Abstract
This study aimed to evaluate essential traits of donkey’s milk and cow’s milk kefir during storage for 28 days at +4 °C. The results showed that the pH decreases significantly during fermentation from 6.75 ± 0.045 to 4.22 ± 0.062 for cow’s milk and from 7.01 ± 0.011 to 4.28 ± 0.030 for donkey’s milk. Acidity values increased significantly during storage from 63 ± 2.08 °D to 170 ± 2.80 °D for cow’s milk and from 92 ± 1.0 °D to 163 ± 1.30 °D for donkey’s milk (p < 0.05). A significant variation in total solids was observed during storage. Stability in protein content was observed for kefirs during storage time. While the level of lactose decreased significantly during storage, the fat content did not vary in kefirs during storage time at 4 °C. For microbiological properties, donkey milk kefir presents a significant difference (p < 0.05) compared to bovine kefir. Donkey’s milk always contains the lowest average germs, suggesting a better microbiological quality than cow’s milk samples. The fermented milks showed an interesting antioxidant activity measured by the DPPH and ABTS assays, which were improved during storage. The Aeromonas hydrophila was the most sensitive bacterium to the action of kefir samples. Results from the sensorial test show that participants prefer kefirs freshly prepared than those stored after 28 days at 4 °C. In conclusion, related to its unique bioactive activities and microbiological properties, donkey’s milk could be an interesting kefir fermentation source materials alternative.
1. Introduction
Milk is an essential component of the human diet because its particular components promote young animals’ nutritional, immunological, and developmental needs [1]. However, people’s intolerance and allergic reactions to cow’s milk (CM) have led to a rising demand for alternative milk sources in recent years [2,3,4,5,6], such as horse and donkey milk (DM), and is classified as a “pharmafood” that is also highly preferred by consumers [7]. Nowadays, the economic worth of DM has been recognized not only for its nutritional value but also for its medicinal and functional capacities due to its chemical composition, which is similar to human milk, particularly for newborns with cow’s milk protein allergy [8,9,10,11]. It has a hypo-allergenic protein composition, high polyunsaturated fatty acid and essential amino acid [6,11,12,13,14,15]. In addition, DM exhibited antiaging, antioxidant, antibacterial, anti-inflammatory, and antiaggregant activities related to its unique composition [16].
Although raw milk has traditionally been favored for consumption, fermented milk products have increased in popularity due to their therapeutic effects and favorable impact on health [17]. Kefir is a fermented milk beverage that originated in Russia’s Caucasus mountains or Mongolia and is made of a unique blend of beneficial microbes [18]. Lactic acid bacteria (LAB) (Streptococcus, Leuconostoc, Lactobacillus, and Lactococcus), yeasts (Saccharomyces, Torula, Kluyveromyces, and Candida spp.), and Acetobacter have been found in fermented milk products based on kefir [19]. All of these bacteria cohabit in kefiran, a water-soluble branched glucogalactan polysaccharide matrix that may boost consumers’ immune systems and increase resistance to some diseases, such as neoplasia and infections [20]. Small clusters of microbe combinations embedded in a special polysaccharide matrix called kefir grains can be inoculated into milk to generate kefir, and fermentation can take up to 24 h [21].
Milk fermentation is not just a traditional preservation method but is also used to improve the quality or taste of dairy products [22]. Through sensory attributions and physicochemical qualities, the textural characteristics of the fermentation process influence customer approval of the product [23]. The composition of the source material is the most important factor in kefir fermentation. As a result, kefir is well-known for its probiotic, prebiotic, antimicrobial, anticarcinogenic, antidiabetic, antiallergic, anticancer, and antioxidant properties [21].
This work aims to study the kefir fermentation of cow and donkey milk by examining the chemical, physicochemical, microbiological, sensory profiles, antioxidant, and antibacterial activities.
2. Materials and Methods
2.1. Samples
Cow’s (Holstein breed) milk and donkey’s (Arabian breed) milk were collected between February and March 2020 under hygienic conditions from intensive cattle breeding farms (n = 120) situated in Manouba (Northern East Tunisia) and modern donkey breeding farms (n = 8) in the Zaghouan (Northerner Tunisia) regions, respectively. Milk samples were collected in dairy farming conditions under Tunisian law (The Livestock Law No. 2005-95 of 18 October 2005) so that the ethical approval of the research protocol was waived. The samples were stored in sterile bottles, immediately cooled, and transported to the laboratory under refrigerated conditions from 6–10 °C.
Kefir grains, obtained from the Animal Production Laboratory of Ecole Supérieure d’Agriculture du Kef, were mainly composed of Kazachstania spp., K. lactis, K. marxianus, S. cerevisiae, L. acidophilus, L. brevis, L. casei, L. delbrueckii, L. helveticus, L. kefiranofaciens, L. kefiri, L. paracasei, L. Plantarum, L. rhamnosus, L. sake, L. lactis, L. mesenteroides, Pseudomonas spp., S. thermophilus, C. humilis [24,25,26,27,28].
2.2. Kefir Manufacture
Raw cow milk (CM) samples (g/100 g, total solids = 11.92 ± 0.65, protein = 3.1 ± 0.04, lactose = 4.60 ± 0.3, fat = 3.65 ± 0.05) and donkey milk (DM) samples (g/100 g, total solids = 8.62 ± 0.11, protein = 1.53 ± 0.02, lactose = 6.15 ± 0.07, fat = 1.33 ± 0.03) were pasteurized at 95 °C for 10 min then cooled down to 25 °C. The fermentation process was carried out according to the method described by Perna et al. (2019) [29] with some modifications. Briefly, the cooled milk sample was inoculated with 5% (w/v) of kefir grains and incubated at 25 °C for 24 h and pH was measured every 2 h. In the end, the grains were separated by filtration through a sieve. The kefir samples were stored in 100 mL plastic containers in a refrigerator (+4 °C) for subsequent analysis. All samples were analyzed at 0, 1, 7, 14, 21, and 28 days of refrigerated storage.
2.3. Physicochemical and Chemical Analysis
The kefir and raw milk samples were mixed and analyzed for pH, Dornic acidity, solid, fat, lactose, and protein contents.
Dornic acidity, expressed in terms of °D [30], was measured on 10 mL of fermented milk. The Dornic acidity was determined by titration with 0.1 N NaOH [31]. In addition, the pH of kefirs was measured using a digital pH meter (Consort PH C860, Belgium) calibrated using commercial pH 4.00 and 7.00 buffers.
The total solids (TS), fat, lactose, and protein contents were determined using the milk analyzer based on the Fourier-transform infrared technology MilkoScan TM Mars (Foss Electric, Hillerod, Denmark).
2.4. Microbiological Analysis
During 28 days of storage at 4 °C of kefir milk, aliquots of the fermented milks were removed under aseptic conditions and decimal dilutions were performed for the enumeration of total aerobic mesophile bacteria (TAMB), total coliforms (TC), fecal coliforms (FC), yeasts (Y), and lactic acid bacteria (LAB). Sterile peptone water was used to prepare the dilutions for the microbiological analyses. TMAB count was determined on plate count agar (PCA, Merck, Germany) after incubation at 30 °C from 48 h to 72 h [32,33,34]. Total and fecal coliforms were counted according to [35]. Yeast counts were quantified on Potato Dextrose Agar with chloramphenicol (0.1 g/L), which was incubated at 30 ± 1 °C for 48 h under aerobic conditions [29,34,36]. LAB counts were determined on De Man, Rogosa, and Sharpe (MRS) agar plates, incubated at 30 ± 1 °C for 48 h under anaerobic conditions [29,34,37].
Afterward, plates containing between 20 and 200 colonies were enumerated, and the results were expressed as colony-forming units per mL of kefir (log10 CFU/mL) [29,34,38]. Finally, the entire microbiologically experiment was repeated in triplicate.
2.5. Antioxidant Activity
2.5.1. Extraction
Kefir samples (2 g) were mixed with 20 mL of aqueous methanol (70:30%, v/v) for 4 h in the dark at room temperature under magnetic stirring. Extracts were centrifuged at 1420× g for 10 min and filtered through qualitative filter paper (Whatman grade 2, 8 µm thick); supernatants were used for ABTS and DPPH assays as described by [39].
2.5.2. ABTS Test
The free radical stock solution ABTS was prepared by reacting 20 mM ABTS•+ with 2.45 mM potassium persulfate solution (1:1, vol/vol) and stored at room temperature from 12 to 16 h in the dark before use. ABTS•+ working solution was prepared by diluting the stock solution 1:10 with ethanol to yield an absorbance of 0.7 ± 0.02 at 734 nm.
Then, 0.25 mL of samples were mixed with 3.75 mL of ethanol and 1 mL of ABTS radical cation solution. The mixture was stood at room temperature for 10 min, and the absorbance was measured after at 734 nm using a UV-visible spectrophotometer (Jenway 6505 UV-Vis spectrophotometer, Berryville, Virginia).
2.5.3. DPPH Test
Firstly, 0.25 mL of extract was mixed with 0.18 mL of DPPH (10−3 M) stock solution, and methanol was added to bring the final volume to 3 mL (6 × 10−5 M). After 30 min in the dark, absorbance readings were taken on reagent blanks at 515 nm using a UV-visible spectrophotometer (Jenway 6505 UV-Vis Spectrophotometer, Berryville, Virginia).
The free radical scavenging activity (SA) was estimated based on the percentage of inhibition of DPPH or ABTS+ radicals, according to the following formula [40].
A1 and A2 were controlled absorbance (ABTS or DPPH solution without extract) and sample absorbance, respectively.
2.6. Antibacterial Activity
2.6.1. Bacterial Strains
To assess the antibacterial activity of pasteurized donkey and cow milk kefirs, four pathogen bacteria available in our laboratory, namely three Gram-negative bacteria, i.e., Escherichia coli (ATCC 35218), Pseudomonas aeruginosa (ATCC 9027), and Aeromonas hydrophila (ATCC1943), and one Gram-positive bacterium, Staphylococcus aureus (ATCC 25923), were used.
2.6.2. Disc Diffusion Test
The antimicrobial activity was assessed according to [41] with slight modifications. First, kefir samples were filtered through 0.22-µm cellulose acetate membrane filters (Solarbio, Beijing, China).
Aeromonas hydrophila, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus were grown at their optimum temperature (37 °C) overnight in a nutrient broth medium (HiMedia, India). After that, the bacteria were diluted to approximately 106 Colony-Forming Units (CFU)/mL and inoculated on the nutrient agar medium (HiMedia, India).
The sterile disks (5 mm diameter) were soaked with filtered kefir samples (150 µL) and placed on the agar surface. Then, the plates were incubated at 37 °C for 24 h. The presence of a clear zone indicates a total inhibition of bacterial growth.
2.7. Sensory Evaluation
The sensory evaluation was performed to evaluate the consumer acceptability of kefir samples freshly prepared (48 h) and after storage (28 days) and the pasteurized raw milks [42]. In addition, coded cow and donkey samples (approximately 15 mL) were tested by 60 untrained panelists chosen by convenience. The following sensory parameters were assessed, including color, flavor, smell, taste, texture, and overall acceptability. A structured 9-point hedonic scale ranging from 1 (highly disliked) to 9 (extremely liked) was used in this test.
2.8. Statistical Analysis
An XL STAT software was used for ANOVA and student’s test to detect significant differences among all kefir samples stored for 28 days at 4 °C for different parameters tested. Microsoft Excel 2010 was used to determine means (± standard deviations) and construct viscosity and flow curves.
3. Results
3.1. Physicochemical and Chemical Analyses
pH Variation during the Fermentation
The pH evolution of the different fermented kinds of milk using the kefir grains is presented in Figure 1. The results show that the pH decreases significantly during fermentation from 6.75 ± 0.045 to 4.22 ± 0.062 for cow’s milk and from 7.01 ± 0.011 to 4.28 ± 0.030 for donkey’s milk.
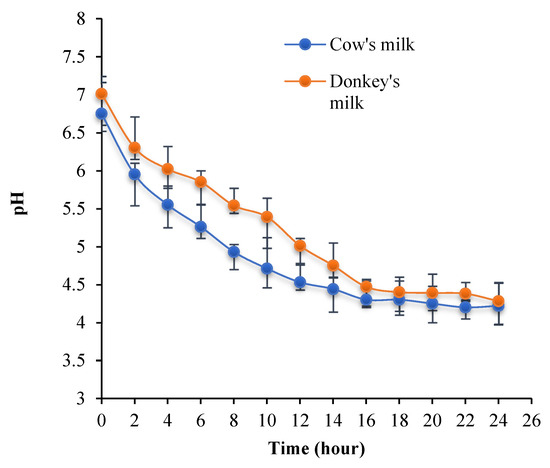
Figure 1.
pH evolution of cow and donkey milks during fermentation with kefir grains at 25 °C.
3.2. Evolution of Physicochemical Parameters during Storage
3.2.1. Dornic Acidity Evolution during Storage
Initially, raw cow and donkey milks have an acidity of 18 ± and 17 ± °D, respectively. However, after fermentation, the acidity of the kefir-fermented milk was 63°D for CM and 92 °D for DM.
The Dornic acidity measurement of fermented milk beverages during storage is presented in Figure 2. Acidity values increase significantly during storage from 63.00 ± 2.08 °D to 170.00 ± 2.80 °D for cow’s milk and from 92.00 ± 1.00 °D to 163.00 ± 1.308 °D for donkey’s milk.
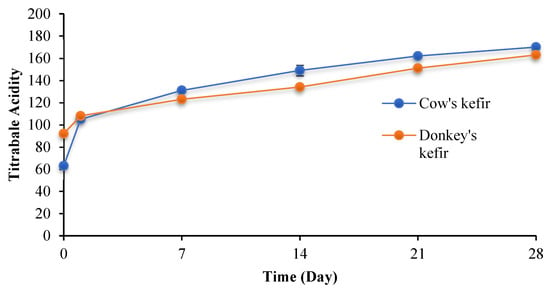
Figure 2.
Dornic acidity evolution of cow and donkey kefirs during storage time at 4 °C.
3.2.2. Biochemical Composition Evolution during Storage Time at 4 °C
After fermentation, the monitoring of biochemical composition during storage at +4 °C is given in Table 1. It can be noted that the significant variation in the biochemical composition of kefirs is due to the variation in the composition of the kinds of milk, and the storage time significantly affects the total solids and lactose contents of the kefirs.

Table 1.
Biochemical composition evolution of cow and donkey kefirs during storage time at 4 °C.
3.3. Microbial Profile of Kefirs
Except for TC and FC, the microbiological analysis of the studied kefirs highlights the presence of significant variations during storage (Table 2). Initially, the number of lactic acid bacteria was high for both samples (8.96 log CFU/mL for CMK and 8.12 log CFU/mL for DMK). However, during storage, the viable LAB count decreased for the two samples reaching 6.80 log CFU/mL (28th day of storage) (Table 2). According to Table 2, the two kefirs do not present fecal coliforms. The absence of these germs indicates the good hygienic quality of kefir.

Table 2.
Viable counts of total aerobic mesophilic bacteria (TAMB), lactic bacteria (LAB), total coliforms (TC), and yeasts (Y) of cow and donkey kefirs during storage at 4 °C.
Moreover, total coliforms were only detected in the CM kefir samples. TC count increases during storage from 4.02 log CFU/mL (day 0) to 4.51 log CFU/mL (day 28). The enumeration of TAMB for the studied samples shows that there is an alteration of the fermented milk during the storage period, with a significant difference (p < 0.05) between the monitoring days and the two types of fermented milk (Table 2). It should be noted that the DM kefir is characterized by a lower level of TAMB (4.18–4.71 log CFU/mL).
For yeast, the CM and DM kefirs show a similar profile. Yeast was not detected from day 14 of storage for CM kefir and day 1 of storage for DM kefir.
3.4. Antioxidant Activity
The studied fermented milk beverages showed an interesting antioxidant activity measured by the DPPH and ABTS tests and expressed as an inhibition percentage of free radicals (Table 3).

Table 3.
Free radical scavenging activity of cow and donkey kefir during storage at 4 °C.
Initially, donkey and cow milk kefirs revealed a similar antioxidant activity against DPPH and ABTS+ free radicals. Then, the antioxidant activity increases significantly during storage for both tests, reaching 84.95% (DPPH test) and 92.73% (ABTS test) for CMK and 78.76% (DPPH test) and 94.01% (ABTS test) for DMK at the end of storage.
3.5. Antibacterial Activity
This analysis allows us to measure the sensitivity of pathogenic strains to the studied kefirs. The inhibitory effect was determined from measurements of the inhibition zone diameter (Table 4). The studied bacteria (A. hydrophila, P. aeruginosa, E. coli, and S. aureus) were chosen for their pathogenic effect causing gastrointestinal disorders, such as diarrhea. This disease is still the most prevalent health problem in developing countries despite scientific progress and management that have taken place in recent years [43]. These authors have shown that ingesting food contaminated with infectious or toxigenic microorganisms such as S. aureus, E. coli, and A. hydrophila leads to death.

Table 4.
Average zone diameter for antibacterial activity of cow and donkey kefirs during storage time at 4 °C.
This study observed that cow and donkey kefirs inhibited most of the studied pathogenic bacteria. However, the evolution of the antibacterial activity of kefir was not significant during storage.
The A. hydrophila is the most sensitive, showing an inhibition zone diameter between 6 mm and 7 mm for cow and donkey kefir during storage at + 4 °C. This Gram-negative bacterium causes gastrointestinal or non-gastrointestinal complications. Kefirs also show an inhibitory effect on S. aureus, followed by E. coli (Table 4).
3.6. Consumer Acceptability
For functional foods, such as kefir, the evaluation of sensorial characteristics and consumer acceptance is important.
The sensorial profiles of samples are illustrated in Figure 3. The results show that raw donkey milk was the most appreciated sample for the most studied sensory characteristics (flavor, taste, and overall appreciation). For the fermented milk, cow and donkey kefir were more appreciated when they were stored for 48 h at 4 °C. Hence, the fermentation of milk with kefir grains improved the appreciation of color, flavor, smell, taste, and texture. However, all sensory characteristics were depreciated by tasters for kefirs stored for 28 days, suggesting a degradation in the sensory parameters during storage.
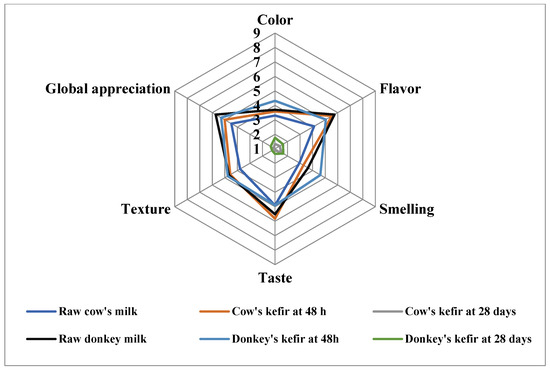
Figure 3.
Sensorial profile of donkey’s and cow’s milk and kefirs.
4. Discussion
The milk composition changes significantly depending on breed, species, diet, lactation period, and genetic polymorphism of the species and breeds [2,44,45]. These results are in agreement with the data presented by [46]. A significant variation in total solids was observed during storage for both kefirs. These results are confirmed by those found in [47], which show that fermentation does not influence the total solids of milk kefir. Indeed, this factor depends on the composition of the used milk and the fermentation process [47]. According to Table 1, non-significant changes in protein content during storage were observed for the two kefirs. This result was similar to data reported by [47] and contrary to data reported by [48], which explained the decrease in protein content due to the presence of enzymes secreted by the bacteria that are found in the kefir grains and hydrolyze the k-caseins by eliminating the hydrophilic part. Then, the hydrophobic parts will, therefore, approach the casein level coagulates and subsequently increase as the fermented milk’s protein level increases. In contrast to data proved by [49], a non-significant decrease in fat content was observed for kefirs during storage time at 4 °C.
Lactose levels decrease significantly during storage, as observed in the case of DM kefir. Lactose is transformed to lactic acid owing to the presence of bacteria and yeasts in kefir grains that stimulate lactic fermentation. The bacteria found in kefir grains secrete galactosidase, which hydrolyzes lactose into simple sugars [46]. Irigoyen et al. [49] found no significant changes in lactose levels in cow milk kefir during storage. The low lactose content encourages lactose intolerant people to drink kefir [26]. The acidity evolution of fermented DM is noticeably lower than that of CM (Figure 2). Indeed, Salimei et al. (2004) [15] speculate that this is due to the low casein and phosphate levels in donkey milk.
pH is an important parameter in the production of fermented milk. It indicates the end of the fermentation process. This involves the increase in the rate of ions (H+) resulting from the lactic and alcoholic fermentations induced by bacteria and yeasts found in kefir grains [50]. Raw cow’s milk is more acidic than donkey’s milk. These phenomena were reported previously by [48,51] and were explained by the high casein content of CM compared to DM. The isoelectric pH of milk is equal to 4.6, and at this value, the milk caseins coagulate. According to Figure 1, the casein precipitation was observed after 14 h for CM and 16 h for DM. This can be related to the low casein concentrations in donkey’s milk leading to slow clotting [44]. According to Bornaz et al. (2010) [46], donkey milk caseins are better digestible than ruminant milk due to the fermentation activities of microorganisms found in milk, mainly lactic acid bacteria, which transform lactose into lactic acid.
For the microbial profile of kefirs, the results showed the absence of fecal coliforms, which indicates the good hygienic quality of the two kefirs [46]. Furthermore, the viable TAMB increased during storage for the two samples, and similar findings were reported by [32] for yogurt. On the contrary, Benmeziane et al. (2021) [45] reported the absence of TAMB and CT during 21 days of storage and similar results were reported by [47,49,50]. The significant decline in LAB count can be likened to the storage temperature. Indeed, one of the important parameters influencing metabolic activity and microorganism growth is storage temperature. The cold temperature may affect cell damage and force cells to be static or have a low metabolism [52]. Furthermore, no yeast was detected on day 1 or day 14 of storage for DM kefir or CM kefir, respectively. This was most likely due to this microorganism’s sensitivity to high acidity levels [53].
Donkey’s milk always contains the lowest average germs, indicating a better microbiological quality than cow’s milk. This is due to the protein profile and richness of DM of lysozyme, which acquires milk antimicrobial activities [11].
The antioxidant activities have increased during the storage time for the two samples for the two tests. This increase is probably due to the low molecular weight bioactive peptides (<5000 KDa) derived from the proteolytic activities of lactic acid bacteria and yeasts [34]. Furthermore, Lima et al. (2017) [54] suggest that the fermented milk kefir is a source of bioactive peptides showing important antioxidant activity. Moreover, they reveal a relationship between the increase in antioxidant activity and the high degree of proteolysis.
The CM and DM kefirs show an inhibitory effect against the growth of pathogenic bacteria. This can be related to the production of bacteriocins and organic acids during fermentation [55]. Further, Nazzaro et al. (2020) [56] estimate the presence of other substances resulting from the digestion of proteins which gives milk an antibacterial power.
P. aeruginosa was the most resistant bacterium to the kefirs effect during storage. It was mentioned that P. aeruginosa is naturally recalcitrant to many antibiotics [57]. Its general resistance is due to a combination of factors, such as the low permeability of its cell wall, the genetic capacity to express a wide repertoire of resistance mechanisms, the capacity of mutation in chromosomal genes, and the acquirement of additional resistance genes from other organisms via plasmids, transposons, and bacteriophages [58].
It is sensitive to a remaining number of antibiotics, explained by the existence of mechanisms with complementary or synergistic actions determined by the chromosomal genes belonging to the heritage of the species [57].
5. Conclusions
Chemical, physicochemical, microbiological, sensorial, and bioactive results show that the composition of kefirs depends on the storage period and the origin of milk. Donkey milk kefir contains less dry matter, fat, and protein; however, it is richer in lactose than cow milk kefir. The microbiological profile reveals a low number of germs and an absence of pathogenic germs in kefirs, particularly for DM kefir. Indeed, the microbial profile of kefirs was improved during storage. The evaluation of the antioxidant and antibacterial activities of kefirs shows a significant improvement during storage. Sensorial results show that raw donkey milk is the most appreciated sample. While the fermentation of milk with kefir grains stored for 48 h has improved the appreciation of color, flavor, smell, taste, and texture.
Thus, donkey kefir production could be a good fermented product because of its composition and high antioxidant and antibacterial activities.
This study is the potential for further investigations focusing on producing fortified kefir with specific functional characteristics.
Author Contributions
M.A.: conceived and designed the experiment, investigation, formal analysis, visualization, and writing—original draft; S.B. and H.B.H.K.: writing, methodology, software, and formal analysis; H.B.H.K., E.S. and S.B.S.: writing—review; M.M., S.B.S. and E.S.: manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We are deeply grateful to the experimental farm and laboratory staff of ESAKef and ESIATunis for the experiment servicing.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DM | donkey milk |
| CM | cow milk |
| TS | total solids |
| TAMB | total aerobic mesophile bacteria |
| TC | total coliforms |
| FC | fecal coliforms |
| Y | yeasts |
| LAB | lactic acid bacteria |
References
- Hitch, T.C.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. J. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Aroua, M. Caractérisation Morpho-Biométrique, Génétique et du Potentiel Laitier des Ressources Asines en Tunisie. Bachelor’s Thesis, Ecole Doctorale Sciences et Techniques de L’Agronomie et de l’Environnement, Aubière, France, 2020. [Google Scholar]
- D’Alessandro, A.G.; Tesse, R.; Montagna, C.; De Leo, V.; Addante, N.; Armenio, L.; Martemucci, G.J. Production of donkey milk for human feeding: Changes of the gross composition and energetic value during lactation in Martina Franca breed. Maced. J. Anim. Sci. 2011, 1, 235–237. [Google Scholar] [CrossRef]
- Guo, H.; Pang, K.; Zhang, X.; Zhao, L.; Chen, S.; Dong, M.; Ren, F. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Vincenzetti, S. The Therapeutic, Nutritional and Cosmetic Properties of Donkey Milk; Cambridge Scholars Publishing: Newcastle-upon-Tyne, UK, 2019. [Google Scholar]
- Salimei, E.; Fantuz, F. Donkey. Encycl. Dairy Sci. 2022, 108, 65–76. [Google Scholar] [CrossRef]
- Derdak, R.; Sakoui, S.; Pop, O.L.; Muresan, C.I.; Vodnar, D.C.; Addoum, B.; Vulturar, R.; Chis, A.; Suharoschi, R.; Soukri, A. Insights on health and food applications of Equus asinus (Donkey) milk bioactive proteins and peptides—An Overview. Foods 2020, 9, 1302. [Google Scholar] [CrossRef]
- Carroccio, A.; Cavataio, F.; Montalto, G.; D’amico, D.; Alabrese, L.; Iacono, G. Intolerance to hydrolyzed cow’s milk proteins in infants: Clinical characteristics and dietary treatment. Clin. Exp. Allergy 2000, 30, 1598–1603. [Google Scholar] [CrossRef]
- Colavita, G.; Amadoro, C.; Rossi, F.; Fantuz, F.; Salimei, E. Hygienic characteristics and microbiological hazard identification in horse and donkey raw milk. Vet. Ital. 2016, 52, 21–29. [Google Scholar]
- Lara-Villoslada, F.; Olivares, M.; Xaus, J. The balance between caseins and whey proteins in cow’s milk determines its allergenicity. J. Dairy Sci. 2005, 88, 1654–1660. [Google Scholar] [CrossRef]
- Papademas, P.; Aspri, M.; Malissiova, E.; Fantuz, F.; Salimei, E. Donkey Milk. Encycl. Dairy Sci. 2022, 5, 522–529. [Google Scholar]
- Aroua, M.; Jemmali, B.; Ben Said, S.; Touati, I.; Mokhtar, M. Milk composition Comparison between donkey, goat and cow breeds. J. New Sci. 2018, 9, 202–206. [Google Scholar]
- Aroua, M.; Jemmali, B.; Said, S.B.; Kbaier, H.B.H.; Mahouachi, M.J.E.J.N. Physicochemical properties of north African donkey milk. Agric. Res. Technol. Open Access J. 2019, 57, 155–166. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F.; Coppola, R.; Chiofalo, B.; Polidori, P.; Varisco, G. Composition and characteristics of ass’s milk. Anim. Res. 2004, 53, 67–78. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Pal, Y.; Legha, R.A.; Sharma, P.; Nayan, V.; Kumar, S.; Tripathi, H.; Tripathi, B. Donkey milk composition and its therapeutic applications. Indian J. Anim. Sci. 2020, 90, 837–840. [Google Scholar] [CrossRef]
- Frias, J.; Martinez-Villaluenga, C.; Peñas, E. Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128023099. [Google Scholar]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a functional beverage gaining momentum towards its health promoting attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Hamida, R.S.; Shami, A.; Ali, M.A.; Almohawes, Z.N.; Mohammed, A.E.; Bin-Meferij, M.M. Kefir: A protective dietary supplementation against viral infection. Biomed. Pharmacother. 2021, 133, 110974. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation-A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Bensmira, M.; Jiang, B. Effect of some operating variables on a novel Kefir formulation’s microstructure and physical properties. J. Food Eng. 2012, 108, 579–584. [Google Scholar] [CrossRef]
- Biadała, A.; Adzahan, N.M. Storage Stability of Antioxidant in Milk Products Fermented with Selected Kefir Grain Microflora. Molecules 2021, 26, 3307. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kesenkaş, H.; Gürsoy, O.; Özbaş, H. Kefir. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 339–361. [Google Scholar]
- Nejati, F.; Junne, S.; Neubauer, P. A big world in small grain: A review of natural milk kefir starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological exploration of different types of kefir grains. Fermentation 2016, 3, 1. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Gambacorta, E. Phenolic content and antioxidant activity of donkey milk kefir fortified with sulla honey and rosemary essential oil during refrigerated storage. Int. J. Dairy Technol. 2019, 72, 74–81. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In Ash of Milk (Gravimetric Method); No. 945.46; Association of Official Analytical Chemists Inc.: Washington, VA, USA, 1990. [Google Scholar]
- Ghasempour, Z.; Alizadeh, M.; Bari, M.R. Optimisation of probiotic yoghurt production containing Zedo gum. Int. J. Dairy Technol. 2012, 65, 118–125. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Kourkoutas, Y.; Stefanis, C.; Vavias, S.; Voidarou, C.; Mantzourani, I.; Bezirtzoglou, E. Experimental effect of ozone upon the microbial flora of commercially produced dairy fermented products. Int. J. Food Microbiol. 2017, 246, 5–11. [Google Scholar] [CrossRef]
- Bachtarzi, N.; Amourache, L.; Dehkal, G. Qualité du lait cru destiné à la fabrication d’un fromage à pâte molle type Camembert dans une laiterie de Constantine (Est algérien) [Quality of raw milk for the manufacture of a Camembert-type soft cheese in a dairy of Constantine (eastern Algeria)]. Int. J. Innov. Sci. Res. 2015, 17, 34–42. [Google Scholar]
- Tzavaras, D.; Papadelli, M.; Ntaikou, I. From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation 2022, 8, 135. [Google Scholar] [CrossRef]
- Shahbandari, J.; Golkar, A.; Taghavi, S.M.; Amiri, A. Effect of storage period on physicochemical, textural, microbial and sensory characteristics of stirred soy yogurt. Int. J. Farming Allied Sci. 2016, 5, 476–484. [Google Scholar]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Fontán, M.C.G.; Martínez, S.; Franco, I.; Carballo, J. Microbiological and chemical changes during the manufacture of Kefir from cows’ milk, using a commercial starter culture. Int. Dairy J. 2006, 16, 762–767. [Google Scholar] [CrossRef]
- Güler-Akın, M.B.; Akın, M.S. Effects of cysteine and different incubation temperatures on the microflora, chemical composition and sensory characteristics of bio-yogurt made from goat’s milk. Food Chem. 2007, 100, 788–793. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of antioxidant capacity of cow and ewe milk kefirs. J. Dairy Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef]
- Bouacida, S.; Koubaier, H.B.H.; Snoussi, A.; Fauconnier, M.-L.; Bouzouita, N. Glucosinolate profiles by HPLC-DAD, phenolic compositions and antioxidant activity of Eruca vesicaria longirostris: Impact of plant part and origin. Mediterr. J. Chem. 2016, 5, 528–539. [Google Scholar] [CrossRef]
- Wang, R.; Han, Z.; Ji, R.; Xiao, Y.; Si, R.; Guo, F.; He, J.; Hai, L.; Ming, L.; Yi, L. Antibacterial activity of trypsin-hydrolyzed camel and cow whey and their fractions. Animals 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- ISO 22935-2:2009; Milk and Milk Products—Sensory Analysis. ISO: Geneva, Switzerland, 2009.
- Cosentino, V.; Fratter, A.; Cosentino, M. Anti-inflammatory effects exerted by Killox®, an innovative formulation of food supplement with curcumin, in urology. Eur Rev. Med. Pharmacol. Sci. 2016, 20, 1390–1398. [Google Scholar]
- Hazebrouck, S. Laits de chèvre, d’ânesse et de chamelle: Une alternative en cas d’allergie au lait de vache. Innov. Agron. 2016, 52, 73–84. [Google Scholar]
- Aroua, M.; Ben Said, S.; Bayrem, J.; Selmi, H.; Touati, I.; Mahouachi, M. Typology and influence of the asinine breeding system on milk composition. SYLWAN 2021, 165, 172. [Google Scholar]
- Bornaz, S.; Guizani, N.; Sammari, J.; Allouch, W.; Sahli, A.; Attia, H. Physicochemical properties of fermented Arabian mares’ milk. Int. Dairy J. 2010, 20, 500–505. [Google Scholar] [CrossRef]
- Yıldız-Akgül, F.; Yetişemiyen, A.; Şenel, E.; Yıldırım, Z. Microbiological, physicochemical, and sensory characteristics of kefir produced by secondary fermentation. Mljekarstvo 2018, 68, 201–213. [Google Scholar] [CrossRef]
- Ochirkhuyag, B.; Chobert, J.-M.; Dalgalarrondo, M.; Haertlé, T. Characterization of mare caseins. Identification of αS1- and αS2-caseins. Dairy J. 2000, 80, 223–235. [Google Scholar]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibanez, F. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]
- Egito, A.; Miclo, L.; Lopez, C.; Adam, A.; Girardet, J.-M.; Gaillard, J.-L. Separation and characterization of mares’ milk αs1-, β-, κ-caseins, γ-casein-like, and proteose peptone component 5-like peptides. J. Dairy Sci. 2002, 85, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Setyawardani, T.; Sumarmono, J. Chemical and microbiological characteristics of goat milk kefir during storage under different temperatures. J. Indones. Trop. Anim. Agric. 2015, 40, 183–188. [Google Scholar] [CrossRef]
- Carbonetto, B.; Nidelet, T.; Guezenec, S.; Perez, M.; Segond, D.; Sicard, D. Interactions between Kazachstania humilis yeast species and lactic acid bacteria in sourdough. Microorganisms 2020, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.d.S.F.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiotics Antimicrob. Proteins 2017, 10, 446–455. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, D.; Kim, H.; Kang, I.-B.; Chon, J.-W.; Song, K.-Y.; Seo, K.-H. Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean J. Food Sci. Anim. Resour. 2016, 36, 787. [Google Scholar] [CrossRef]
- Nazzaro, F.; Orlando, P.; Fratianni, F.; Coppola, R. Isolation of components with antimicrobial property from the donkey milk: A preliminary study. Open Food Sci. J. 2010, 4, 43–47. [Google Scholar] [CrossRef]
- Jeannot, K.; Sobel, M.L.; El Garch, F.; Poole, K.; Plésiat, P. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 2005, 187, 5341–5346. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002, 95, 22. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).