Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
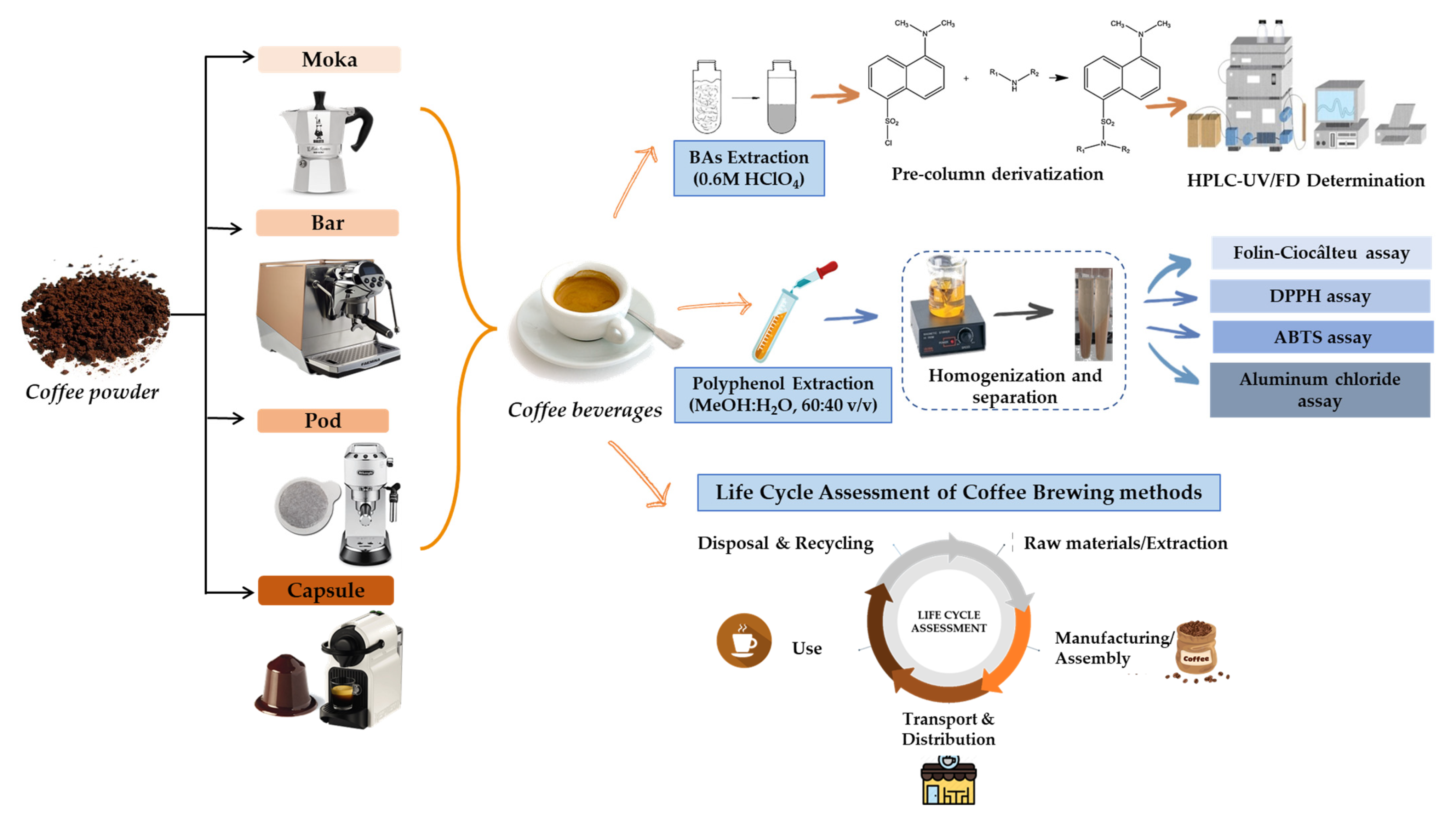
2.3. Sampling
2.4. Determination of Biogenic Amines
2.5. Polyphenol, Flavonoid, and Antioxidant Activity Determination
2.5.1. Total Polyphenols Content
2.5.2. Determination of Total Flavonoid Content
2.5.3. Determination of Antioxidant Activity
2.6. Life Cycle Assessment (LCA)
2.6.1. Goal and Scope Definition
2.6.2. Life Cycle Inventory (LCI)
2.7. Statistic Analysis
3. Results and Discussion
3.1. Biogenic Amiens Content in Coffee Samples
3.2. Total Polyphenol Content
3.3. Total Flavonoid Content
3.4. Determination of Antioxidant Activity
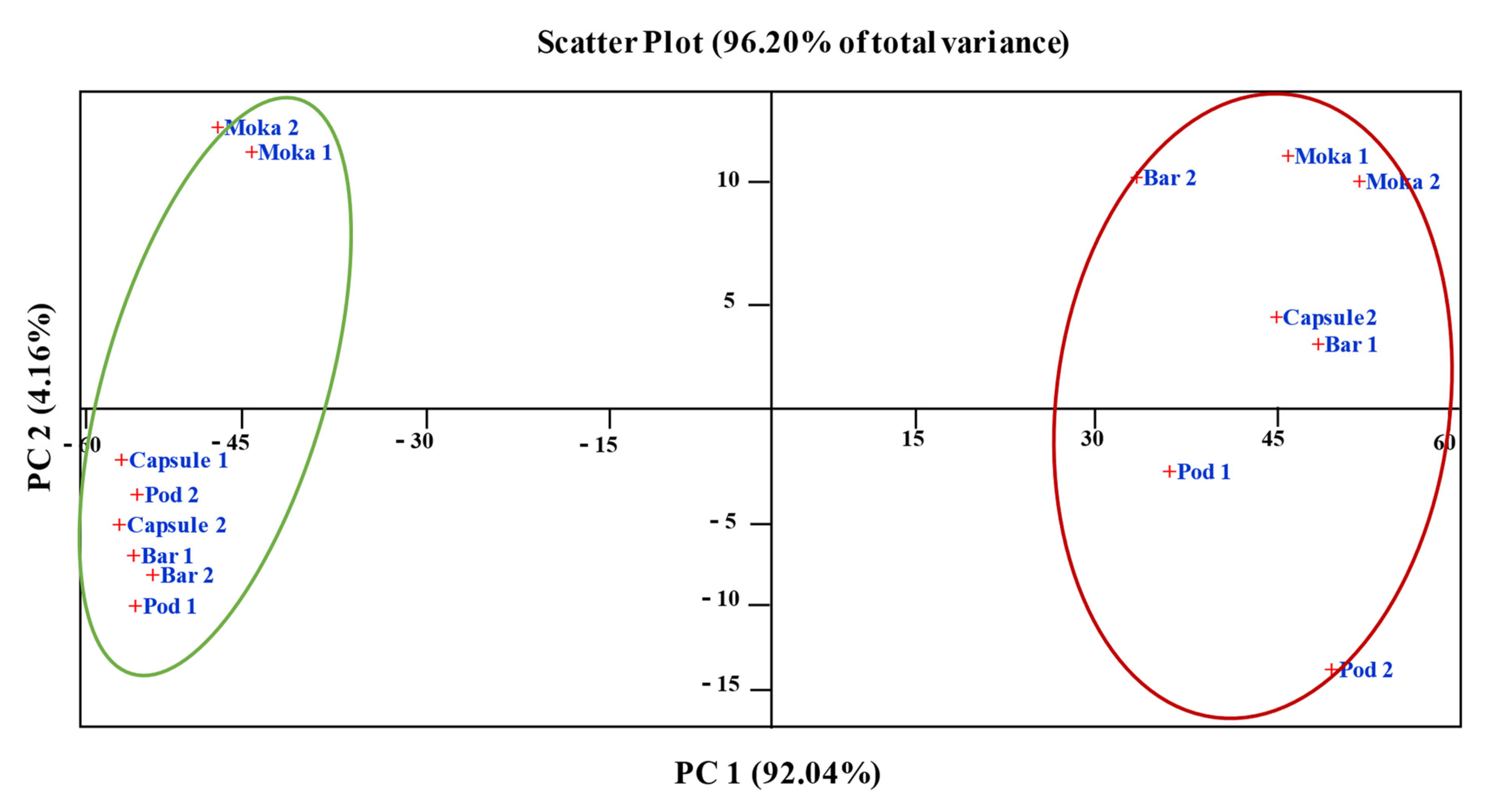
3.5. Statistics Analysis
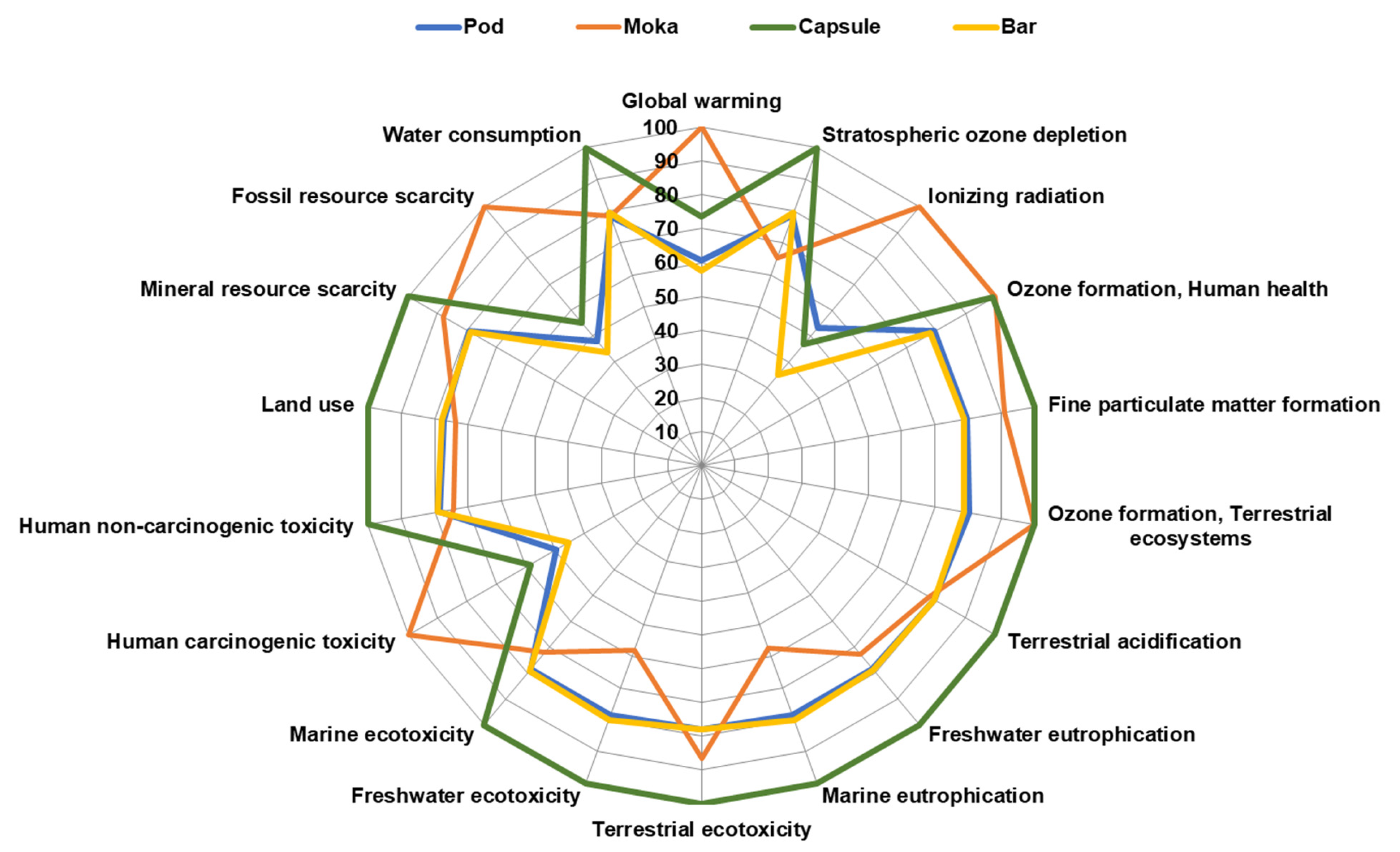
3.6. Life Cycle Assessment Impact Assessment of Coffee Brewing Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing Effect on Levels of Biogenic Amines in Different Coffee Samples as Determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef]
- Mitraka, G.C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response SurfaceMethodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- International Coffee Organisation (ICO). Cocoa Market Report—October 2022. Available online: https://www.ico.org/Market-Report-22-23-e.asp (accessed on 12 October 2022).
- Zarebska, M.; Stanek, N.; Barabosz, K.; Jaszkiewicz, A.; Kulesza, R.; Matejuk, R.; Andrzejewski, D.; Biłos, Ł.; Porada, A. Comparison of Chemical Compounds and Their Influence on the Taste of Coffee Depending on Green Beans Storage Conditions. Sci. Rep. 2022, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of Coffee Brewing Methods on the Chromatographic and Spectroscopic Profiles, Antioxidant and Sensory Properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz-Szymá Nska, A.; Nowak, A.; Wira, D.; Klimowicz, A.; Oszmianski, J.; Lachowicz, S.; Cacciola, F. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent Coffee Grounds: A Potential Commercial Source of Phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J.; Fernández-Ruiz, V.; Morales, P. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [PubMed]
- Carvalho Neto, D.P.D.; Gonot-Schoupinsky, X.P.; Gonot-Schoupinsky, F.N. Coffee as a naturally beneficial and sustainable ingredient in personal care products: A systematic scoping review of the evidence. Front. Sustain. 2021, 2, 697092. [Google Scholar] [CrossRef]
- Muzaifa, M.; Hasni, D.; Febriani; Patria, A.; Abubakar, A. Chemical Composition of Green and Roasted Coffee Bean of Gayo Arabica Civet Coffee (Kopi Luwak). IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 012001. [Google Scholar] [CrossRef]
- Oliveira, S.D.; Franca, A.S.; Glória, M.B.A.; Borges, M.L.A. The Effect of Roasting on the Presence of Bioactive Amines in Coffees of Different Qualities. Food Chem. 2005, 90, 287–291. [Google Scholar] [CrossRef]
- Cortés-Macías, E.T.; López, C.F.; Gentile, P.; Girón-Hernández, J.; López, A.F. Impact of Post-Harvest Treatments on Physicochemical and Sensory Characteristics of Coffee Beans in Huila, Colombia. Postharvest Biol. Technol. 2022, 187, 111852. [Google Scholar] [CrossRef]
- Casal, S.; Oliveira, M.B.P.P.; Ferreira, M.A. Determination of Biogenic Amines in Coffee by an Optimized Liquid Chromatographic Method. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 2535–2549. [Google Scholar] [CrossRef]
- Chongsrimsirisakhol, O.; Pirak, T. Total Polyphenol Content and Antioxidant Properties of Cold Brew Coffee Extracts as Affected by Ultrasound Treatment and Their Application in Low Fat Pork Sausage. Int. J. Food Prop. 2022, 2022, 813–826. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.d.T. Roasting Process Affects Differently the Bioactive Compounds and the Antioxidant Activity of Arabica and Robusta Coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Brommer, E.; Stratmann, B.; Quack, D. Environmental Impacts of Different Methods of Coffee Preparation. Int. J. Consum. Stud. 2011, 35, 212–220. [Google Scholar] [CrossRef]
- Dubois, C.; Humbert, S.; Margni, M. Comparative Full Life Cycle Assessment of B”C Cup of Espresso Made Using a Packaging and Distribution system from Nespresso Espresso and Three Generic Products; Quantis: Lausanne, Switzerland, 2011. [Google Scholar]
- Vinci, G.; Maddaloni, L.; Prencipe, S.A.; Ruggieri, R. Natural Contaminants in Wines: Determination of Biogenic Amines by Chromatographic Techniques. Int. J. Environ. Res. Public Health 2021, 18, 10159. [Google Scholar] [CrossRef]
- Mietz, J.L.; Karmas, E. Chemical quality index of canned tuna as determined by high-pressure liquid chromatography. J. Food Sci. 1977, 42, 155–158. [Google Scholar] [CrossRef]
- Duflos, G.; Inglebert, G.; Himber, C.; Degremont, S.; Lombard, B.; Brisabois, A. Validation of Standard Method EN ISO 19343 for the Detection and Quantification of Histamine in Fish and Fishery Products Using High-Performance Liquid Chromatography. Int. J. Food Microbiol. 2019, 288, 97–101. [Google Scholar] [CrossRef]
- Vinci, G.; D’Ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The Influence of Green and Black Tea Infusion Parameters on Total Polyphenol Content and Antioxidant Activity by ABTS and DPPH Assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Sallam, K.I.; Malak, N.M.L. Improvement of the Microbial Quality, Antioxidant Activity, Phenolic and Flavonoid Contents, and Shelf Life of Smoked Herring (Clupea harengus) during Frozen Storage by Using Chitosan Edible Coating. Food Control. 2021, 130, 108317. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Yuan, H.; He, N. From Tea Leaves to Factories: A Review of Research Progress in l -Theanine Biosynthesis and Production. J. Agric. Food Chem. 2021, 69, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Rodrigues, F.; Antónia Nunes, M.; Vinha, A.F.; Oliveira, M.B.P.P. State of the Art in Coffee Processing By-Products. In Handbook of Coffee Processing By-Products: Sustainable Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–26. ISBN 9780128112915. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- Cibelli, M.; Cimini, A.; Moresi, M. Carbon Footprint of Different Coffee Brewing Methods. Chem. Eng. Trans. 2021, 87, 373–378. [Google Scholar] [CrossRef]
- Cibelli, M.; Cimini, A.; Cerchiara, G.; Moresi, M. Carbon Footprint of Different Methods of Coffee Preparation. Sustain. Prod. Consum. 2021, 27, 1614–1625. [Google Scholar] [CrossRef]
- Pré Consultants, P. SimaPro (Version 9.2.0.2.) [Computer Software]; Pré Sustainability: Amersfoort, The Netherlands, 2017; Available online: Https://Simapro.Com (accessed on 5 October 2022).
- Spizzirri, U.G.; Picci, N.; Restuccia, D. Extraction Efficiency of Different Solvents and LC-UV Determination of Biogenic Amines in Tea Leaves and Infusions. J. Anal. Methods Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Greener Extraction of Anthocyanins and Antioxidant Activity from Blackberry (Rubus Spp) Using Natural Deep Eutectic Solvents. LWT 2022, 158, 113184. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ibrahim, S.A.; Koca, I.; Galanakis, C.M. Green and Highly Extraction of Phenolic Compounds and Antioxidant Capacity from Kinkeliba (Combretum Micranthum G. Don) by Natural Deep Eutectic Solvents (NADESs) Using Maceration, Ultrasound-Assisted Extraction and Homogenate-Assisted Extraction. Arab. J. Chem. 2022, 15, 103752. [Google Scholar] [CrossRef]
- EU COMMISSION REGULATION (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Brussels, Belgium. Available online: http://data.europa.eu/eli/reg/2005/2073/oj (accessed on 10 November 2022).
- Zargar, B.; Majeed, D.; Ganai, S.A.; Mir, S.A.; Dar, B.N. Effect of Different Processing Parameters on Antioxidant Activity of Tea. Food Meas. 2018, 12, 527–534. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R. The Effect of Water Mineralization on the Extraction of Active Compounds from Selected Herbs and on the Antioxidant Properties of the Obtained Brews. Foods 2021, 10, 1227. [Google Scholar] [CrossRef]
- Niseteo, T.; Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Budeč, M. Bioactive Composition and Antioxidant Potential of Different Commonly Consumed Coffee Brews Affected by Their Preparation Technique and Milk Addition. Food Chem. 2012, 134, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Uslu, N. The Influence of Decoction and Infusion Methods and Times on Antioxidant Activity, Caffeine Content and Phenolic Compounds of Coffee Brews. Eur. Food Res. Technol. 2022, 248, 2021–2030. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 10, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan Coffee Brew Chemical Analysis in Comparison to Espresso, Moka and American Brews. FRIN 2014, 61, 152–160. [Google Scholar] [CrossRef]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant Capacity of Cocoa Beans and Chocolate Assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of Ultrasound-Assisted Extraction of Polyphenols from Chicory Grounds under Different Operational Conditions. J. Clean Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Sualeh, A.; Tolessa, K.; Mohammed, A. Biochemical Composition of Green and Roasted Coffee Beans and Their Association with Coffee Quality from Different Districts of Southwest Ethiopia. Heliyon 2020, 6, e05812. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminum Toxicosis: A Review of Toxic Actions and Effects. Interdiscip. Toxicol. 2020, 12, 45–70. [Google Scholar] [CrossRef]
- Hicks, A.L.; Halvorsen, H. Environmental Impact of Evolving Coffee Technologies. Int. J. Life Cycle Assess. 2019, 24, 1396–1408. [Google Scholar] [CrossRef]
- Hicks, A.L. Environmental Implications of Consumer Convenience: Coffee as a Case Study. J. Ind. Ecol. 2018, 22, 79–91. [Google Scholar] [CrossRef]

| Extraction Condition | Coffee Powder Weight (g) | Water Volume (mL) | Pressure (Pa) | Time (s) | Temperature (°C) |
|---|---|---|---|---|---|
| Moka | 5 | 25 | 10 × 105 | 300 | 95 |
| Espresso Professional | 7 | 25 | 18 × 105 | 25 | 96 |
| Espresso Pods | 7.5 | 20 | 18 × 105 | 30 | 90 |
| Espresso Capsules | 5.5 | 20 | 15 × 105 | 30 | 90 |
| INPUTS | Coffee Brewing Methods | ||||
|---|---|---|---|---|---|
| Unit | Coffee Moka | Coffee Bar | Coffee Capsule | Coffee Pod | |
| Roasted and ground coffee | g | 8.0 | 11.2 | 14.0 | 11.0 |
| Water for preparation | g | 100 | 100 | 54.08 | 40.3 |
| Electricity | kW·h | 0.266 | 0.014 | 0.022 | 0.021 |
| Primary packaging | |||||
| Low-density polyethylene (LDPE) | g | 0.352 | 0.493 | - | - |
| Paper filters | g | - | - | 2.47 | |
| Poly-laminated bag (PE-Al-PP) | g | - | - | 2.01 | |
| OUTPUTS | |||||
| 1 cup of coffee beverage | mL | 40 | 40 | 40 | 40 |
| Samples | Biogenic Amines (µg/g of Coffee Powder) | BAs Tot | BAQI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ser | B-Pea | Put | His | Cad | Tyr | Spd | Spm | ||||
| Coffee powder | Bar 1 | 71.77 ± 1.02 c | 8.97 ± 0.23 b | 0.33 ± 0.07 a | 2.78 ± 0.23 b | nd | nd | 0.72 ± 0.09 b | 0.74 ± 0.12 a | 84.59 | 1.26 |
| Bar 2 | 75.50 ± 1.21 a | 11.93 ± 0.33 a | 0.35 ± 0.04 a | 2.32 ± 0.20 a | nd | nd | 0.51 ± 0.07 b | 0.67 ± 0.17 b | 90.77 | 1.22 | |
| Capsule 1 | 63.46 ± 0.76 b | 3.26 ± 0.12 a | 0.52 ± 0.13 c | 3.62 ± 0.14 c | nd | nd | 0.19 ± 0.03 c | 0.78 ± 0.11 c | 71.85 | 2.10 | |
| Capsule 2 | 57.87 ± 0.84 c | 3.65 ± 0.17 a | 0.45 ± 0.11 a | 4.19 ± 0.17 a | nd | nd | 0.10 ± 0.01 a | 0.74 ± 0.13 a | 67.01 | 2.52 | |
| Moka 1 | 82.61 ± 1.32 a | 5.81 ± 0.36 b | 0.43 ± 0.06 a | 7.30 ± 0.51 b | nd | nd | 0.29 ± 0.02 a | 0.38 ± 0.03 a | 96.83 | 4.60 | |
| Moka 2 | 84.24 ± 0.91 b | 5.35 ± 0.36 b | 0.42 ± 0.01 a | 5.78 ± 0.32 a | nd | nd | 0.21 ± 0.05 b | 0.39 ± 0.05 b | 96.38 | 3.89 | |
| Pod 1 | 62.13 ± 0.53 c | 2.75 ± 0.25 a | 0.40 ± 0.03 b | 2.22 ± 0.13 b | nd | nd | 0.34 ± 0.01 b | 0.55 ± 0.07 b | 67.88 | 1.89 | |
| Pod 2 | 72.47 ± 1.32 c | 2.22 ± 0.12 a | 0.35 ± 0.02 a | 3.20 ± 0.21 a | nd | nd | 0.33 ± 0.02 b | 0.57 ± 0.08 a | 81.15 | 1.87 | |
| Coffee beverages | Bar 1 | 14.38 ± 0.56 b | 0.52 ± 0.08 b | 0.18 ± 0.02 b | 8.29 ± 0.58 a | nd | nd | 0.14 ± 0.02 a | 0.24 ± 0.11 c | 23.60 | 6.81 |
| Bar 2 | 12.75 ± 0.41 c | 0.52 ± 0.05 b | 0.16 ± 0.03 a | 7.97 ± 0.74 c | nd | nd | 0.12 ± 0.03 b | 0.20 ± 0.09 b | 21.61 | 6.76 | |
| Capsule 1 | 14.42 ± 0.65 a | 0.41 ± 0.31 a | nd | 0.77 ± 0.21 b | nd | nd | 0.11 ± 0.03 a | 0.32 ± 0.08 a | 16.02 | 0.54 | |
| Capsule 2 | 19.95 ± 0.25 c | 0.48 ± 0.11 b | nd | 0.85 ± 0.26 b | nd | nd | 0.06 ± 0.01 c | 0.22 ± 0.14 b | 21.75 | 0.57 | |
| Moka 1 | 33.46 ± 0.84 b | 0.51 ± 0.08 b | nd | 14.66 ± 0.54 b | nd | nd | 0.04 ± 0.02 a | 0.25 ± 0.06 a | 48.92 | 11.38 | |
| Moka 2 | 31.82 ± 0.67 c | 0.44 ± 0.05 a | nd | 20.57 ± 0.86 a | nd | nd | 0.07 ± 0.01 b | 0.26 ± 0.09 b | 53.15 | 15.50 | |
| Pod 1 | 21.38 ± 0.35 a | 0.85 ± 0.14 a | nd | 0.37 ± 0.36 b | nd | nd | 0.03 ± 0.01 c | 0.20 ± 0.04 a | 22.83 | 0.30 | |
| Pod 2 | 18.46 ± 0.62 b | 0.31 ± 0.04 a | nd | 0.22 ± 0.05 a | nd | nd | 0.14 ± 0.02 b | 0.15 ± 0.06 c | 19.28 | 0.17 | |
| Sample | TPC (mg GAE/g of Coffee Powder) | |
|---|---|---|
| Coffee Powder | Coffee Beverages | |
| Bar 1 | 25.37 ± 0.52 b | 1.51 ± 0.26 a |
| Bar 2 | 23.33 ± 0.61 c | 1.72 ± 0.21 a |
| Capsule 1 | 22.96 ± 0.38 b | 1.46 ± 0.02 b |
| Capsule 2 | 23.87 ± 0.71 b | 1.49 ± 0.05 a |
| Moka 1 | 23.03 ± 0.23 c | 2.71 ± 0.25 b |
| Moka 2 | 24.06 ± 0.41 b | 3.52 ± 0.08 a |
| Pod 1 | 29.61 ± 0.26 b | 1.30 ± 0.06 b |
| Pod 2 | 28.46 ± 0.53 a | 1.44 ± 0.07 c |
| Sample | TFC | |
|---|---|---|
| Coffee Powder | Coffee Beverages | |
| Bar 1 | 86.78 ± 0.42 c | 5.13 ± 0.21 c |
| Bar2 | 79.90 ± 0.56 a | 4.77 ± 0.36 b |
| Capsule 1 | 82.33 ± 0.84 b | 4.39 ± 0.25 b |
| Capsule 2 | 90.02 ± 0.72 a | 5.01 ± 0.14 b |
| Moka 1 | 93.64 ± 0.63 a | 8.55 ± 0.24 a |
| Moka 2 | 97.67 ± 0.82 b | 8.60 ± 0.31 c |
| Pod 1 | 113.69 ± 1.05 b | 4.41 ± 0.09 a |
| Pod 2 | 99.40 ± 0.94 b | 3.94 ± 0.11 b |
| Sample | ABTS | DPPH | ||
|---|---|---|---|---|
| Coffee Powder | Coffee Beverages | Coffee Powder | Coffee Beverages | |
| Bar 1 | 99.03 ± 0.21 a | 97.63 ± 0.11 a | 61.09 ± 0.23 b | 27.03 ± 0.12 a |
| Bar 2 | 99.12 ± 0.23 c | 97.71 ± 0.13 a | 60.89 ± 0.11 a | 27.42 ± 0.18 a |
| Capsule 1 | 97.59 ± 0.11 b | 99.35. ± 0.21 b | 48.76 ± 0.15 a | 7.84 ± 0.06 c |
| Capsule 2 | 96.31 ± 0.22 c | 99.42 ± 0.25 b | 49.04 ± 0.13 a | 9.06 ± 0.09 a |
| Moka 1 | 99.88 ± 0.25 b | 99.62 ± 0.13 b | 64.65 ± 018 b | 16.01 ± 0.25 c |
| Moka 2 | 99.96 ± 0.27 b | 99.73 ± 0.17 b | 64.82 ± 0.16 c | 14.78 ± 0.17 a |
| Pod 1 | 98.23 ± 0.19 a | 97.68 ± 0.15 a | 54.75 ± 0.09 b | 24.23 ± 0.24 a |
| Pod 2 | 98.52 ± 0.17 b | 97.76 ± 0.14 a | 55.21 ± 0.05 a | 22.04 ± 0.15 b |
| ABTS | DPPH | TPC | TFC | Ser | B-Pea | Put | His | Spd | |
|---|---|---|---|---|---|---|---|---|---|
| ABTS | 0.163 | 0.187 | 0.195 | 0.414 | 0.448 | 0.034 | 0.087 | 0.465 | |
| DPPH | 0.878 | 0.904 | 0.900 | 0.810 | 0.906 | −0.272 | 0.607 | ||
| TPC | 0.990 | 0.925 | 0.678 | 0.912 | −0.213 | 0.555 | |||
| TFC | 0.933 | 0.713 | 0.927 | −0.228 | 0.542 | ||||
| Ser | 0.809 | 0.847 | −0.090 | 0.667 | |||||
| B-Pea | 0.649 | −0.232 | 0.879 | ||||||
| Put | −0.195 | 0.444 | |||||||
| His | −0.247 |
| Impact Category | Unit | Pod | Moka | Capsule | Bar |
|---|---|---|---|---|---|
| Global warming | kg CO2 eq | 1.14 × 10−1 | 1.87 × 10−1 | 1.38 × 10−1 | 1.08 × 10−1 |
| Stratospheric ozone depletion | kg CFC11 eq | 7.78 × 10−7 | 6.48 × 10−7 | 9.90 × 10−7 | 7.89 × 10−7 |
| Ionizing radiation | kBq Co−60 eq | 6.05 × 10−4 | 1.14 × 10−3 | 5.33 × 10−4 | 3.98 × 10−4 |
| Ozone formation, Human health | kg NOx eq | 3.57 × 10−4 | 4.49 × 10−4 | 4.46 × 10−4 | 3.50 × 10−4 |
| Fine particulate matter formation | kg PM2.5 eq | 2.75 × 10−4 | 3.13 × 10−4 | 3.45 × 10−4 | 2.72 × 10−4 |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | 3.71 × 10−4 | 4.62 × 10−4 | 4.63 × 10−4 | 3.64 × 10−4 |
| Terrestrial acidification | kg SO2 eq | 1.33 × 10−3 | 1.29 × 10−3 | 1.67 × 10−3 | 1.33 × 10−3 |
| Freshwater eutrophication | kg P eq | 1.57 × 10−5 | 1.46 × 10−5 | 2.00 × 10−5 | 1.58 × 10−5 |
| Marine eutrophication | kg N eq | 2.25 × 10−4 | 1.65 × 10−4 | 2.86 × 10−4 | 2.29 × 10−4 |
| Terrestrial ecotoxicity | kg 1.4-DCB | 3.28 × 10−1 | 3.64 × 10−1 | 4.21 × 10−1 | 3.28 × 10−1 |
| Freshwater ecotoxicity | kg 1.4-DCB | 4.05 × 10−3 | 2.99 × 10−3 | 5.16 × 10−3 | 4.12 × 10−3 |
| Marine ecotoxicity | kg 1.4-DCB | 9.02 × 10−4 | 8.29 × 10−4 | 1.15 × 10−3 | 9.10 × 10−4 |
| Human carcinogenic toxicity | kg 1.4-DCB | 1.18 × 10−3 | 2.39 × 10−3 | 1.40 × 10−3 | 1.09 × 10−3 |
| Human non-carcinogenic toxicity | kg 1.4-DCB | 8.78 × 10−2 | 8.31 × 10−2 | 1.12 × 10−1 | 8.86 × 10−2 |
| Land use | m2a crop eq | 1.04 × 10−1 | 9.95 × 10−2 | 1.35 × 10−1 | 1.05 × 10−1 |
| Mineral resource scarcity | kg Cu eq | 5.17 × 10−4 | 5.74 × 10−4 | 6.52 × 10−4 | 5.14 × 10−4 |
| Fossil resource scarcity | kg oil eq | 2.38 × 10−2 | 4.94 × 10−2 | 2.72 × 10−2 | 2.15 × 10−2 |
| Water consumption | m3 | 7.96 × 10−3 | 7.95 × 10−3 | 1.01 × 10−2 | 8.08 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbi, L.; Maddaloni, L.; Prencipe, S.A.; Vinci, G. Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment. Beverages 2023, 9, 3. https://doi.org/10.3390/beverages9010003
Gobbi L, Maddaloni L, Prencipe SA, Vinci G. Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment. Beverages. 2023; 9(1):3. https://doi.org/10.3390/beverages9010003
Chicago/Turabian StyleGobbi, Laura, Lucia Maddaloni, Sabrina Antonia Prencipe, and Giuliana Vinci. 2023. "Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment" Beverages 9, no. 1: 3. https://doi.org/10.3390/beverages9010003
APA StyleGobbi, L., Maddaloni, L., Prencipe, S. A., & Vinci, G. (2023). Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment. Beverages, 9(1), 3. https://doi.org/10.3390/beverages9010003










