Abstract
The efficient extraction of phenols from grapes is an important step for their reliable quantification. The aim was to optimise the lyophilisation process and the extraction of phenols from grape skins and seeds. The phenol extraction yield from lyophilised tissues was investigated with different accelerated solvent extraction (ASE) operating conditions. Skins and seeds were separated from frozen berries and lyophilised without being ground. The weight loss during lyophilisation was followed daily. Phenols were extracted from lyophilised, cryo-ground seeds and skins with ASE at room temperature and 10.3 MPa using 80% aqueous acetone and 60% aqueous methanol. The effects of ASE operational parameters (the number of extraction cycles (ECs) and static time (ST) duration) were investigated. The yield of extracted phenols was evaluated spectrophotometrically by determining total phenolic index at 280 nm (TPI). The weight of skins and seeds significantly dropped after 24 h of lyophilisation and continued to decrease, although not significantly, up until the 9th day. The optimal lyophilisation time was estimated to be 3 days and 5 days for skins and seeds, respectively. The phenol extraction yield was significantly affected after changes of ASE conditions. Based on TPI, the optimal ASE conditions were as follows: (i) lyophilised seeds—eight ECs with 10 min ST using aqueous acetone and then four ECs with 20 min ST using aqueous methanol; (ii) lyophilised skins—eight ECs with 1 min ST using aqueous acetone and then one EC with 20 min ST using aqueous methanol.
1. Introduction
Phenolic compounds are secondary metabolites that contain benzene rings, with one or more hydroxyl substituents, and range from simple to highly polymerised molecules called polyphenols [1]. They are divided into flavonoids and non-flavonoids. Flavonoids are based on a C6-C3-C6 skeleton that includes flavan-3-ols, proanthocyanidins (condensed flavan-3-ols), flavonols and anthocyanins. Proanthocyanidin structures vary in terms of their sub-units, the mean degree of polymerisation (mDP) and the linkage position. Non-flavonoids include hydroxycinnamic acids (C6-C3), stilbenes and hydroxybenzoic acids (C6-C1) [2]. Polyphenols are crucial for red wine quality, especially flavan-3-ols, proanthocyanidins and anthocyanins, which are extracted from the skins and, except of anthocyanins, from the seeds of grapes during alcoholic fermentation. They make a strong contribution to the sensory characteristics (colour, astringency, bitterness, etc.) and the ageing behaviour of red wines and have also been shown to have beneficial effects on human health [3].
Quantitative extraction is the first step required to characterise phenol concentration in grapes. The quantitative extraction of phenolic compounds from plant tissues has been a constant challenge, due to interfering parameters involving the particle size of the sample, type of solvent(s), tissue solvent ratio, time and temperature of extraction, pH of the extraction medium, number of extractions, degradation of compounds during extraction, etc. [4]. The so-called total extraction of phenols from grape skins and seeds is usually performed from lyophilised ground tissue using aqueous organic solvents. The lyophilisation of non-ground or cryo-milled skins and seeds has been reported to last 2 days [5,6,7] and up to 5 days [8]. Previously published methods for phenol extraction from grape skins and seeds with organic–aqueous solvents are summarised in Table 1.

Table 1.
Methods for the phenol extraction from grape seeds and skins with organic-aqueous solvents.
The choice of solvent plays a crucial role in quantitative extraction [10]. There is no solvent generally accepted as the best for the extraction of polyphenols since the structure of phenolic compounds has a strong influence on their solubility [16]. Solvents of higher polarity often perform best in terms of polyphenol extraction because of the high solubility of polyphenols in such solvents [16]. A study performed on extraction of different phenols from ground grape seeds indicated that aqueous solutions of ethanol, methanol or acetone were better than a single-compound solvent system for the extraction of total phenols [11]. For proanthocyanidin extraction from solid parts of grape berries, 70% aqueous acetone was reported to be most efficient [10,12]. The maximum concentration of extracted proanthocyanidins from grape skins was reached between 50 and 70% acetone and started to decrease above 70% acetone [10]. On the other hand, the optimal conditions for mechanical flavan-3-ols extraction from ground seeds were by the use of 80% methanol [9]. Two-step extraction, with aqueous acetone followed by aqueous methanol, was mostly used for extraction of both proanthocyanidins and flavan-3-ols from ground skins and seeds of grape berries [5,6,7,8]. However, the quantitative extraction of proanthocyanidins from grape tissues may still have limitations even when strong solvents are used. Part of the proanthocyanidins of higher molecular mass could be adsorbed by skin cell walls and become unextractable in acetone [17].
The tissue solvent ratio also affects extraction efficiency. These ratios often vary considerably between reported extraction protocols. The efficiency of flavan-3-ol extraction from ground grape seeds in 80% aqueous methanol and tissue: solvent ratio 1:30 had the highest yield [9]. For solid–liquid extraction of flavan-3-ols and proanthocyanidins from lyophilised ground seeds and skins, a solvent to sample ratio of 1:9 has been reported in several studies [5,6,7,8].
To reduce both extraction time and solvent consumption and to increase extraction efficiency, new techniques have been developed, such as ultrasound, pressurised liquid, electrical and microwave-assisted extraction [18]. Pressurised liquid extraction, also known as accelerated solvent extraction (ASE), is a technique introduced by the Dionex Corporation in 1995 [19]. This technique involves extraction using liquid solvents at an elevated temperature and higher pressure and allows the extraction of analytes in an inert and closed environment. In this way, the pressurised solvents remain in a liquid state above their boiling point and the higher temperature of extraction provides better analyte solubility [19,20]. ASE operates by moving the extraction solvent through an extraction cell containing the sample, and extraction is performed by direct contact of the sample with the solvent in both static and dynamic modes [20]. When extraction is complete, compressed nitrogen moves all the solvent from the cell to the vial for analysis. During the static cycle (extraction cycle), fresh solvent is introduced to maintain the extraction equilibrium. The static time (ST) and the number of extraction cycles (ECs) can be adjusted to optimise both extraction time and efficiency. When more than one EC is used in a method, the flush volume is divided by that number. When the first ST is complete, the divided portion of the flush volume is delivered to the cell, with the “used” solvent directed to the collection vial. The system then holds the sample and solvent for a second static period. The nitrogen purge step is initiated only after the final EC. In recent studies, a protocol for ASE of phenols from ground lyophilised stems, or skins or seeds, included eight ECs with 80% aqueous acetone and three to four ECs with 60% aqueous methanol, automatic pressure control at 25 °C or at 40 °C, 5 min pre-heat time and 4 min of ST (the time of one EC) [13,14,15]. Temperature is another parameter that favours ASE extraction. However, operation at high temperature may worsen extract quality if the high temperature period is too long [21]. In the case of analytes sensitive to thermal degradation, extraction at a lower temperature with multiple ECs is proposed [22]. Furthermore, the extension of ST is proposed, enabling the analytes to pass into the extraction solvent [22]. Due to the possibility of the thermal degradation of phenolic compounds [23], a temperature of 40 °C is usually not exceeded.
The aim of this study was to reveal optimal lyophilisation time under certain conditions for structurally different tissues, such as grape seeds and skins. Lyophilised skins and seeds were cryo-milled, and the optimisation of the extraction of phenolic compounds was studied in different ASE operational conditions. Because phenolic compounds are highly unstable and rapidly transformed when the plant cells are damaged, sample preparation steps were followed the requirements of metabolomic studies [24].
2. Materials and Methods
2.1. Chemicals
HPLC grade acetone and methanol were from Sigma-Aldrich (St. Louis, MO, USA) and diatomaceous earth from Dionex™ (Thermo Scientific, Idstein, Germany), compressed nitrogen 5.0 and liquid nitrogen were provided by Messer (Ruše, Slovenia)
2.2. Lyophilisation of Grape Seeds and Skins
“Merlot” grape berries were randomly sampled from bunches at maturation and stored at −80 °C. Lyophilisation was performed in four replicates for both skins and seeds. For each replicate the skins and seeds of 100 frozen berries were separated using a scalpel, immediately dropped into liquid nitrogen, weighed, and lyophilised for nine days with VirTis BenchTop Pro with Omnitronics freeze dryer (SP Scientific, Gardiner, NY, USA) with a maximum condenser capacity of 9 L, lowest condenser temperature from −82 to −85 °C and constant pressure at 5 Pa. Skins were lyophilised in 100 mL flasks, connected to the freeze dryer, and seeds in open 100 × 20 mm glass petri dishes on a shelf rack to maximise surface area. Skin and seed weight was checked each day from day 1 to day 9 of lyophilisation. The difference between the fresh and dry weight determined the % of water in skins and seeds. After 9 days of lyophilisation, seeds and skins were milled under liquid nitrogen into a fine powder with an A11 analytical mill (Ika, Staufen, Germany) and immediately extracted using aqueous organic solvents.
2.3. Accelerated Solvent Extraction of Phenols from Ground Seeds and Skins
The extractions were performed with an ASE 350 Accelerated Solvent Extraction System (Thermo Scientific) equipped with 10 mL stainless steel cells and 60 mL collection vials. ASE 350 provides both static and dynamic modes in the same run, with the ability of the instrument to introduce fresh solvents during the extraction process [19]. A total of 1 g of dry seed or fine skin powder and 1 g of diatomaceous earth (acts as dispersant and drying agent) were mixed and loaded into a 10 mL extraction cell with a cellulose paper filter (Dionex) placed at the bottom of the ASE cell. The cell was tightly closed and placed into the ASE system. Extractions were performed without preheating the cell. Pressure increases the contact between the extracting solvent and the sample, but it generally has a negligible effect on the extraction yield [25]. Therefore, the extraction pressure was set to standard operating pressure 10.3 MPa (1500 psi), the flush volume was 60% and the purge time was set to 100 s. Extractions were performed at room temperature with 10 mL of 80% aqueous acetone and 10 mL of 60% aqueous methanol. The extraction of phenols from grape seeds is slower than from skins and requires stronger solvents [26]. Therefore, ASE conditions were first investigated to optimise phenol extraction efficiency from grape seeds. The number of ECs and the ST duration of each cycle were optimised for grape seeds. Afterwards, the optimal number of ECs and ST durations were investigated for the extraction of phenols from both seeds and skins.
2.3.1. Assessment of the Effect of Extraction Solvent Order for Extraction of Phenols from Grape Seeds
A total of 1 g of lyophilised seed powder was extracted ten times with one EC, with 15 min ST in triplicate. With the first approach, the EC was performed first with 80% aqueous acetone five times and then with 60% aqueous methanol five times. With the second approach, 1 g of lyophilized seed powder was extracted with 80% aqueous acetone for one EC and then with 60% aqueous methanol for the next EC. This order was repeated five times. After each EC, aqueous acetone or methanol seed extracts were collected and total phenolic index (TPI) was determined (as described in Section 2.5) to verify the efficiency of phenol extraction.
2.3.2. Assessment of the Number of Extraction Cycles for Extraction of Phenols from Grape Seeds
A total of 1 g of seeds was extracted in triplicate using one, four, eight and nine ECs and 1 min ST, first with aqueous acetone and then with aqueous methanol. A N2 flush was used between ECs to prevent oxidation during extraction. The TPI values were determined separately in extracts from aqueous acetone and aqueous methanol.
2.3.3. Assessment of Static Time Duration for Extraction of Phenols from Grape Seeds
A total of 1 g of lyophilised seeds was extracted in triplicate with one EC using aqueous acetone, followed by one EC using aqueous methanol. The ST durations (time of one EC) were 1 min, 10 min, 20 min and 30 min. After each EC, the extract was collected for determination of the TPI value.
2.3.4. Assessment of the Optimal Number of Extraction Cycles and Static Time Durations for Extraction of Phenols from Seeds and Skins
Extractions were performed with 10 mL of 80% aqueous acetone and 10 mL of 60% aqueous methanol using different number of ECs and different ST durations for each solvent. Acetone and methanol extracts were combined and TPI value was determined and results were compared to those obtained with manual solid–liquid extraction, as described in Section 2.4.
2.4. Extraction of Phenols from Seeds and Skins by Mechanical Shaking (Extraction by Maceration)
Manual solid–liquid extraction was performed as described previously [8]. Lyophilised and cryo-milled skins and seeds were extracted at a ratio 1:9 with 80% acetone for 4 h by mechanical shaking at room temperature and 70 rpm with an OrbitTM 1900 high-capacity lab shaker (Labnet International, Edison, NJ, USA). Samples were then centrifuged at 4 °C, 3500× g for 15 min. Sediments were extracted by shaking again at a 1:9 ratio in 60% methanol for 3 h and centrifuged. Both supernatants were pooled to determine TPI value as described in Section 2.5.
2.5. Spectrophotometric Evaluation of Total Phenolic Index in Seed and Skin Extracts
After each extraction, solvents were evaporated till dryness at 40 °C with a Genevac EZ-2 centrifugal evaporator (Genevac Ltd., Ipswich, UK). Acetone absorbs the UV light and was removed before spectrophotometric analyses. Samples were then re-dissolved in 20 mL of methanol and diluted one hundred times with milli-q water at a ratio of 1:100. TPI was determined spectrophotometrically in a 10 mm quartz cuvette by measuring the absorbance at 280 nm (A280). The TPI value corresponds to the A280 times the dilution factor. The determination of TPI is based on the characteristic absorption of the benzene cycles of most phenols and represents a fast and reproducible assay for the determination of phenols [27,28].
2.6. Statistical Analysis
Statistical analysis was performed with R [29] and plotted with the ggplot2 package [30]. Before analysis, the normality of data was checked using the Shapiro–Wilk test and Levene test to verify the homogeneity of variance of ordinal variables (days of lyophilisation, extraction times, numbers of ECs and extraction programmes). Seed and skin weight after different lyophilisation days were analysed using the nonparametric rank Kruskal–Wallis test, with days as a fixed factor (p < 0.016, Bonferroni correction). Descriptive statistics (mean and standard deviation) were calculated for each individual extraction, to study the effect of the order of extraction solvents and different extraction programmes for skins and seeds. For the mean comparison of total TPI, the two-sample t-test (p < 0.05) was used. Analysis of variance (ANOVA) was carried out to test for the main effects of solvents and extraction time and numbers of ECs as fixed factors. ANOVA was performed to determine the similarities between different extraction programmes. In the case of significant differences, Tukey’s honest significance test was applied for means comparison, using a 5% significance level.
3. Results and Discussion
3.1. Lyophilisation of Grape Skins and Seeds
To prevent phenol losses due to enzymatic oxidation, seeds and skins were first lyophilised and then cryo-ground just before extraction in organic solvents. The largest average weight loss occurred after 24 h of lyophilisation, being 60.13% and 26.68% for skins and seeds, respectively (Figure 1). The weight loss after 24 h until the 9th day of lyophilisation was not statistically significant, either for skins or seeds (Figure 1). However, from 24 h until the 9th day of lyophilisation there was still an average weight loss for skins and seeds of 1.17% and 4.29%, respectively (Figure 1). In the case of seeds, 4.29% of weight loss might still have impacted the quantification of analytes. Therefore, the lyophilisation of non-ground seeds should be longer than 24 h.
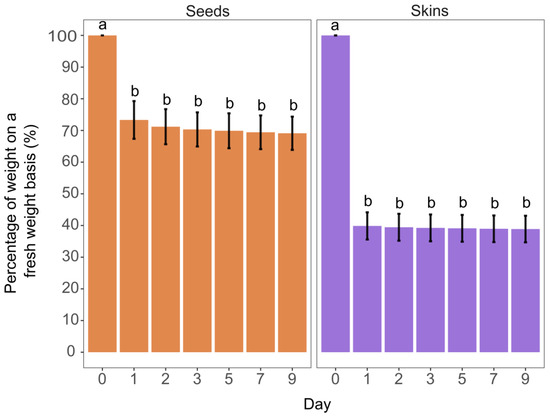
Figure 1.
Percentage of weight as regard to fresh weight of non-ground grape skins and seeds during the lyophilisation process. Error bars represent the standard deviation of three replicates and different letters indicate statistically significant differences between lyophilisation days in terms of sample weight (Kruskal–Wallis test, p < 0.05).
Lyophilisation thus demands both extensive time and energy, and to outweigh the costs and for analytical precision, 3 days and 5 days of lyophilisation at −85 °C and in a rough vacuum (5 Pa) were chosen for non-ground skins and seeds, respectively. The average percentage of water determined in these conditions was 53.47% and 29.25% in skins and seeds, respectively. From day 3 of lyophilisation to day 9, there was, on average, 0.43% and 0.83% of additional weight loss for skins and seeds, respectively, which could be considered negligible. In previous studies, 2 [5,6,31,32] and 5 days of lyophilisation [8] of non-ground seeds and skins were used; however, technical parameters, such as the pressure and temperature regime of lyophilisation, were not reported.
3.2. Assessment of the Effect of Extraction Solvent Order
Seed powder was extracted ten times with one EC, with 15 min ST in triplicate (Table 2). After each EC, extracts were collected and TPI was evaluated in 1 g of dry seeds. The phenol extraction yield after ten ECs (evaluated as the total of TPI from each EC) was higher when five ECs were performed with aqueous acetone, followed by another five ECs with aqueous methanol, compared to the procedure when acetone and methanol were exchanged after each cycle (Table 2). The difference in phenol extraction yield (Total TPI) after ten ECs was not statistically significant for the two extraction approaches (Table 2). However, after the ninth and tenth ECs, there was no absorption at 280 nm when first acetone was used and then methanol. On the other hand, TPI was still determined after ten ECs when the solvents were exchanged (Table 2). From the data obtained, it can be concluded that extraction was more efficient when starting with several ECs using aqueous acetone, followed by several ECs with aqueous methanol. This solvent order is consistent with previous studies, which reported phenol extraction from lyophilised ground seeds or skins using eight ECs with 80% aqueous acetone, followed by three to four ECs with 60% aqueous methanol, automatic pressure control at 25 °C and 4 min ST [13,14]. Most phenols expressed as TPI were already extracted from lyophilised ground seeds in the first EC with aqueous acetone (Table 2). Aqueous acetone is an efficient solvent for proanthocyanidins [10,12]. On the other hand, besides proanthocyanidins, the majority of free grape flavan-3-ols are reportedly located in the seeds [33]. Aqueous methanol is an effective solvent for free flavan-3-ols [9]. On observing TPI, phenols were extracted from grape seeds with aqueous methanol, even when five ECs with 15 min ST had already been performed with aqueous acetone (Table 2, left column).

Table 2.
Determination of total phenolic index (TPI) in 1 g of lyophilised grape seed powder extracted with accelerated solvent extraction at 10.3 MPa and room temperature, ten times with one extraction cycle (EC) and 15 min static time (ST). Left—the EC was performed five times with 80% aqueous acetone (AC) and then five times with 60% aqueous methanol (MeOH). Right—AC and MeOH were exchanged after each EC. The results represent means of three replicates ± standard deviation.
3.3. Assessment of the Number of Extraction Cycles and Static Time Duration
It was found that extraction starting with several ECs using aqueous acetone, followed by several ECs with aqueous methanol was more efficient than if the solvents were exchanged alternately. Therefore, the assessment of the number of ECs needed and the ST duration required for this solvent order was carried out.
To assess the most efficient number of ECs for each solvent ground lyophilised seeds were extracted with either one, four, eight or nine ECs, first with aqueous acetone with 1 min ST, followed by either one, four, eight or nine ECs with aqueous methanol with 1 min ST (Figure 2a). The yield of extracted phenols (evaluated as TPI in 1 g of dry seeds) with aqueous acetone was significantly higher after eight and nine ECs than after just one (Figure 2a), whereas the TPI values between four, eight or nine ECs did not significantly differ. ECs have proven to be useful for sample types with a very high concentration of analyte or samples with difficult to penetrate matrices [34]. The yield of extracted phenols with aqueous methanol was the highest after only one EC, however not significantly different for one, four and eight ECs with 1 min ST (Figure 2a). Surprisingly, TPI was statistically lower after nine ECs for 1 min than after just one (Figure 2a). Results are compliant with findings of Álvarez-Casas et al. who extracted polyphenols from white grape march with methanol. They found that extraction efficiency was optimal after two ECs while the use of five ECs was not efficient due to an increased background [35].
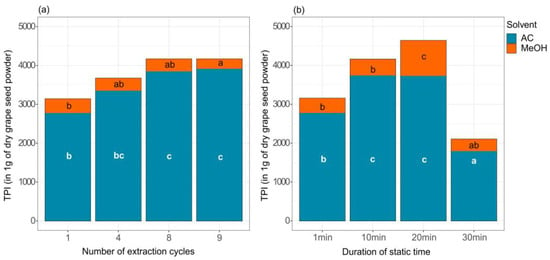
Figure 2.
Total phenolic index (TPI) in 1 g of lyophilised grape seed powder extracted with accelerated solvent extraction with 80% aqueous acetone (AC) and 60% aqueous methanol (MeOH). (a) TPI with different numbers of ECs (n = 3), (b) TPI with different STs (n = 3). Different letters indicate statistically significant differences between TPI within each solvent used (ANOVA, p < 0.05).
To assess the most efficient ST (duration of pressurised extraction), ground lyophilized seeds were extracted in triplicate using one EC with aqueous acetone and then one EC with aqueous methanol for either 1 min, 10 min, 20 min or 30 min (Figure 2b). After each EC, the extracts were collected and TPI was determined. Using aqueous acetone, TPI values were significantly higher in the case of both 10- and 20-min STs compared to 1 min and 30 min. With the use of aqueous methanol, TPI values were significantly higher after 20 min of extraction (Figure 2b). In the case of 30 min ST, the lowest extraction yield was obtained using both aqueous acetone and aqueous methanol.
Our results showed a significantly increased yield of extracted phenols in aqueous acetone extracts after at least eight ECs with 1 min ST. The effect of ST on phenol extraction yield with aqueous acetone was highest between 1 min and 10 min, while thereafter the yield did not change significantly up to 20 min ST. In agreement with the literature [13,15], it is more efficient to perform a larger number of ECs with aqueous acetone with shorter STs. Considering subsequent extraction in aqueous methanol, the phenol extraction yield did not increase after a single EC. However, the ST significantly impacted the extraction yield, being highest after 20 min and then decreasing. The methanol fraction of phenols was efficiently extracted even if ST were not divided to more ECs which is the advised procedure for the most efficient extraction [34]. Flavan-3-ols are monomeric compounds with less complex structure than proanthocyanidins, which may affect their extractability. The extraction efficiency was the lowest for both solvents if the ST was 30 min. It could be postulated that either phenols oxidized (unlikely because of organic solvents and N2 flush) or extraction conditions with longer STs (which are not divided by ECs) are not efficient for lyophilised grape seed powder.
3.4. Yield of Extracted Phenols from Grape Skins and Seeds with Manual Solid-Liquid Extraction and Accelerated Solvent Extraction under Different Extraction Conditions
Table 3 represents the extraction yield of phenols (evaluated as TPI) from 1 g of lyophilised ground seeds and skins, starting with aqueous acetone and continuing with aqueous methanol. Manual and ASE extractions under different conditions were performed in four replicates. Interestingly, the TPI values obtained after manual solid–liquid extractions did not significantly differ to those obtained with ASE. Indeed, ASE performed under similar conditions as previously reported [13,14] and previously reported conditions of manual solid–liquid extractions [5,8] had a similar phenol extraction yield in the case of both seeds and skins (Table 3). However, the duration of manual extraction was 450 min with similar solvent consumption (the tissue solvent ratios were the same). Furthermore, manual extraction requires manual work and therefore higher human exposure to organic solvents.

Table 3.
Determination of total phenolic index (TPI) in 1 g of lyophilised grape seed and skin powder extracted with manual solid–liquid extraction and accelerated solvent extraction (ASE) at 10.3 MPa and room temperature. The results represent means ± standard deviation of four replicates.
It was again confirmed that exceeding eight ECs was not efficient when aqueous acetone was used for lyophilised seed powder (Table 3). The highest extraction yield of phenols from seed powder was obtained by repeating eight ECs with 10 min ST using 80% aqueous acetone and repeating four ECs with 20 min ST using 60% aqueous methanol. Although the differences in the yield of phenols were not statistically significant between ASE conditions, the latter approach was chosen as the most efficient in the case of seeds. The estimated time for ASE was 162 min for one sample and total solvent use was 24 mL (Table 3, Figure 3). The volume of solvents used was the same as for previously published protocols, whereas the time for one extraction was longer. Since ASE is a fully automated and closed process, the longer time could be accepted for the sample preparation protocol.
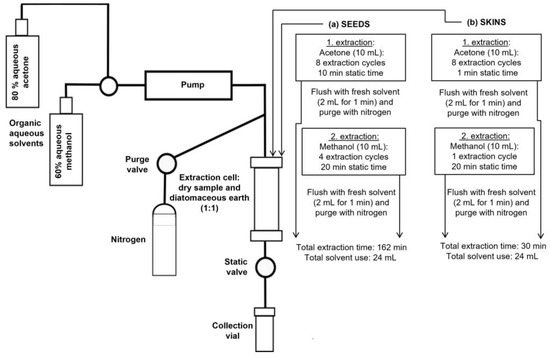
Figure 3.
Schematic outline of proposed accelerated solvent extraction at room temperature and 10.3 MPa for extraction of phenols from (a) lyophilised grape seed powder and (b) lyophilised grape skin powder. Acetone −80% aqueous acetone; Methanol −60% aqueous methanol.
In the case of lyophilised grape skin powder, the TPI value was among the highest after eight ECs with 1 min ST using 80% aqueous acetone and one EC with 20 min ST using 60% aqueous methanol (Table 3). However, the yield of phenols did not significantly differ in different extraction protocols. At the end of ASE, acetone and methanol extracts were pooled in a collection vial for analyses. The estimated extraction time for one sample is 30 min and total solvent consumption is 24 mL (Table 3, Figure 3).
4. Conclusions
By providing specific data about sample preparation, the study contributes to overcoming analytical limitations for the accurate quantitation of phenols in grapes. This study investigated the conditions for the lyophilisation and extraction of phenolics from grape skins and seeds. The guidelines of metabolomic studies were considered in sample preparation steps, aiming to ensure metabolic quenching, efficient extraction and the reproducible, accurate analysis of samples. For the efficient drying of non-ground seeds and skins, the optimal lyophilisation time proposed is five days and three days, respectively. Accelerated solvent extraction at a lower temperature with multiple extraction cycles and longer static times is the proposed procedure for thermolabile compounds, such as phenols. It was found that the extraction of phenols from lyophilised ground seeds first with aqueous acetone, followed by aqueous methanol, was more efficient than if the solvents were exchanged alternately. Considering aqueous acetone (the extraction solvent for proanthocyanidins) eight extraction cycles with 10 min static time was the most efficient protocol for the extraction from seeds at room temperature. Considering the subsequent extraction in aqueous methanol (the extraction solvent for flavan-3-ols), the phenol extraction yield did not increase significantly after the first extraction cycle, but longer static time (20 min) significantly improved the extraction yield.
Author Contributions
Conceptualization, K.L., A.V. and A.M.; data curation, A.M. and A.V.; formal analysis, A.M.; investigation, A.M., A.V. and K.L.; methodology, K.L., A.V. and A.M.; software, A.M.; supervision, A.V. and K.L.; writing—original draft, A.M. and A.V.; writing—review and editing, A.V. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency, grant numbers 51919 (young researcher), L4-1841 (research project) and P4-0133 (research programme), and by the Acquavitis (Interreg) project within the Programme Interreg V-A Italy-Slovenia 2014–2020 programme, funded by the European Regional Development Fund.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge wine producers from Vipava Valley for both their donation of grapes and cooperation in the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vermerris, W.; Nicholson, R. Phenolic Compounds and Their Effects on Human Health. In Phenolic Compound Biochemistry; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 235–255. ISBN 978-1-4020-5163-0. [Google Scholar]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 115–124. ISBN 978-1-119-15804-2. [Google Scholar]
- Li, L.; Sun, B. Grape and Wine Polymeric Polyphenols: Their Importance in Enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M.; Salunkhe, D.K. Tannin Analysis of Food Products. Crit. Rev. Food Sci. Nutr. 1986, 24, 401–449. [Google Scholar] [CrossRef]
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.-L. Grape Variety Effect on Proanthocyanidin Composition and Sensory Perception of Skin and Seed Tannin Extracts from Bordeaux Wine Grapes (Cabernet Sauvignon and Merlot) for Two Consecutive Vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef]
- Sivilotti, P.; Falchi, R.; Vanderweide, J.; Sabbatini, P.; Bubola, M.; Vanzo, A.; Lisjak, K.; Peterlunger, E.; Herrera, J.C. Yield Reduction through Cluster or Selective Berry Thinning Similarly Modulates Anthocyanins and Proanthocyanidins Composition in Refosco Dal Peduncolo Rosso (Vitis vinifera L.) Grapes. Sci. Hortic. 2020, 264, 109166. [Google Scholar] [CrossRef]
- Lorrain, B.; Chira, K.; Teissedre, P.-L. Phenolic Composition of Merlot and Cabernet-Sauvignon Grapes from Bordeaux Vineyard for the 2009-Vintage: Comparison to 2006, 2007 and 2008 Vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef]
- Calderan, A.; Sivilotti, P.; Braidotti, R.; Mihelčič, A.; Lisjak, K.; Vanzo, A. Managing Moderate Water Deficit Increased Anthocyanin Concentration and Proanthocyanidin Galloylation in “Refošk” Grapes in Northeast Italy. Agric. Water Manag. 2021, 246, 106684. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Jiang, J.; Duan, W.; Fan, P.; Li, S.; Wang, L. Flavan-3-Ols in Vitis Seeds: Their Extraction and Analysis by HPLC-ESI-MS/MS. Food Res. Int. 2021, 139, 109911. [Google Scholar] [CrossRef]
- Downey, M.O.; Hanlin, R.L. Comparison of Ethanol and Acetone Mixtures for Extraction of Condensed Tannin from Grape Skin. S. Afr. J. Enol. Vitic. 2010, 31. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy, J.A. Factors Affecting Skin Tannin Extractability in Ripening Grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of Seed and Skin Polyphenolic Extracts of Two Red Grape Cultivars Grown in Croatia and Their Sensory Perception in a Wine Model Medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Ma, W.; Waffo-Téguo, P.; Jourdes, M.; Li, H.; Teissedre, P.-L. First Evidence of Epicatechin Vanillate in Grape Seed and Red Wine. Food Chem. 2018, 259, 304–310. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.-L. Proanthocyanidin Composition and Antioxidant Potential of the Stem Winemaking Byproducts from 10 Different Grape Varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-Specific and Developmental Modifications of Grape Cell Walls Influence the Adsorption of Proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.-L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal Degradation of Grape Marc Polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
- Dionex. Dionex ASE 350 Accelerated Solvent Extractor Operator’s Manual; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2011. [Google Scholar]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Fiehn, O. Combining Genomics, Metabolome Analysis, and Biochemical Modelling to Understand Metabolic Networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized Liquid Extraction in the Analysis of Food and Biological Samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lisjak, K.; Lelova, Z.; Žigon, U.; Bolta, Š.V.; Teissedre, P.-L.; Vanzo, A. Effect of Extraction Time on Content, Composition and Sensory Perception of Proanthocyanidins in Wine-like Medium and during Industrial Fermentation of Cabernet Sauvignon. J. Sci. Food Agric. 2020, 100, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L. Wessel du Toit The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solís-Oviedo, R.L., de la Cruz Pech-Canul, Á., Eds.; IntechOpen: Rijeka, Croatia, 2018; p. 3. ISBN 978-1-78985-496-1. [Google Scholar]
- Chris Somers, T.; Evans, M.E. Spectral Evaluation of Young Red Wines: Anthocyanin Equilibria, Total Phenolics, Free and Molecular SO2, “Chemical Age”. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.-L. Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules 2011, 16, 1519–1532. [Google Scholar] [CrossRef]
- Chira, K.; Zeng, L.; Le Floch, A.; Péchamat, L.; Jourdes, M.; Teissedre, P.-L. Compositional and Sensory Characterization of Grape Proanthocyanidins and Oak Wood Ellagitannin. Tetrahedron 2015, 71, 2999–3006. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the Amount and Structure of Extractable Skin and Seed Tannins amongst Red Grape Varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Dionex. Methods Optimization in Accelerated Solvent Extraction (ASE); Technical Note 208; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2004. [Google Scholar]
- Álvarez-Casas, M.; García-Jares, C.; Llompart, M.; Lores, M. Effect of Experimental Parameters in the Pressurized Solvent Extraction of Polyphenolic Compounds from White Grape Marc. Food Chem. 2014, 157, 524–532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).