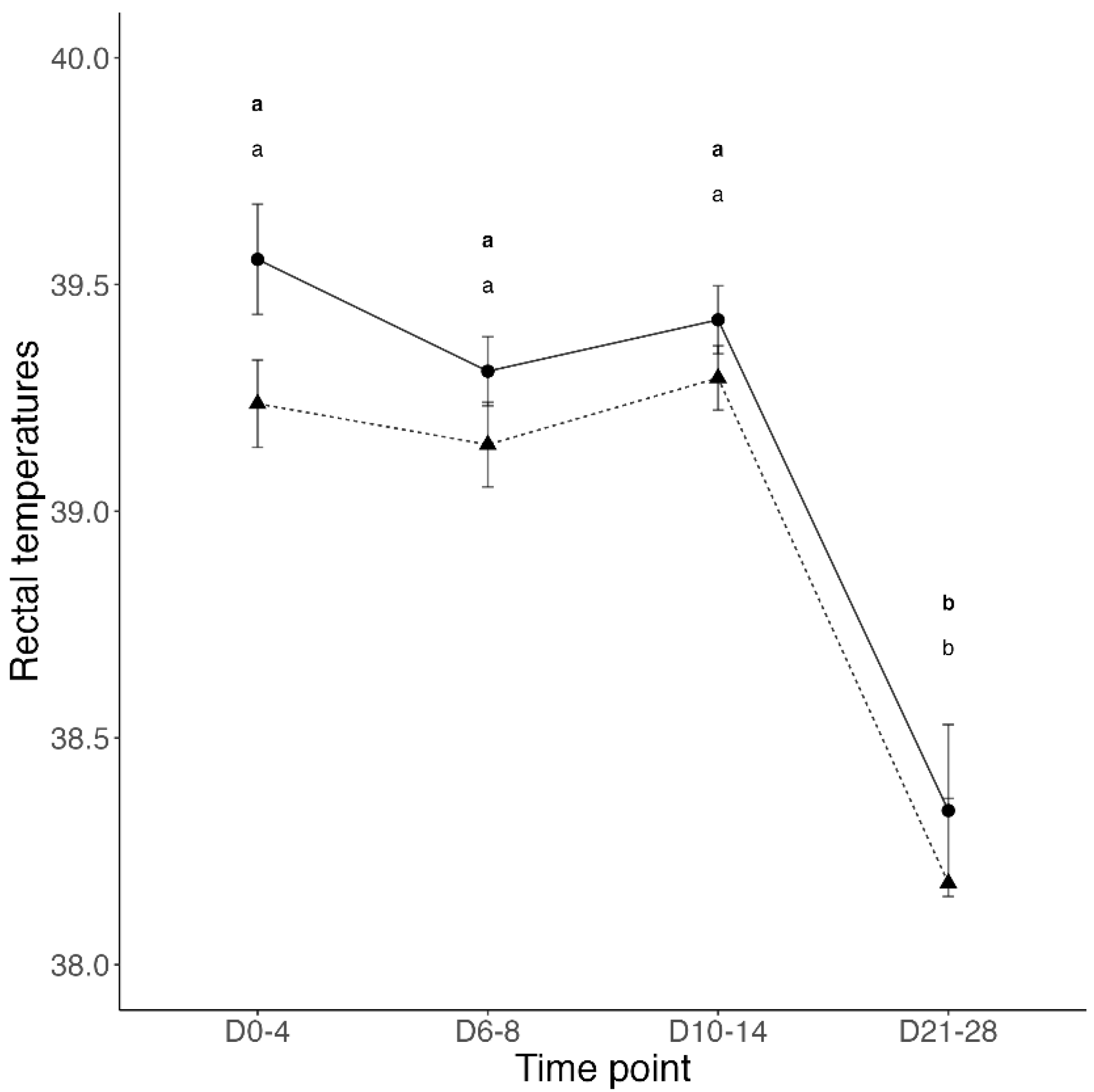
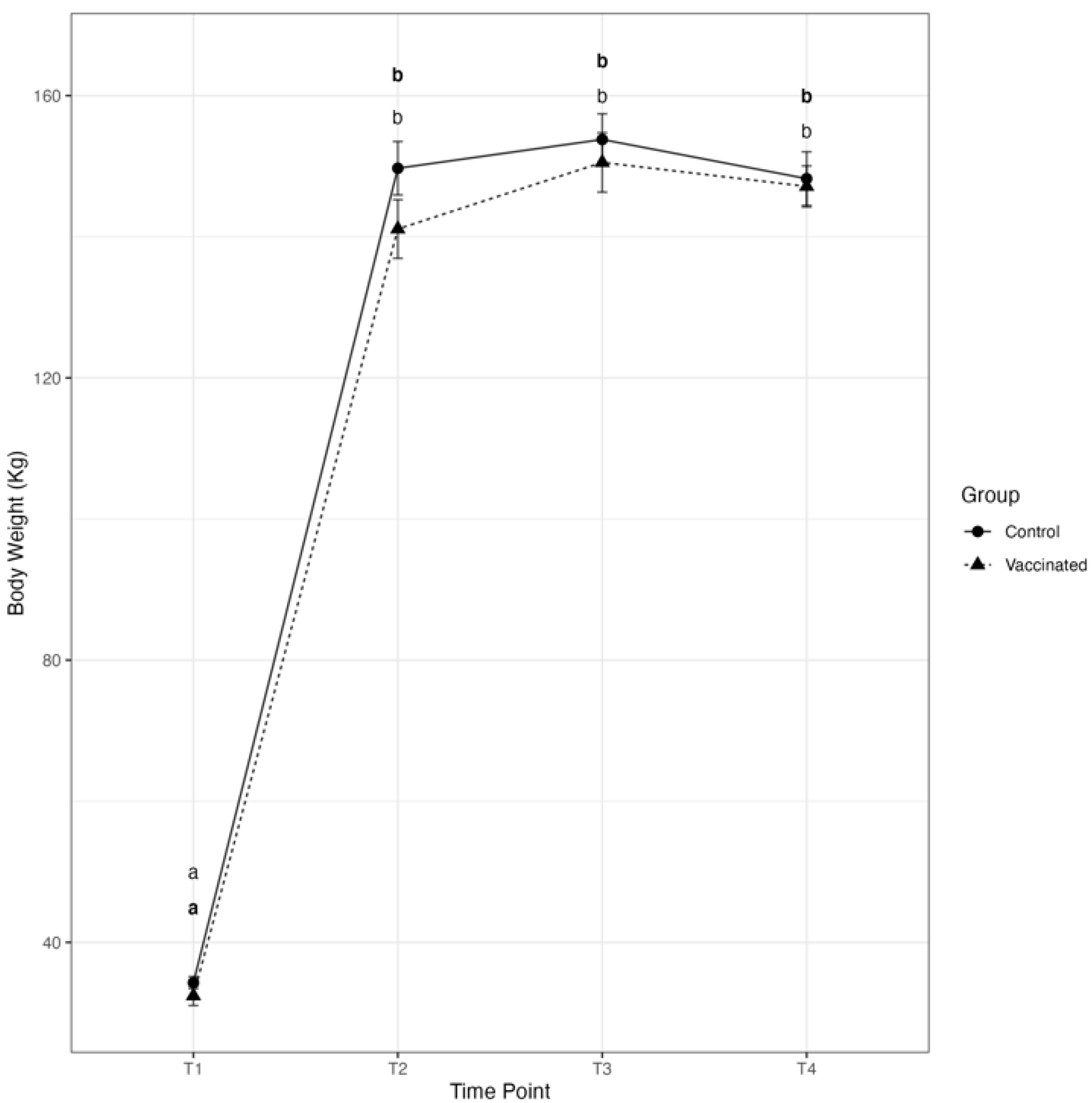
3.1. Transfer of Passive Immunity and Clinical Outcomes
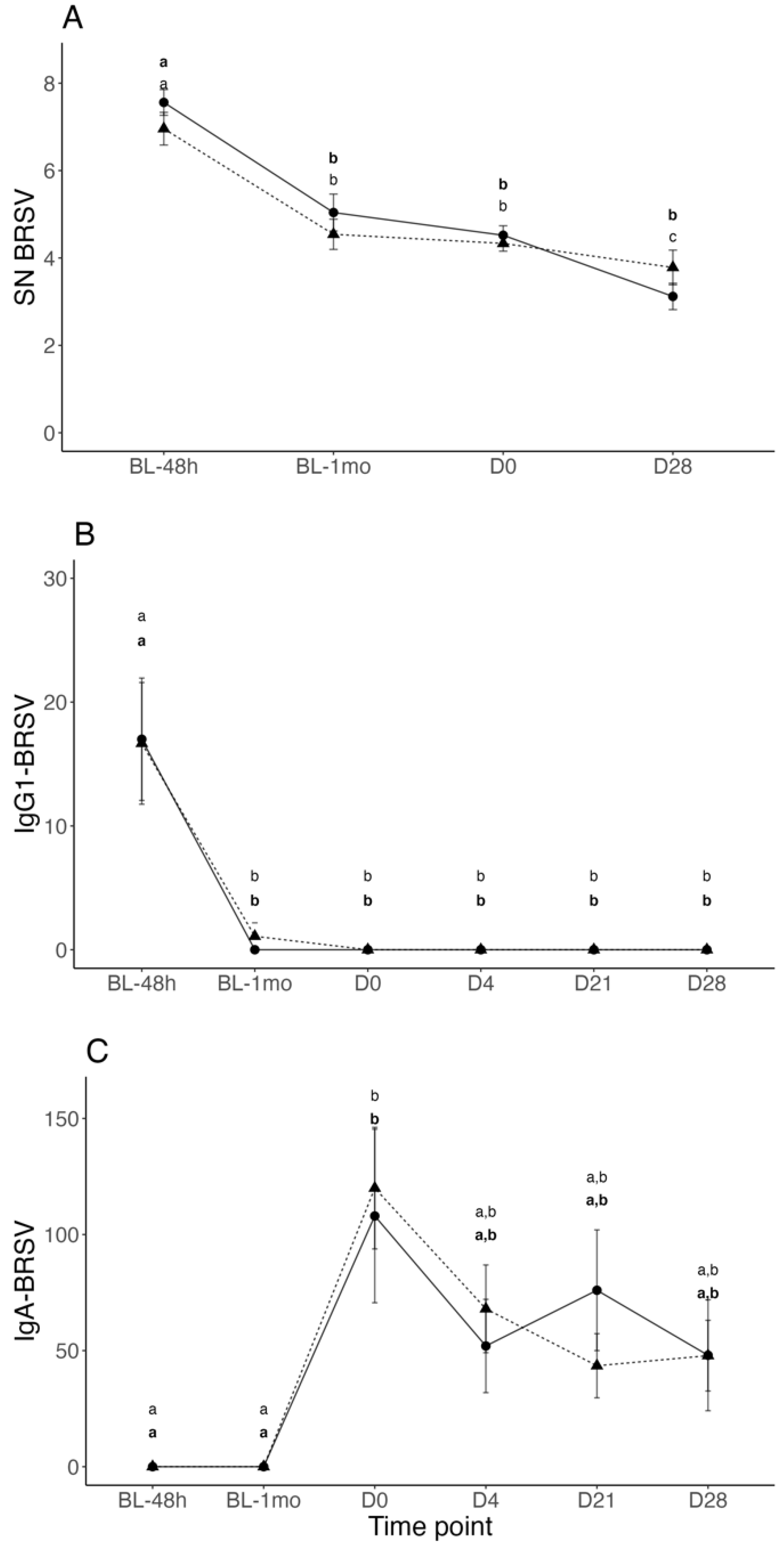
3.2. BRSV Neutralizing Antibodies in Serum
3.3. BRSV-IgG-1 and IgA Titers in Nasal Secretions
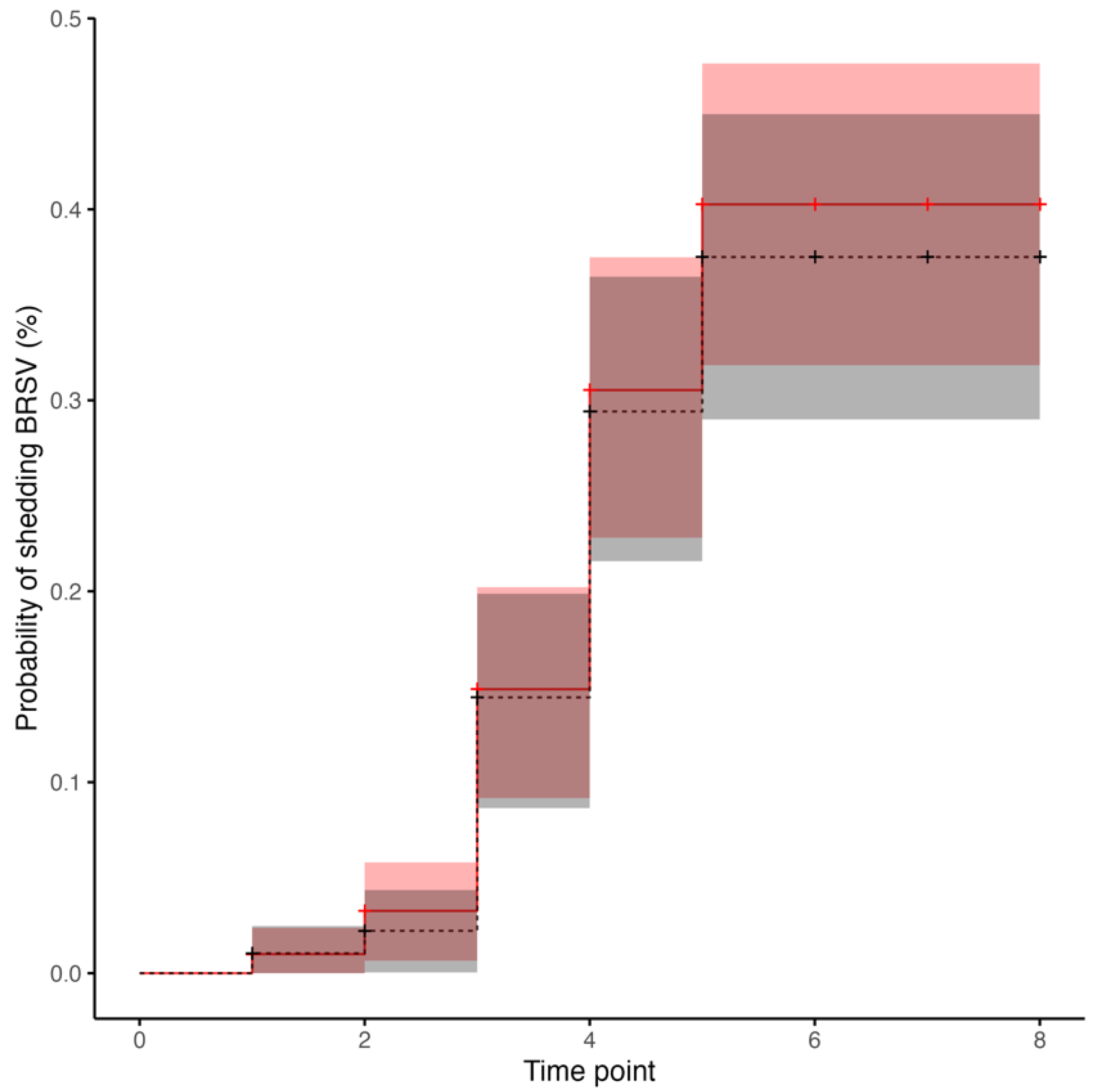
3.4. BRSV Real-Time RT-PCR in Nasal Secretions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, K.K.; Pendell, D.L. Market Impacts of Reducing the Prevalence of Bovine Respiratory Disease in United States Beef Cattle Feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Schneider, L.G.; Hubbard, K.J.; Smith, D.R. Cost of bovine respiratory disease in preweaned calves on US beef cow-calf operations (2011–2015). J. Am. Vet. Med. Assoc. 2018, 253, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Theurer, M.E.; Larson, R.L.; White, B.J. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J. Am. Vet. Med. Assoc. 2015, 246, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C.; Ames, T.R.; Werdin, R.E. Seroepizootiologic study of bovine respiratory syncytial virus in a beef herd. Am. J. Vet. Res. 1986, 47, 246–253. [Google Scholar] [PubMed]
- Ellis, J.; Gow, S.; Berenik, A.; Lacoste, S.; Erickson, N. Comparative efficacy of modified-live and inactivated vaccines in boosting responses to bovine respiratory syncytial virus following neonatal mucosal priming of beef calves. Can. Vet. J. 2018, 59, 1311–1319. [Google Scholar] [PubMed]
- Ellis, J.; Gow, S.; West, K.; Waldner, C.; Rhodes, C.; Mutwiri, G.; Rosenberg, H. Response of calves to challenge exposure with virulent bovine respiratory syncytial virus following intranasal administration of vaccines formulated for parenteral administration. J. Am. Vet. Med. Assoc. 2007, 230, 233–243. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Goji, N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J. Am. Vet. Med. Assoc. 2010, 236, 991–999. [Google Scholar] [CrossRef]
- Ellis, J.; Gow, S.; Bolton, M.; Burdett, W.; Nordstrom, S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can. Vet. J. 2014, 55, 1180–1185. [Google Scholar]
- Martinez, D.A.; Chamorro, M.F.; Passler, T.; Huber, L.; Walz, P.H.; Thoresen, M.; Raithel, G.; Silvis, S.; Stockler, R.; Woolums, A.R. The titers, duration, and residual clinical protection of passively transferred nasal and serum antibodies are similar among beef calves that nursed colostrum from vaccinated or unvaccinated dams and were challenged experimentally with bovine respiratory syncytial virus at three months of age. Am. J. Vet. Res. 2022, 83, 1–9. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Mahan, S.; Leyh, R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J. Am. Vet. Med. Assoc. 2013, 243, 1602–1608. [Google Scholar] [CrossRef]
- Poulsen, K.P.; McGuirk, S.M. Respiratory disease of the bovine neonate. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 121–137, vi–vii. [Google Scholar] [CrossRef] [PubMed]
- Bornheim, H.N.; Chamorro, M.F.; Cernicchiaro, N.; Reppert, E.J.; Larson, R.L.; Huser, S.; Thoresen, M.; Jones, K.; Weaber, R.L.; Woolums, A.R. Evaluation of specific immunoglobulin A in nasal secretions and neutralizing antibodies in serum collected at multiple time points from young beef calves following intranasal or subcutaneous administration of a modified-live bovine respiratory syncytial virus vaccine. Am. J. Vet. Res. 2021, 82, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Thonur, L.; Maley, M.; Gilray, J.; Crook, T.; Laming, E.; Turnbull, D.; Nath, M.; Willoughby, K. One-step multiplex real time RT-PCR for the detection of bovine respiratory syncytial virus, bovine herpesvirus 1 and bovine parainfluenza virus 3. BMC Vet. Res. 2012, 8, 37. [Google Scholar] [CrossRef]
- Kimman, T.G.; Westenbrink, F.; Schreuder, B.E.; Straver, P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J. Clin. Microbiol. 1987, 25, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Westenbrink, F.; Straver, P.J. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: Effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet. Immunol. Immunopathol. 1989, 22, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.F.; Walz, P.H.; Passler, T.; Palomares, R.; Newcomer, B.W.; Riddell, K.P.; Gard, J.; Zhang, Y.; Galik, P. Efficacy of four commercially available multivalent modified-live virus vaccines against clinical disease, viremia, and viral shedding in early-weaned beef calves exposed simultaneously to cattle persistently infected with bovine viral diarrhea virus and cattle acutely infected with bovine herpesvirus 1. Am. J. Vet. Res. 2016, 77, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.F.; Walz, P.H.; Passler, T.; van Santen, E.; Gard, J.; Rodning, S.P.; Riddell, K.P.; Galik, P.K.; Zhang, Y. Efficacy of multivalent, modified- live virus (MLV) vaccines administered to early weaned beef calves subsequently challenged with virulent Bovine viral diarrhea virus type 2. BMC Vet. Res. 2015, 11, 29. [Google Scholar] [CrossRef]
- Collins, J.K.; Teegarden, R.M.; MacVean, D.W.; Salman; Smith, G.H.; Frank, G.R. Prevalence and specificity of antibodies to bovine respiratory syncytial virus in sera from feedlot and range cattle. Am. J. Vet. Res. 1988, 49, 1316–1319. [Google Scholar]
- De Jong, M.C.; van der Poel, W.H.; Kramps, J.A.; Brand, A.; van Oirschot, J.T. Quantitative investigation of population persistence and recurrent outbreaks of bovine respiratory syncytial virus on dairy farms. Am. J. Vet. Res. 1996, 57, 628–633. [Google Scholar]
- Walz, P.H.; Newcomer, B.W.; Riddell, K.P.; Scruggs, D.W.; Cortese, V.S. Virus detection by PCR following vaccination of naive calves with intranasal or injectable multivalent modified-live viral vaccines. J. Vet. Diagn. Investig. 2017, 29, 628–635. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C.; Ames, T.R.; Markham, R.J. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. Am. J. Vet. Res. 1986, 47, 240–245. [Google Scholar] [PubMed]
- Van der Poel, W.H.; Kramps, J.A.; Middel, W.G.; Van Oirschot, J.T.; Brand, A. Dynamics of bovine respiratory syncytial virus infections: A longitudinal epidemiological study in dairy herds. Arch. Virol. 1993, 133, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Gamsjager, L.; Elsohaby, I.; Pearson, J.M.; Levy, M.; Pajor, E.A.; Haines, D.M.; Windeyer, M.C. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J. Vet. Intern. Med. 2020, 34, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Gamsjager, L.; Elsohaby, I.; Pearson, J.M.; Levy, M.; Pajor, E.A.; Windeyer, M.C. Evaluation of 3 refractometers to determine transfer of passive immunity in neonatal beef calves. J. Vet. Intern. Med. 2021, 35, 632–643. [Google Scholar] [CrossRef]
| Time Point | BRSV Real Time RT-PCR CT (Median, (IQR)) | p-Value | |
|---|---|---|---|
| Control | Vacc | ||
| Day 0 (Challenge Day) | 0 | 0 | 1.0 |
| Day 4 | 32.7 (29.1, 34) | 32.7 (31, 34.3) | 0.85 |
| Day 6 | 33.7 (32.2, 34.9) | 34.5 (32.3, 36.2) | 0.13 |
| Day 8 | 32.9 (31.1, 34.1) | 33.9 (32.1, 35) | 0.36 |
| Day 10 | 36.1 (33.2, 36.9) | 34.9 (33.8, 36.8) | 0.09 |
| Day 14 | 0 | 0 | 1.0 |
| Day 21 | 0 | 0 | 1.0 |
| Day 28 | 0 | 0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, D.A.; Chamorro, M.F.; Passler, T.; Huber, L.; Walz, P.H.; Thoresen, M.; Raithel, G.; Silvis, S.; Stockler, R.; Woolums, A.R. Local and Systemic Antibody Responses in Beef Calves Vaccinated with a Modified-Live Virus Bovine Respiratory Syncytial Virus (BRSV) Vaccine at Birth following BRSV Infection. Vet. Sci. 2023, 10, 20. https://doi.org/10.3390/vetsci10010020
Martínez DA, Chamorro MF, Passler T, Huber L, Walz PH, Thoresen M, Raithel G, Silvis S, Stockler R, Woolums AR. Local and Systemic Antibody Responses in Beef Calves Vaccinated with a Modified-Live Virus Bovine Respiratory Syncytial Virus (BRSV) Vaccine at Birth following BRSV Infection. Veterinary Sciences. 2023; 10(1):20. https://doi.org/10.3390/vetsci10010020
Chicago/Turabian StyleMartínez, David A., Manuel F. Chamorro, Thomas Passler, Laura Huber, Paul H. Walz, Merrilee Thoresen, Gage Raithel, Scott Silvis, Ricardo Stockler, and Amelia R. Woolums. 2023. "Local and Systemic Antibody Responses in Beef Calves Vaccinated with a Modified-Live Virus Bovine Respiratory Syncytial Virus (BRSV) Vaccine at Birth following BRSV Infection" Veterinary Sciences 10, no. 1: 20. https://doi.org/10.3390/vetsci10010020
APA StyleMartínez, D. A., Chamorro, M. F., Passler, T., Huber, L., Walz, P. H., Thoresen, M., Raithel, G., Silvis, S., Stockler, R., & Woolums, A. R. (2023). Local and Systemic Antibody Responses in Beef Calves Vaccinated with a Modified-Live Virus Bovine Respiratory Syncytial Virus (BRSV) Vaccine at Birth following BRSV Infection. Veterinary Sciences, 10(1), 20. https://doi.org/10.3390/vetsci10010020






