Abstract
Momordica grosvenorii saponin (MGS), as a promising dietary supplement with remarkable biological properties, has poor stability under acidic conditions and thus hinders its application in functional foods. In this study, capsules of chitosan and sodium alginate were successfully prepared to enhance the stability of MGS. The optimized parameters for preparing MGS capsules were established. Sodium alginate of 20.8 mg/mL and triplication of MGS powder were added to chitosan of 4 mg/mL and calcium chloride of 10 mg/mL at a volume ratio of 3:1, stirring at 1000 r/min for 30 min to form the capsules. In this case, the fresh particles averaged 1687 μm with an encapsulation efficiency (EE) of 80.25% MGS. The capsule tolerated acidic environments better, and in vitro MGS could be controlled to release in a stimulated gastrointestinal tract system. The antioxidant activity and delayed release of MGS could be achieved by microencapsulation of chitosan/sodium alginate. Moreover, one drink containing 19 mg/mL MGS was successfully developed for the fruit.
1. Introduction
Nowadays, with the increasing awareness of health among consumers and manufacturers, natural sweetener is taking a more important position in the beverage industry. Momordica grosvenorii saponin (MGS) is the characteristic functional component in its fresh fruits consisting of essentially a triterpene glucoside shown in Figure 1 [1,2,3]. Right now, MGS has been recognized as one of the natural sweeteners which are widely used in beverage production [4] and attracted more attention as an ingredient for producing functional beverages or pharmaceutical supplements [5]. Equal 0.01 mg/mL Momordica grosvenorii saponin (MGS) solution is reported to be more than 400 times sweeter than 5 mg/mL sucrose aqueous solution [6]. Meanwhile, MGS as a functional ingredient has been indicated to offer such health benefits as cough expectorant [7], anti-inflammatory [8], blood glucose homeostasis and regulating [9], immune enhancing [10], and anticancer [11] for human beings. Interestingly, oxidation resistance from MGS was observed to improve the nutritional value and delay the oxidative rancidity of carambola fruit juice [12]. However, several research works have demonstrated a significant decrease in antioxidant activity when its saponin is exposed to acidic conditions. It was reported that a mild acidic condition caused the side chain double bond of saponin to be hydrated so easily [13], and thus when soaked in a 10% HCl solution, MGS could be completely decomposed within one hour [14]. Studies from rat experiments indicated that MGS, after passing through the gastrointestinal tract, was completely digested after 120 min [15]. It means MGS does not work in a desirable time and targeted position after challenging the gastrointestinal stresses. Micro-encapsulation consisting of sodium alginate and chitosan often has been confirmed to be available for the protection and delayed release of various nutrients, flavors, antioxidants, and specific food additives [16,17,18]. This technique not only improves the stability of the core materials like MGS but allows the desirable compounds to be well-controlled and released in gastrointestinal fluid as well [19,20]. To our knowledge, few studies have been carried out to investigate the enhanced tolerance of MGS encapsulated by sodium alginate plus chitosan to acidic conditions, especially gastrointestinal stresses.
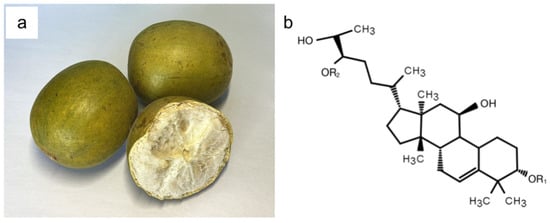
Figure 1.
The fruit of Momordica grosvenorii (a) and the chemical structure of MGS (b).
Therefore, the objectives of this work were to: (1) optimize the parameters of MGS encapsulated by sodium alginate and chitosan; (2) analyze the antioxidant capacity and content of MGS after embedded; (3) evaluate the potentials of the embedded MGS in stimulated gastrointestinal tract system as well as one MGS-based drinking.
2. Materials and Methods
2.1. Materials
Fresh Momordica grosvenorii fruits were purchased from Xiamen Zhongmin Tea Co. Ltd. (Xiamen, China) MGS (98%) was provided by Xi An Shouherb Biotech Co., Ltd. (Xi’an, China). Vanillin, sodium bicarbonate (NaHCO3), potassium dihydrogen phosphate (KH2PO4), saline sodium citrate (SSC), and sodium hydroxide (NaOH) were purchased from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China). Concentrated sulfuric acid, glacial acetic acid, hydrochloric acid (HCl), anhydrous ethanol were obtained from Beijing Chemical Works (Beijing, China). Pepsin (1:3000), trypsin (1:250), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 1,10-Phenanthroline (O-Phen), and 2, 2’-azino-bis (ABTS) were purchased from Beijing Ke’Ao Technology Co., Ltd. (Beijing, China). Chitosan (CTS) and calcium chloride (CaCl2) were acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium alginate (SA) was procured from Guangdong Guanghua Sci-Tech Co., Ltd. (Shantou, China).
2.2. Preparation of MGS Capsules
Process construction: According to Cheow’s report, 18 mL CTS and CaCl2 were mixed as the shell material. MGS and SA were mixed as the core material [16]. MGS capsules were formed by dropping the latter into the former at a rate of 1 mL/min. Then, the mixture was allowed to stir to make more capsules.
Process optimization: To obtain capsules with optimal properties, independent variables including CTS concentration (cCTS), CaCl2 concentration (cCaCl2), SA concentration (cSA), stirring time (ST), stirring speed (SS), the ratio of external to internal materials (REI), and the ratio of SA to MGS in the core material (RSM) were optimized using single factors and orthogonal experiments [21]. The detail gradients are shown in Table 1, and the values in italics in each group were fixed values for the other groups [20]. A response surface analysis was further designed to optimize the parameters of three principal factors affecting the encapsulation efficiency of MGS, i.e., SA (15 and 25 mg/mL), REI (1:2 and 1:4), and RSM (1:3 and 1:5).

Table 1.
Designation of possible factors affecting the encapsulation efficiency of MGS.
2.3. Analysis of Circular Dichroism Spectra (CD)
To explore the connection between core material and MGS, the CD spectra of sodium alginate plus MGS were detected using circular dichroism apparatus (Chirascan-plus, Applied Photophysics Ltd., Leatherhead, UK). The working parameters for CD spectra inspection were set as 190–260 nm wavelength, 0.2 s steps, and 1 cm cuvette.
2.4. Characterization of MGS Capsules
Particle size: The morphology of MGS capsules was observed and photographed by optical microscope (CX23L, Olympus Corporation, JPN). Diameters collected from 50 capsules were recorded for the analysis of the particle size via Minitab software.
Scanning electron microscopy (SEM): Scanning electron microscope (jsm-6700F, Japan Electronics Co., Ltd., Beijing, China) is used to observe the morphology of MGS capsules under magnification of 300× and 1200×. The accelerating voltage ranges from 15 to 30 kV. The samples were immobilized on copper plates with double-sided adhesive tape and sputter-coated with metal conductive film in order to reduce the charging effect and improve the image quality.
Capsules soaking in gastric and intestinal fluid: MGS capsules were mixed with 20 mL simulated gastric fluid (16.4 mL HCl and 10 g pepsin, and pH was adjusted to 1.5) and photographed at 0 h and 2 h, respectively [22]. Then the capsules were collected and soaked in simulated intestinal fluid (3.4 g KH2PO4, 5 g trypsin, and 0.1 mg/mL NaOH, and pH was adjusted to 6.8) for another 4 h, and photos showing the change of MGS capsules were taken at the end of reaction.
2.5. Evaluation of Loading Efficiency
2.5.1. Loading Efficiency (LE)
LE means the weight ratio of MGS to the whole capsules [23]. LE (%) was calculated according to Equation (1).
where m and M represent the theoretical level of MGS in each capsule and the weight of one capsule, respectively.
2.5.2. Encapsulation Rate (ER)
ER means actual/theoretical MGS content. The determination of MGS was carried out using a modified vanillin-concentrated sulfuric acid colorimetric method [24]. Briefly, 0.5 mL of vanillin ethanol solution (50 mg/mL), 4 mL of concentrated sulphuric acid (72%), and 0.5 mL MGS solution (0.00, 0.24, 0.36, 0.48, 0.6, 0.72, 0.84, and 0.96 mg/mL, respectively) were added to test tubes. The reaction was carried out in water bath at 60 °C for 15 min to reach dark green, and the curve was set up with 1.1718 as coefficient (R2 = 0.9993). The capsules were immersed in 250 mL aqueous mixture of NaHCO3 (33 mg/mL) and SSC (35 mg/mL) for 2 h to dissolve. Then the mixture was subjected to centrifuge at 7000 r/min for 8 min. The supernatant was collected to calculate the saponin content. The calculation of ER (%) was made in terms of Equation (2).
where A, V, and m represent the absorbance value, the weight of each capsule, the volume of solution after decapsulating, and the theoretical level of MGS in each capsule, respectively.
2.6. Determination of MGS Antioxidant Capacity
Three different methods were used to characterize the antioxidant capacity of MGS for a more complete and accurate evaluation. All three tests used Trolox as the positive control.
ABTS [2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic Acid Ammonium Salt] determination: The K2S2O8 and ABTS solution was diluted until the absorbance reached 0.7 ± 0.02 at 734 nm. Each 0.4 mL mixture was blended with 1 mL MGS (0, 0.5, 1, 2, 3, 4, 5 mg/mL), and the absorbance was tested.
DPPH (1,1-diphenyl-2-picrylhydrazyl) determination: The absorbance of 2 mL DPPH and 2 mL MGS (2, 5, 10, 15, 20, 25 mg/mL) was measured at 514 nm.
Phen (1,10-Phenanthroline) determination. The mixture of 1 mL 5 mmol/L O-Phen, 2 mL 0.2 mol/L PBS and 1 mL MGS (1, 2, 4, 6, 8, 10, 20, 30, 40 mg/mL) was heated to 37 °C in the water bath, and then 1 mL 7.5 mol/mL FeSO4 and 1 mL 0.1% H2O2 were added to the mixture. The absorbance was measured at 536 nm. Three of the antioxidant activities were calculated according to Equation (3).
where A0, A, and kv represent the absorbance of the control group, the absorbance of the sample, and the ratio of the diluted solution to the original solution, respectively.
2.7. Release of MGS
Twelve sets of equal-quality MGS capsules were prepared, four of which were mixed with simulated gastric fluid and stopped the reaction at 0.5, 1, 1.5, and 2 h for the determination of the total saponin content, respectively. The remaindering 8 sets were reacted in simulated gastric fluid for 2 h, and then transferred into simulated intestinal fluid and kept soaking for another 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 h for the determination of the total saponin content, respectively. An equal amount of saponin power was set as the control group.
2.8. Protective Effect of Capsules under Gastric Acid Conditions
ABTS free radical scavenging ability was used as an antioxidant activity index with reference to Miller’s method [25]. Briefly, after soaking the MGS capsules in simulated gastric fluid for 2 h, the mixture was collected at different time intervals, followed by centrifugation at 8000 r/min for 5 min to collect the supernatant (0.1 mol/mL NaOH, and pH was adjusted to 7). Then, the antioxidant activity in the supernatant was determined by calculating the absorbance value after mixing with ABTS, as did Equation (3) in 2.6.
2.9. Production of the Momordica grosvenorii Beverage
Fresh Momordica grosvenorii was cut into pieces and boiled for 20 min in water. Then the filtered solution was mixed with 0.03% citric acid, 1.5% sugar, 3% xylitol, 0.1% sodium alginate, and the capsules. The contents of MGS, total sugar, total acid, and soluble solids in the beverage were determined, and the stability of the beverage system was analyzed.
Content of MGS: High Performance Liquid Chromatography (HPLC) (Shimadzu. Ltd., JPN, SPD-M20A, Kyoto, Japan) was used for analyzing MGS content by a modified method of Nowicka’s [26]. A 10 μm sample was injected into the C18 chromatographic column (250 nm × 4.6 nm, 5 μm). The elution was carried out at 30 °C at the flow rate of 0.6 mL/min under 203 nm detection wavelength (Equation (4)).
where A, c, and As represent the sample MGS peak area, control MGS concentration, and control MGS peak area, respectively.
Determination of pH, Total Titratable Acidity (TTA): The pH was measured periodically by a digital pH meter (PHS-3c, Shanghai Xiao Sheng Instrument Manufacturing, Co., Ltd., Shanghai, China). Total titratable acid was measured by NaOH 0.1 N up to reach pH 8.2, and the milliequivalents (mEq) of citric acid were used to determine TTA (%) (Equation (5)) [27].
where C, V1, V2, and S1 represent the concentration of NaOH, volume of NaOH, volume of sample, and mEq of citric acid, respectively.
Total sugar quantification: Analysis of sugars was performed according to the method described by Kadir et al. [28]. Fehling’s reagent azeotropically boiled with the sample to produce a red precipitate of cuprous oxide. The reaction ended when the blue precipitate disappeared (Equation (6)).
where F, V1, V2, and V3 represent the mass of 5 mL of Fehling’s reagent converted to glucose, the volume of the original sample, the volume of the diluted sample, and the volume of Felling, respectively.
Soluble solids content: Soluble solids content was measured by using a handheld saccharimeter following the National Standards of the People’s Republic of China (GB12295-1990, China).
Stability of the capsules: The samples were tested using the Turbiscan (AGS, Formulation. Ltd., Toulouse, France) to analyze the stability of the capsules following Cristián’s steps [29]. The test lasted for 12 h, and scanning was performed every 5 min at a time. The standard sample was set as a control group.
2.10. Statistical Analysis
All experiments were carried out at least in triplicate, with data expressed as mean values ± standard deviation (SD). The data fitting and figures were performed on Origin 2017 software (Origin Lab Corporation, Northampton, MA, USA). ANOVA followed by Tukey’s test was used to determine the significant level between treatments (p < 0.05) using SPSS 25.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Optimization Conditions
To systematically evaluate the impacts of cCTS, cSA, cCaCl2, SS, ST RSM, and REI on the ER and LE, single-factor experiments were carried out, and the results were presented (Figure 2). As shown in Figure 2a–c, CTS, SA, and CaCl2 solutions were prepared at different concentrations. Firstly, regarding the shell material, the increase in cCTS led to a higher ER, but a lower LE and a more viscous solution happened (Figure 2a). Secondly, there was a consistent trend of cSA on ER and LE, showing a relatively high level at 20 mg/mL and a significant difference compared to the other concentrations. Regarding SA used as the core material that was proportional to MGS, an increase in cSA encapsulated the core material better within a reasonable dosing range. However, exceeding cSA of over 20 mg/mL reduced the encapsulation of the core material, as shown in Figure 2b. Thirdly, CaCl2 acted as a cross-linking agent for SA, which accelerated capsule formation, but changes in cCaCl2 did not make a significant difference in the results (Figure 2c). Hence, CTS (4 mg/mL) and CaCl2 (10 mg/mL) were adopted as the optimal conditions in the following experiment, but the best cSA level required to be analyzed using the orthogonal tests.
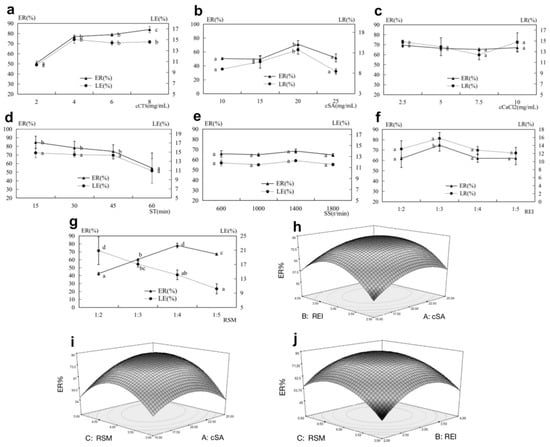
Figure 2.
Factors affecting MGS capsule preparation. Effect of (a) cCTS; (b) cSA; (c) cCaCl2; (d) ST; (e) SS; (f) REI; (g) RSM on the encapsulation rate (ER) and loading efficiency (LE); three-dimensional surface plots showing the effect of varying components (h) cSA; (i) REI; (j) RSM on ER; a,b,c,d, ab, and bc in the line chart means the difference of significance among the groups.
Thereafter, as shown in Figure 2d, ST did not significantly affect ER and LE before 60 min, and the optimal ST was set as 30 min. Figure 2e shows that the increase of the SS led to the increase of ER and LE to some degree. However, the over-fast rotation would cause the outer wall solution to become bubbly, which was not conducive to stable capsule titration. Therefore, the best SS was chosen as 1000 r/min.
Subsequently, Figure 2f shows that both ER and LE were significantly affected by REI and peaked at 1:3. Eventually, RSM was evaluated, as depicted in Figure 2g. There were significant differences between ER and LE. However, when RSM was <1:4, the ER increased dramatically, and the LE decreased. When it reached 1:5, the ER would also drop.
The above-mentioned analysis indicated that the main factors affecting the preparation of MGS capsules included cCTS, cCaCl2, SS, and ST. Thus, the optimal conditions for MGS capsule preparation were determined with ER as evaluation indices using a response surface analysis in terms of the three factors cSA, REI, and RSM. It was seen that the magnitude of the effect of the three factors on the ER was RSM (p = 0.0058 < 0.01) > cSA (p = 0.0167 < 0.05) > REI (p = 0.0245 < 0.05). The interaction between cSA (A) and RSM (C) had a significant effect on the ER. To explore the interactions among the three factors, they were plotted in pairs on a response surface diagram of Figure 2h–j. Response surface plots demonstrated that the interaction did have a pronounced effect on ER. The AC group had the steepest response surface, equal to having the greatest impact on ER, which was consistent with the results of the ANOVA (Figure 2i). The optimal conditions predicted by the response surface analysis were 20.8 mg/mL for cSA, 1:414 for RSM, and 1:3.06 for REI, with a predicting ER of 79.0062%. Moreover, the validation experiments, based on the optimal conditions of cSA (20.8 mg/mL), RSM (1:4), and REI (1:3), produced 80.25% of ER with an RSD = 1.8% < 3%. Completely, the real results were in good agreement with the predicted values.
3.2. Analysis of Circular Dichroism Spectra
To show the potential of sodium alginate in wrapping MGS, a CD analysis was performed. As shown in Figure 3, the signal of CD increased with the concentration of MGS, and the peak had a sight wavelength shift. This kind of shift would be concluded as the changes of unit G in SA, resulting in a better encapsulation effect [30].
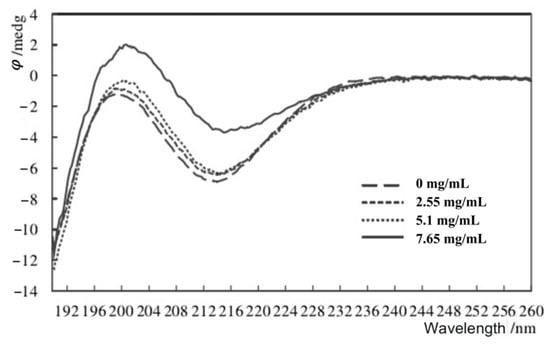
Figure 3.
CD spectra of sodium alginate with different concentrations of MGS with the wavelength as the horizontal coordinate and the ellipticity as the vertical coordinate.
3.3. MGS Capsule Morphology
Particle size. As seen in Figure 4a, the particle size of prepared MGS capsules averaged 1725 µm and mainly occupied between 1650 µm and 1750 µm. MGS capsules were evenly spherical, showing a slightly opaque milky white, smooth surface, tough and elastic texture, with many small internal bubbles (Figure 4b). Clearly, MGS capsules with small and round surfaces should be easy-to-use in drinking products [31].
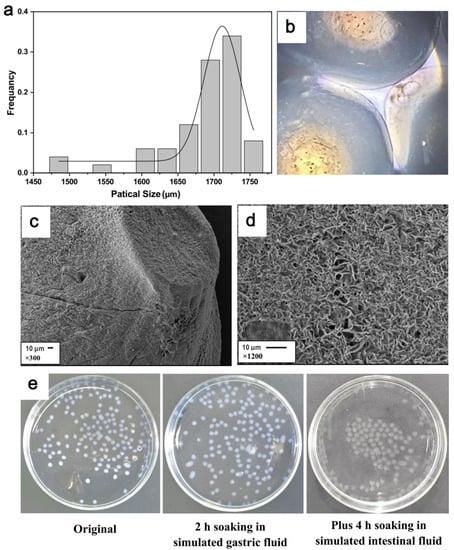
Figure 4.
Characteristics of MGS capsule appearances. (a) particle size distribution; (b) micro-graph; (c) SEM morphology under 300 magnifications; (d) SEM morphology under 1200 magnification; (e)macroscopic characteristics of MGS capsule’s changes after stimulated gastrointestinal environments.
Scanning electron microscopy (SEM). The surface of MGS capsules was nearly round and complete, with only a few tiny cracks under 300× magnification, shown in Figure 4c. After magnifying 1200 times, the surface structure was observed much more clearly. As shown in Figure 4d, there was a continuous arrangement of micro-holes, which could be considered as the channel for MGS to release slowly.
Capsules soaking in gastric and intestinal fluid. It was seen that the MGS capsules, soaked in the simulated gastric fluid after 2 h, did not produce a significant change in their appearance (Figure 4e). However, after four hours of disposing of the intestinal fluid, their transparency was further enhanced, and the MGS capsule volume increased dramatically. Hence, it could be inferred that the MGS capsules were broken more quickly in intestinal fluid than in gastric fluid environments.
3.4. MGS Capsule Antioxidant Capacity
The antioxidant activity of MGS capsules is presented in Figure 5. When 5 mg/mL MGS-containing capsules were selected, the ABTS radical scavenging reached 80.72%. Their IC50 was 1.82 mg/mL and corresponded to the Trolox yield of 26.50 μmol/g (Figure 5a,b). The MGS capsules had a DPPH radical scavenging of up to 82.89% when the concentration of MGS was 25 mg/mL. Their IC50 was 9.61 mg/mL and corresponded to a Trolox yield of 3.58 μmol/g (Figure 5c,d). O-Phen test showed that 10 mg/mL MGS-containing capsules had a maximal antioxidant capacity of 19.54% (Figure 5e,f). In our case, the encapsulated MGS showed good antioxidant activity, depending on its concentration.
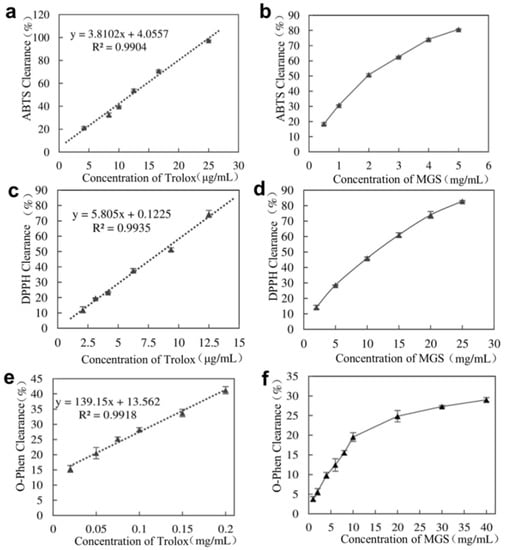
Figure 5.
Evaluation of encapsulated MGS antioxidant capacity. (a,b) ABTS determination; (c,d) DPPH determination; and (e,f) O-Phen determination.
3.5. Improvement of Antioxidant Capacity in Acidic Conditions
The comparison of the antioxidant activity of total MGS under gastric acid conditions with and without encapsulation is shown in Figure 6. The results indicated that the harsh acidic environment did lead MGS to lose its antioxidant activity, ABTS free radical scavenging effect of MGS became significantly worse. After 2 h of acidic treatment, the MGS lost 77.14% antioxidant capacity, compared with the original state. However, the encapsulated MGS retained nearly 70% of its antioxidant resistance, which could be considered successful conservation. Therefore, microencapsulation of MGS by sodium alginate and chitosan should be a promising strategy to make them work better in the human body.
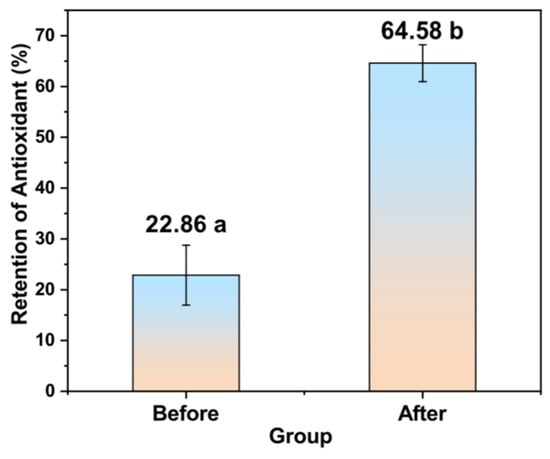
Figure 6.
The comparison of antioxidants of MGS before and after embedding in acidic conditions,the letter a and b represents a significant difference between the two groups.
3.6. Effects of Gastric and Intestinal Fluids on MGS Content
It can be seen from Figure 7 that without embedding, the content of MGS decreased linearly in gastric acid conditions, dropping below 20% at 2 h. After another 0.5 h soaking in intestinal fluid, the MGS was completely decomposed. On the contrary, the MGS capsules were prone to be stable and slow-release in an acidic environment from the inclusion complex. In the gastric fluid, the release of capsules was much slower than non-encapsulate MGS. There were still more than 60% saponins after 2 h of soaking in an acid solution. Some of the released MGS in the first 0.5 h may be attached to the surface of capsules, so it was combined loosely and more easily dissolved. In the first hour in intestinal fluid, the content of MGS dropped quickly due to the high concentrations of acid. Later, the curve flattened out gradually under dilution. Therefore, these data demonstrated the effectiveness of microencapsulation in protecting MGS from challenging gastrointestinal environments and delaying its release in a desirable position.
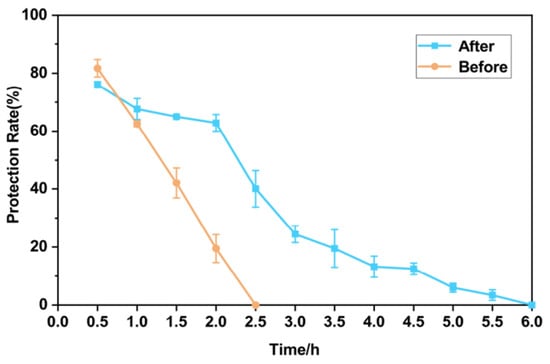
Figure 7.
The comparison of the MGS release before and after embedding in gastric and intestinal conditions.
3.7. The Application of Momordica grosvenorii Beverage
The potential of MGS capsules in drinking with soft and sweet tastes is presented in Figure 8. It was seen that this beverage contained 19 mg/mL MGS determined by the HPLC method (Figure 8a). Its content of total titratable acid was 0.28 mg/mL with a pH of 3.91. The total sugar content was 19.22 mg/mL, and the soluble solids were 5.0%. This drinking had high stability, as observed in Figure 8b,c, demonstrating the stability of the capsules. Compared with the standard sample, the light scattering dropped clearly, which indicated that the MGS embedded in the sodium alginate and chitosan kept a good dispersibility. Our current results proved that the specific beverage containing MGS capsules is worthy of promotion for health direction regarding the ability of the wrapped compound to resist adverse environments and the potential functionality.
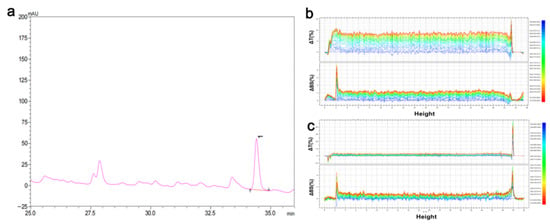
Figure 8.
The properties of MGS-based drinking. (a) MGS content determined by HPLC; the light scattering spectra of (b) standard solution and (c) MGS-based drinking sample.
4. Discussion
In the present study, CTS and SA were generally recognized as natural polysaccharides and were widely used in micro-encapsulation technologies [32,33,34]. This study showed that MGS capsules made by CTS (4 mg/mL), CaCl2 (10 mg/mL), and SA (20.8 mg/mL) exhibited a maximum ER of 80.25% and a superior LE, which was optimally developed as RES (1:4) and REI (1:3) with stirring at 1000 r/min for 30 min. Several reports have indicated that the SA and CTS capsules are effective in embedding oil and egg yolk [35,36,37]. In addition to good embedding performance, CTS (+) and SA (−) have unique advantages, such as antibacterial properties, easy decomposition, and low toxicity that can be used to form polyelectrolyte complexes particles due to their strong opposite charges [38,39]. The optimized conditions allowed the wrapped MGS to be released through permeation [38] because MGS interacting with SA was more tightly packed, as confirmed by the CD test [40]. As revealed by others, CTS and SA capsules can control the release of capsules and enhance the stability of active materials [41,42,43,44]. In this study, the MGS capsules possessed a remarkable MGS sustained-release property in gastrointestinal fluids compared with the non-encapsulated form, extending the release process from 2.5 h to 6 h. Moreover, MGS capsules could effectively protect the antioxidant ability against the acidic condition, making it three times higher than the non-encapsulated state.
Additionally, fresh Momordica grosvenorii fruits and MGS capsules were used to make one beverage with soft and sweet mouth feels. This beverage obtained 19 mg/mL MGS, which was higher than the ginseng saponins beverage [45]. Like other drinking containing sugar beet saponins, the MGS-based drinking with a pH of 3.91 had higher TTA% (0.28 mg/mL) [46]. Regarding the MGS capsules showing effectiveness in less than pH 1.5 environments, it is believed that the encapsulated MGS should work well in acidic juices, offering potential benefits to health promotion [31]. Additionally, this MGS-based juice holds high stability, one of the key factors affecting the acceptance of consumers [47]. Meanwhile, the involvement of MGS capsules not only protected the saponins from oxidants and adverse interactions with other ingredients in the drink [41] but also enabled the precise and effective release of saponins and improved the functionality of the drink greatly [48]. Therefore, the addition of encapsulated MGS should be one innovation for the development of fruit juice products. To our knowledge, this is the first report that MGS microcapsules with enhanced antioxidant activity are supplemented to the beverage products processed by the fruits.
In summary, the formation of MGS capsules effectively strengthens the antioxidant activity of MGS, overcoming the limitation of its sensitivity to acidic surroundings and becoming available for the successful manufacture of Momordica grosvenorii fruits-based juice.
Author Contributions
Conceptualization, L.L., Y.W. and B.Z. (Bolin Zhang); data curation, L.L., Y.W. and H.X.; formal analysis, L.L., H.X., B.Z. (Bo Zhang), and B.Z. (Bolin Zhang); funding acquisition, B.Z. (Bolin Zhang); investigation, L.L., Y.W. and B.Z. (Bolin Zhang); methodology, L.L., Y.W., B.Z. (Bo Zhang), and B.Z. (Bolin Zhang); project administration, B.Z. (Bolin Zhang); resources, L.L., Y.W. and H.X.; software, L.L. and B.Z. (Bo Zhang); visualization, L.L.; writing—original draft, L.L., Y.W. and H.X.; writing—review and editing, L.L. and B.Z. (Bolin Zhang). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2015ZCQ-SW-05).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, B.; Chang, L.; Park, E.; Cuendet, M.; Santarsiero, B.; Mesecar, M.; Mehta, R.; Fong, H. Bioactive constituents of the seeds of Brucea javanica. Planta Med. 2002, 68, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, W.; Gao, M.; Liu, X.; Ma, X. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr. Polym. 2013, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef]
- Kim, N.C.; Kinghorn, A.D. Highly sweet compounds of plant origin. Arch. Pharm. Res. 2002, 25, 725–746. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. J. Magn. Magn. Mater. 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Hossen, M.A.; Shinmei, Y.; Jiang, S.; Takubo, M.; Tsumura, T.; Murata, Y.; Sugiura, M.; Kamei, C. Effect of lo han kuo (Siraitia grosvenori Swingle) on nasal rubbing and scratching behavior in icr mice. Biol. Pharm. Bull. 2005, 28, 238. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Qi, X.; Chen, W.; Jia, W.; Yao, P.; Nussler, A.K.; Sun, X.; Liu, L. Effect of Momordica grosvenori on oxidative stress pathways in renal mitochondria of normal and alloxan-induced diabetic mice involvement of heme oxygenase-1. Eur. J. Nutr. 2010, 46, 61–69. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Yang, J.; Tsai, M.; Lai, C.; Hod, C.; Pan, M. Inhibitory effects of Momordica grosvenori swingle extracts on 12-o-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mouse skin. Food Funct. 2014, 5, 257–264. [Google Scholar] [CrossRef]
- Pan, M.; Yang, J.; Tsai, M.; Sang, S. Anti-inflammatory effect of Momordica grosvenori swingle extract through suppressed lps-induced upregulation of inos and cox-2 in murine macrophages. J. Funct. Foods 2009, 1, 145–152. [Google Scholar] [CrossRef]
- Takasakia, M.; Konoshimaa, T.; Muratab, Y.; Sugiurab, M.; Nishinoc, H.; Tokudac, H.; Matsumotod, K.; Kasaid, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides from Momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Dai, Z.R.; Liang, D.P.; Qin, H.Q.; Zhang, X.C.; Wang, P. Antioxidation and Stability of Compound Beverage of Momordica grosvenori and Carambola Fruit. J. Food. Res. Dev. 2019, 40, 31–35. [Google Scholar] [CrossRef]
- Han, B.; Park, M.; Han, Y.; Woo, L.; Sankawa, U.; Yahara, S. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982, 44, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.L.; Huang, Z.C.; Yan, X.J.; Chen, Y.Y.; Xu, F.; Cai, S.Q.; Li, D.P. Stability of mogroside V in artificial gastric juice and its metabolism in vitro. J. Guihaia 2015, 35, 792–795. [Google Scholar] [CrossRef]
- Murata, Y.; Ogawa, T.; Suzuki, Y.A.; Yoshikawa, S.; Inui, H.; Sugiura, M.; Nakano, Y. Digestion and Absorption of Siraitia grosvenori Triterpenoids in the Rat. Biosci. Biotech. Biochem. 2010, 74, 673–676. [Google Scholar] [CrossRef]
- Cheow, W.; Kiew, T.; Hadinoto, K. Controlled release of Lactobacillus rhamnosus biofilm probiotics fromalginate-locust bean gum microcapsules. Carbohydr. Polym. 2014, 103, 587–595. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus acidophilus in zein-alginate core shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Leong, M.; Tan, C.; Nyam, L. Effects of accelerated storage on the quality of kenaf seed oil in chitosan-coated high methoxyl pectin-alginate microcapsules. J. Food Sci. 2016, 81, 2367–2372. [Google Scholar] [CrossRef]
- Morishita, M.; Goto, T.; Peppas, N.A.; Joseph, J.I.; Torjman, M.C.; Munsick, C.; Nakamura, K.; Yamagata, T.; Takayama, K.; Lowman, A.M. Mucosal insulin delivery systems based on complexation polymer hydrogels: Effect of particle size on insulin enteral absorption. J. Control. Release 2004, 97, 115–124. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Ali, J.; Arora, S.; Ahuja, A. Formulation and development of floating capsules of celecoxib: In vitro and in vivo evaluation. AAPS PharmSciTech 2007, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Consolini, M.; Sega, M.; Zanetti, C. Erratum to: Emulsification of Simulated Gastric Fluids Protects Wheat α-Amylase Inhibitor 0.19 Epitopes from Digestion. Food Anal. Methods 2011, 4, 446. [Google Scholar] [CrossRef]
- Dwamena, A.K.; Woo, S.H.; Kim, C.S. Enzyme immobilization on porous chitosan hydrogel capsules formed by anionic surfactant gelation. Biotechnol. Lett. 2020, 42, 845–852. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; VanderGheynst, J.S. Rapid Quantitative Analysis of Lipids Using a Colorimetric Method in a Microplate Format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Evans, C.R.; Davies, M.J. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant statusin premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur. Food Res. Technol. 2019, 245, 929–938. [Google Scholar] [CrossRef]
- Dabbagh, M.A.; Garavand, F.; Razavi, S. Production of saffron-based probiotic beverage by lactic acid bacteria. J. Food. Meas. Charact. 2018, 12, 2708–2717. [Google Scholar] [CrossRef]
- Ugurtan, Y.K.; Ercisli, S.; Cam, M. Fruit Weight, Total Phenolics, Acidity and Sugar Content of Edible Wild Pear (Pyrus elaeagnifolia Pall.) Fruits. Erwerbs Obstbau 2015, 57, 179–184. [Google Scholar] [CrossRef]
- Huck-Iriart, C.; Rincón-Cardona, J.A.; Herrera, M.L. Stability of Whey Protein Concentrate/Sunflower Oil Emulsions as Affected by Sucrose and Xanthan Gum. Food Bioprocess Technol. 2014, 7, 2646–2656. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, X.; Chen, W.; Song, Y. Study on the antioxidant activity of Momordica grosvenorii extract. Food Sci. 2006, 206, 213–216. [Google Scholar] [CrossRef]
- Rama, G.R.; Führ, A.J.; da Silva, J.A.B.S. Encapsulation of Lactobacillus spp. using bovine and buffalo cheese whey and their application in orange juice. 3 Biotechnol. 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, A.; Kubiak, P.; Białas, W.; Jankowski, T. Improvement of oxidative stability of menhaden fish oil by microencapsulation within biocapsules formed of yeast cells. J. Food Eng. 2015, 167, 2–11. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Zhang, D.; Jing, T.; Li, B.; Liu, F. Porous microcapsules with tunable pore sizes provide easily controllable release and bioactivity. J. Colloid Interface Sci. 2018, 517, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Atkin, R.; Davies, P.; Hardy, J.; Vincent, B. Preparation of aqueous core polymer shell microcapsules by internal phase separation. Macromolecules 2004, 37, 7979–7985. [Google Scholar] [CrossRef]
- Wang, W.; Waterhouse, G.I.N.; Waterhouse, D.S. Co-extrusion encapsulation of canola oil with alginate: Effect of quercetin addition to oil core and pectin addition to alginate shell on oil stability. Food Res. Int. 2013, 54, 837–851. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Mcallister, T.; Stanford, K.; Xu, J.; Lu, Y.; Zhen, Y.; Sun, Y.; Xu, Y. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (igy). J. Agric. Food Chem. 2017, 55, 2911. [Google Scholar] [CrossRef]
- Waterhouse, S.D.; Zhou, J.; Miskelly, G.M.; Wibisono, R.; Wadhwa, S.S. Stability of encapsulated olive oil in the presence of caffeic acid. Food Chem. 2011, 126, 1049–1056. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Rakotozafy, L.; Mackova, B.; Delcros, J.F.; Boussard, A.; Davidou, S.; Potus, J.; Nicolas, J. Effect of adding exogenous oxidative enzymes on the activity of three endogenous oxidoreductases during mixing of wheat flour dough. Cereal Chem. 1999, 76, 213–218. [Google Scholar] [CrossRef]
- Gutiérrez, K.G.; Varaldo, H.M.P.; García, F.E.; Rendón, J.I.; Cortés, J.B. Small microcapsules of crystal proteins and spores of Bacillus thuringiensis by an emulsification/internal gelation method. Bioprocess Biosyst. Eng. 2011, 34, 701–708. [Google Scholar] [CrossRef]
- Silva, C.M.; Ribeiro, A.J.; Figueiredo, M.; Ferreira, D.; Veiga, F. Microencapsulation of hemoglobin in chitosan-coated alginate microspheres prepared by emulsification/internal gelation. AAPS J. 2006, 7, E903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Zhang, Y.; Pan, P.; Zhang, Q. Magnetic auto-fluorescent microspheres for a drug delivery system. Mater. Lett. 2014, 119, 143–145. [Google Scholar] [CrossRef]
- Gray, A.; Egan, S.; Bakalis, S.; Zhang, Z. Determination of microcapsule physicochemical, structural and mechanical properties. Particuology 2016, 132, 24. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, W.; Li, D.; Shi, K.; Li, R.; Han, Y.; Jing, E.; Ding, J.; Chen, X. Functional Polymer-Based Nerve Guide Conduits to Promote Peripheral Nerve Regeneration. Adv. Mater. Interfaces 2020, 7, 2000225. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, J.Y.; Liu, H.M.; Ci, H. The Determination of Content of Ginseng Saponins in Ginseng Beverage. Mod. Food 2018, 2, 77–80. [Google Scholar] [CrossRef]
- Motlagh, A.H.; Nasirpour, A.; Saeidy, S. Physicochemical and sensory properties of malt beverage containing sugar beet saponins. J. Food Sci. Technol. 2022, 59, 4380–4389. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Almeida, F.D.L.; de Jesus, A.L.T. Storage Stability and Acceptance of Probiotic Beverage from Cashew Apple Juice. Food Bioprocess Technol. 2013, 6, 3155–3165. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).