Abstract
Waste orange peels (WOP) are a food processing residue rich in bioactive polyphenols. However, data on aqueous extraction processes for efficient polyphenol recovery are rather limited. The present study dealt with the optimization of WOP polyphenols, using a batch stirred-tank mode and water as solvent. After a preliminary single-factor examination, the implementation of response surface methodology revealed that optimum extraction time and temperature were 60 min and 55 °C, respectively. Under these conditions, the extraction afforded a total polyphenol yield of 26.13 ± 0.78 mg gallic acid equivalents per g dry mass. The use of aqueous solutions of citric, tartaric and lactic acid showed that total polyphenol yield may be significantly increased with 1% tartaric acid or equally with 2.5% citric acid. However, the effect on individual polyphenolic constituents was rather negligible, as determined by high-performance liquid chromatography. The discrepancies observed in the antioxidant properties of the extracts produced with water and acidified water were putatively attributed to some differences in the polyphenolic composition. The extraction methodology proposed is environmentally green and of low cost, and it could certainly be used as an effective means of recovering WOP polyphenols.
1. Introduction
The agri-food sector is central to the world economy, embracing cultivation and processing activities that pertain to plant and animal exploitation, and the production of associated food commodities. Nowadays, the unprecedented, intensified production of crops and animals to cover the needs of the world’s population has led to a severe misuse and depletion of natural resources, and environmental aggravation. On the recognition that the linear economy model has now overlived its initial objectives, several countries around the globe are increasingly adopting circular bioeconomy models, in order to establish sustainable routes of development [1]. In such a framework, the valorization of food waste through various biorefinery concepts has become an indispensable element in soliciting eco-friendly strategies for the recovery and production of high value-added chemicals [2].
Globally, citrus production amounted 158 million tons in 2019, including oranges, lemon, grapefruit, lime, tangerines, etc. [3]. Oranges are the largest citrus crop in all citrus-producing countries, accounting for almost 50% of the global citrus production, and they are destined mainly for juice manufacturing. The juice represents approximately 50% of the fruit weight, whereas the pulp, peels, and seeds, accounting for the remaining 50%, are considered as waste. It has been estimated that about 8–20 million tons of orange waste are produced each year during orange juice production, of which 60–65% (w/w) is composed of orange peels [4]. Due to their particularly high organic load, which may pose environmental risks, orange peels should receive appropriate treatment prior to disposing to landfills. However, orange peels are a unique plant material containing an array of precious phytochemicals, including essential oil, pectins, carotenoids, and polyphenols [5,6].
Polyphenols are major bioactives in orange peels, and the polyphenolic profile is dominated by flavanones [7], which are usually accompanied by lower amounts of flavones and phenylpropanoids [8]. The versatile bioactivity exhibited by orange peel polyphenols has long been recognized [9], and may include chemopreventive, anti-inflammatory, antioxidant and antimicrobial effects [10]. It is, therefore, not surprising that a multitude of methodologies have been developed to produce orange peel extracts enriched in polyphenolic substances [8]. However, several methodologies are dealing either with conventional petroleum-based solvent extraction [8], or integration of methods requiring expensive equipment and laborious processes [4]. In opposition, there has been limited evidence on the bioactivities of orange peel extracts, generated with simple stirred-tank, aqueous extraction [11]. In compliance with the Green Chemistry principles, the usage of green and recyclable solvents, such as water, should be a key priority in establishing eco-friendly extraction procedures [12]. Based on these grounds, the investigation presented herein is aimed at performing the optimization of an extraction methodology for polyphenol recovery from waste orange peels (WOP), using water as the sole solvent. Furthermore, the role of the addition of food-grade common organic acids was also considered, to examine whether such additives could affect polyphenol extraction yield, as well as extract composition and antioxidant properties. To the best of the authors’ knowledge, such an approach of aqueous polyphenol extraction from WOP has never been previously reported.
2. Materials and Methods
2.1. Chemicals
Sodium carbonate anhydrous and tartaric acid were from Penta (Prague, Czech Republic). Furthermore, 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was from Alfa Aesar (Karlsruhe, Germany). Citric acid anhydrous and iron(III) chloride hexahydrate (FeCl3) were from Merck (Darmstadt, Germany). L-Ascorbic acid was purchased from Carlo Erba (Milano, Italy). Absolute ethanol, Folin-Ciocalteu reagent and gallic acid monohydrate were from Panreac (Barcelona, Spain). 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) was from Fluka (Steinheim, Germany). Luteolin 7-O-rutinoside, ferulic acid, narirutin, neochlorogenic acid, chlorogenic acid, caffeic acid and hesperidin were from Sigma-Aldrich (Steinheim, Germany). All solvents used for chromatography were HPLC grade.
2.2. Waste Orange Peels
The orange residues consisted of orange peels and orange peels bearing parts of flesh, and were collected within two days from several catering premises around the town of Larissa (Central Greece). The collected material was transported to the laboratory facility and screened to remove the flesh-bearing parts and those contaminated with foreign materials. The remaining matter, composed of only waste orange peels (WOP) including the flavedo and albedo tissues, was dried in an oven (Binder BD56, Bohemia, NY, USA) at 80 °C for 24 h, and ground in a table domestic mill. The resulting powder was sieved and only material with mean particle diameter of <300 μm was selected, and stored at −40 °C in sealed, glass containers, until used.
2.3. Aqueous Polyphenol Extraction
A 25-mL Duran glass vial was used to carry out all the extractions. The exact volume of 20 mL of deionized water was placed in the vial, which was immersed in oil bath, set at the desired temperature. After the water acquired the temperature set (usually 5 min), accurately weighted WOP powder was added, the vial was screw-capped, and the extraction was performed under stirring (500 rpm), for predetermined resident time. Both heating and stirring were provided by a hotplate (Witeg, Wertheim, Germany). After this time, the extract was cooled and centrifuged at 10,000× g for 10 min, in a REMI NEYA 16R (REMI ELEKTROTECHNIK LTD, Palghar, India) centrifuge.
2.4. Response Surface Optimization
The scope of the optimization was to examine the effect of the two most influential extraction variables, extraction time (t) and temperature (T), on the effectiveness of aqueous extraction of polyphenols from WOP. The ranges of both variables (t, T) were selected on the ground of preliminary experiments. To accomplish this, a response surface methodology was deployed, using Box–Behnken experimental design with three central points, to enable the establishment of a mathematical model with high reliability. The total polyphenol yield (YTP) was used as the screening response. The codification of the independent variables (t, T) between-1 (lower limit) and 1 (upper limit) (Table 1), as well the determination of all statistical parameters associated with the model, were carried out as described in detail elsewhere [13].

Table 1.
Codification and actual values of the independent variables used to set up the experimental design.
2.5. Determination of Total Polyphenols, Total Flavonoids, and Antioxidant Activity
The Folin-Ciocalteu assay was used to determine total polyphenols [14]. The calibration curve was constructed with gallic acid and the results were expressed as mg gallic acid equivalents (GAE) per g dry mass (DM). The chromophore radical probe DPPH was used to assess the antiradical activity (AAR) of the extracts. The results were given as μmol DPPH per g DM, using a previously described protocol [15]. Likewise, the ferric-reducing power (PR) was estimated with TPTZ as the probe and results were reported as μmol ascorbic acid equivalents (AAE) per g DM [15].
2.6. Chromatographic Determinations
The tentative identification of certain polyphenolic metabolites in WOP was accomplished with liquid-chromatography-mass spectrometry. The chromatography settings regarding the elution program and mass spectra acquisition have been given in detail in an earlier study [16]. Quantitative analyses were performed using the equipment and methodology previously reported [17]. The calibration curves used for quantitation were caffeic acid (R2 = 0.9980), ferulic acid (R2 = 0.9980), neochlorogenic acid (R2 = 0.9980), chlorogenic acid (R2 = 0.9990), narirutin (R2 = 0.9999), hesperidin (R2 = 0.9999) and luteolin 7-O-glucoside (R2 = 0.9980).
2.7. Statistical Processing
SigmaPlot™ 12.5 (Systat Software Inc., San Jose, CA, USA) was used for all linear regressions. JMP™ Pro 13 software (SAS, Cary, NC, USA) was used to carry out the experimental design, response surface-associated statistics (analysis of variance, lack-of-fit), and distribution analysis. All extractions were performed at least twice, and the analytical determinations were carried out in triplicate. The values given represent average ± standard deviation.
3. Results and Discussion
3.1. Single Factor Testing
In order to obtain a picture regarding the effect of the two key variables in solid-liquid extraction, time, t, and temperature, T, single-factor assays were undertaken. This was deemed critical, considering that t and T may define both the efficiency [18] and the severity [19] of the process. Increasing the temperature from 50 to 90 °C was shown to provoke non-significant effect on YTP (Figure 1). This finding indicated that WOP polyphenols readily soluble in water may be solubilized at temperatures as low as 50 °C, and that further temperature raising would not provide an increased total polyphenol yield.
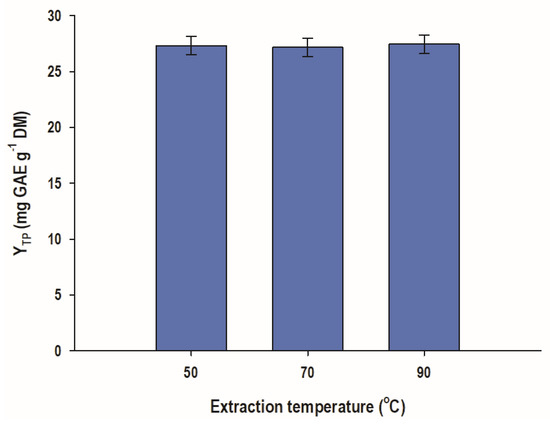
Figure 1.
WOP aqueous extraction to test temperature effects on total polyphenol yield. The extractions were carried out with a liquid-to-solid ratio of 20 mL g−1, for 1 h, under stirring at 500 rpm.
On the contrary, when the extractions were performed at different time regimes, it was recorded that prolonging extraction period over than 1 h provoked a gradual decline in YTP (Figure 2). After 5 h of extraction, a reduction by 12.1% in YTP was noticed. This outcome strongly suggested that extraction periods longer than 1 h would not favor increased polyphenol recovery.
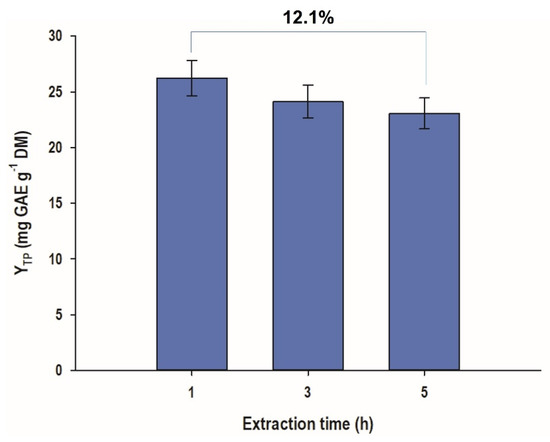
Figure 2.
WOP aqueous extraction to test time effects on total polyphenol yield. The extractions were carried out with a liquid-to-solid ratio of 20 mL g−1, at 50 °C, under stirring at 500 rpm.
3.2. Extraction Process Optimization
On the grounds of the evidence that emerged from the single-factor assays, the ranges of both variables t and T were delimited, and a design of experiment was implemented, using response surface methodology. Using this approach, the effect of the process variables may be individually appraised, but possible synergistic functions between them may also be detected. The analysis of variance (ANOVA) along with lack-of-fit test enabled a credible assessment of the fitted model (Figure 3), taking into consideration the proximity of the predicted and measured values (Table 2).
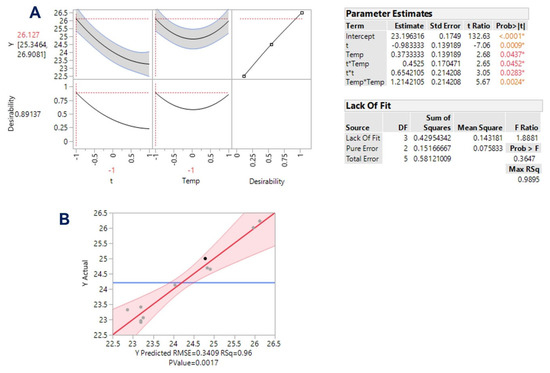
Figure 3.
Statistical analyses pertaining to the response surface optimization of WOP aqueous polyphenol extraction. (A), desirability function; (B), plot showing actual vs. predicted YTP values. The values marked with asterisks in the inset table “Parameter estimates” are statistically significant. The values in different color denote different level of significance. Pink area in B plot indicates the confidence interval of the model.

Table 2.
Measured and predicted values of the response (YTP) corresponding to the design points used for the response surface methodology.
The second-degree polynomial equation (mathematical model) derived was as follows:
YTP = 23.19 − 0.98X1 + 0.37X2 + 0.45X1X2 + 0.65X12 + 1.21X22 (R2 = 0.96, p = 0.0017)
Both R2 and p (confidence interval of 95%) of the model concurred that Equation (1) showed an excellent fit regarding the experimental data. Therefore, it could be supported that the model derived could be used for reliable predictions, concerning the effect of t and T on YTP. The three-dimensional diagram constructed based on the model depicted at-a-glance how the experimental variables affected YTP (Figure 4).
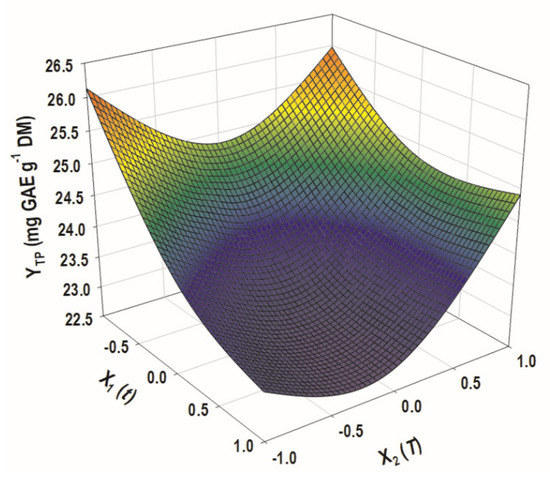
Figure 4.
The three-dimensional diagram illustrating the effect of process (independent) variables on the response (YTP).
Based on the prediction of the desirability function, the optimum values for t and T were 60 min and 55 °C, respectively. This fact clearly showed that prolonged resident time would not favor increased yield in total polyphenols. On the other hand, increases in temperature would not offer a statistically higher effect. Regarding extraction time, this outcome was in absolute accordance with a recent thorough study on several different plants, which indicated that, on average, aqueous extraction required 60 min for maximum performance [18]. Likewise, specifically for aqueous polyphenol extraction from orange peels aided by cyclodextrin addition, it was demonstrated that temperatures higher than 40 °C did not contribute to attaining higher extraction yield [17]. Thus, it could be argued that aqueous polyphenol extraction from WOP would be rather favored at relatively low temperatures, as opposed to extraction from other plants, which usually require an optimum higher than 75 °C [18]. This point is supported by recent examinations, which indicated that polyphenols in orange peel extracts may significantly decrease at temperatures higher than 50 °C [20]. Other studies proposed temperatures as low as 25 °C for the effective ultrasound-assisted aqueous extraction of polyphenols from WOP [21], yet optimum temperatures as high as 70 °C have also been proposed [22].
Under the optimized conditions (t = 60 min, T = 55 °C), the maximum predicted response was 26.13 ± 0.78 mg GAE g−1 DM. The validity confirmation of this model prediction was tested by performing three individual extractions under optimized conditions. The YTP determined was 26.73 ± 3.50 mg GAE g−1 DM, which clearly pointed to a high model credibility. The optimum YTP value was identical to that reported recently (26.30 ± 1.49 mg GAE g−1 DM) for aqueous polyphenol extraction from WOP with the use of hydroxypropyl β-cyclodextrin [17]. Furthermore, identical values of 26.88 mg GAE g−1 [23] and 23.63 mg GAE g−1 DM [24], both achieved with microwave-assisted extraction, have been reported.
3.3. Effect of Organic Acid Addition
To examine the effect of organic acids on the extraction performance, three organic acids, namely citric, tartaric and lactic, commonly encountered in foods, were selected. These acids were incorporated into the aqueous medium at concentrations varying from 1 to 5% [25], and extractions were carried out under optimized conditions, as reported in paragraph 3.2.
It can be seen in Figure 5 that 1 and 2.5% citric acid, as well as 1% tartaric acid were significantly more effective than water, in attaining higher YTP (p < 0.05). To the contrary, 5% citric acid had a significantly lower performance (p < 0.05), while the addition of lactic acid had no statistically significant influence. This finding indicated that the effect that the organic acids exerted depended on both the type of acid and its concentration. This assumption was in line with previous examinations, which suggested that aqueous solutions of acetic acid and citric acid may have significantly different efficacy for flavanol extraction from red grape pomace [26]. In addition, a particular role in this regard was attributed to acid concentration. Following thorough studies employing response surface methodology demonstrated that aqueous lactic acid solutions may be more effective means of flavonoid extraction from red grape pomace compared to tartaric, acetic, and citric acids [25]. A similar outcome for the role of lactic acid was also reported for the pressurized-liquid extraction of polyphenols from saffron floral residues [27]. The effect the acids exerted may be ascribed to the decomposition of orange peel tissues, which in turn could facilitate polyphenol liberation and increased entrainment into the liquid phase. In support of this argument are data on orange peel treatment with dilute organic acids, where it was demonstrated that flavedo and albedo disintegration led to increased recovery of flavonoids [28].
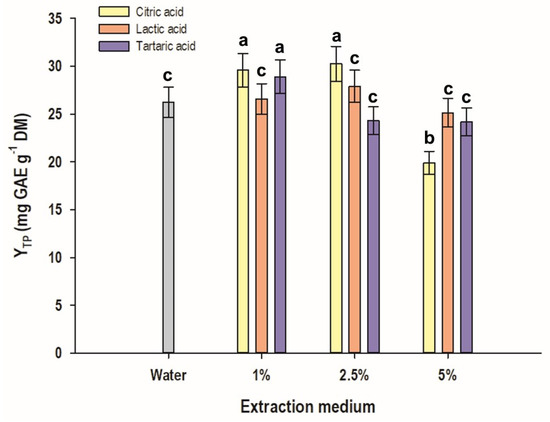
Figure 5.
The bar plot presenting the effect of various acids and their concentration on YTP. The extractions were performed at 55 °C, for 60 min, under stirring at 500 rpm. The bar denoted with different letters represent statistically different values (p < 0.05).
3.4. Polyphenolic Composition of the Extracts
To shed more light onto the polyphenolic composition of the extract produced under optimized conditions, and to spot differences arising by the use of organic acids, the aqueous extract and the extracts generated with the highest-performing systems (1% tartaric acid and 2.5% citric acid) were analyzed by high-performance liquid chromatography. A typical chromatogram may be seen in Figure 6.
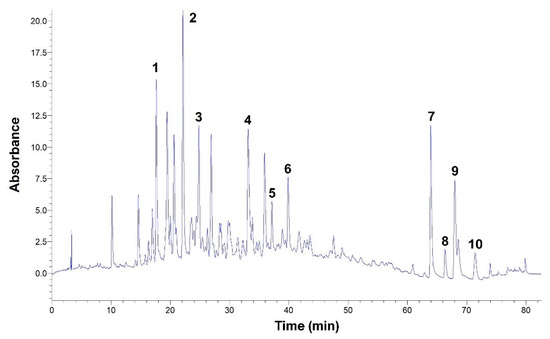
Figure 6.
A typical chromatogram of a WOP aqueous extract, obtained under optimized conditions (55 °C, 60 min). The eluent was monitored at 320 nm. Peak assignment: 1, neochlorogenic acid; 2, chlorogenic acid; 3, caffeic acid; 4, ferulic acid; 5, narirutin; 6, hesperidin, 7, didymin; 8, sinensetin; 9, nobiletin; 10, dimethylnobiletin.
Compounds 1–6, corresponding to neochlorogenic, chlorogenic, caffeic, and ferulic acids, and to narirutin and hesperidin, were tentatively identified by comparing their retention times and UV-vis spectra with those of original standards. Compounds 7–10 corresponding to didymin, sinensetin, nobiletin and dimethylnobiletin were tentatively identified by employing liquid chromatography-mass spectrometry, as recently reported [19]. The quantitative analysis revealed that 2.5% citric acid was unfavorable compared to water, for the recovery of both neochlorogenic and chlorogenic acids (Table 3). For all other compounds considered, the differences among the three extraction media were marginal and statistically non-significant (p > 0.05). Overall, the aqueous extraction afforded 1933.70 μg g−1 DM of total polyphenols, which was higher, yet not statistically significant, from the yields achieved with either 1% tartaric acid or 2.5% citric acid. Such a result suggested that acidification with either acid offered no advantage regarding the recovery of specific polyphenolic substances.

Table 3.
The quantitative data on the polyphenolic composition of WOP extracts. TA and CA denote tartaric and citric acid, respectively. The values given represent means of triplicate determination ± standard deviation.
3.5. Antioxidant Characteristics
The three extracts analyzed for their polyphenolic composition were further evaluated for their antioxidant properties. With reference to the antiradical activity (AAR), the aqueous extract exhibited 7.7 and 15.8% higher performance rates, compared to the extracts obtained with 1% tartaric acid and 2.5% citric acid, respectively (Figure 7A). On the other hand, based on the data for the ferric-reducing power (PR), the aqueous extract displayed by far the weakest activity, being over 60% lower than those of the extracts produced either with 1% tartaric or 2.5% citric acid (Figure 7B).
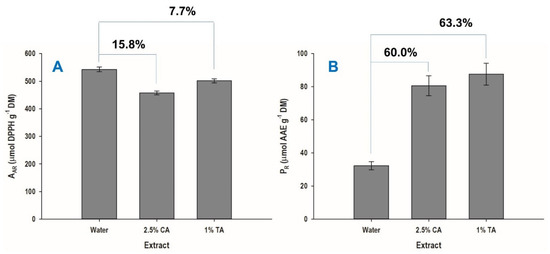
Figure 7.
Antiradical activity (AAR) (A) and ferric-reducing power (PR) (B) of WOP extracts. All extracts were produced at 55 °C, for 60 min. TA and CA denote tartaric and citric acid, respectively.
Such an outcome is rather inconclusive regarding the antioxidant behavior of the extracts. Recent investigations on the antioxidant characteristics of orange peel extracts supported that polar polyphenols were of lower abundance, but they showed stronger antioxidant effects compared to the more abundant and less polar constituents extracted in ethanol [29]. These authors also concluded that the polyphenols occurring in orange peel extracts may preferably act through a single-electron transfer mechanism, rather than hydrogen atom transfer one. Thus, considering that the aqueous extract contained significantly higher concentration of the polar neochlorogenic and chlorogenic acids, it could be assumed that these compounds played an important role in the DPPH scavenging, which is a single-electron transfer mechanism [30]. On the other hand, antioxidants that may perform well in DPPH assays react relatively slowly with Fe3+, and therefore may give diversified results in a ferric-reducing assay. On this ground, the discrepancies observed might reflect differences in both the mechanisms and the reaction kinetics of the various WOP constituents.
4. Conclusions
The present study dealt with the optimization of the aqueous polyphenol extraction from WOP. Using water as solvent and a batch stirred-tank extraction mode, a significant yield in total polyphenols was achieved, under benign temperature and a relatively short extraction period. The acidification of water with 1% tartaric acid and 2.5% citric acid was shown to offer advantage over pure water, regarding attaining a higher total polyphenol yield. On the other hand, the polyphenolic composition was not impacted to a significant extent, and only a few differences were observed for neochlorogenic and chlorogenic acid. It was assumed that these differences might be responsible for the discrepancies observed in the antioxidant characteristics of the extracts. The method developed is a fast, environmentally green and low-cost means of extracting polyphenols from WOP, and it could certainly be used as an alternative to more sophisticated, time-consuming and expensive ones.
Author Contributions
Conceptualization, D.P.M.; data curation, D.K., D.P. and V.A.; formal analysis, D.K., D.P. and V.A.; investigation, D.K., D.P. and V.A.; methodology, D.K., D.P. and V.A.; supervision, D.P.M.; writing—original draft, D.P.M. and S.I.L.; writing—review and editing, D.P.M. and S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
The project received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.; Lourenço-Lopes, C.; Prieto, M.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O. Citrus peels Odyssey: From the waste bin to the lab bench to the dining table. Appl. Food Res. 2022, 2, 100083. [Google Scholar] [CrossRef]
- Mohsin, A.; Hussain, M.H.; Zaman, W.Q.; Mohsin, M.Z.; Zhang, J.; Liu, Z.; Tian, X.; Salim-ur-Rehman; Khan, I.M.; Niazi, S. Advances in sustainable approaches utilizing orange peel waste to produce highly value-added bioproducts. Crit. Rev. Biotech. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Khan, M.K.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Inter. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- González-Gómez, D.; Cardoso, V.; Bohoyo, D.; Ayuso, M.; Delgado-Adamez, J. Application of experimental design and response surface methodology to optimize the procedure to obtain a bactericide and highly antioxidant aqueous extract from orange peels. Food Control 2014, 35, 252–259. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Mourtzinos, I.; Makris, D.P. Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Inter. J. Ind. Chem. 2015, 6, 85–92. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A.N.; Papageorgiou, V.P. Analysis of antioxidant compounds in sweet orange peel by HPLC–diode array detection–electrospray ionization mass spectrometry. Biomed. Chrom. 2005, 19, 138–148. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-cyclodextrin as a green co-solvent in the aqueous extraction of polyphenols from waste orange peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Arom. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process optimization and stability of waste orange peel polyphenols in extracts obtained with organosolv thermal treatment using glycerol-based solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Y.; Liu, D.; Sheng, Z.; Liu, J.; Chen, H.; Feng, W. The kinetic behavior of antioxidant activity and the stability of aqueous and organic polyphenol extracts from navel orange peel. Food Sci. Technol. 2022, 42, e90621. [Google Scholar] [CrossRef]
- Dalmau, E.; Rosselló, C.; Eim, V.; Ratti, C.; Simal, S. Ultrasound-assisted aqueous extraction of biocompounds from orange byproduct: Experimental kinetics and modeling. Antioxidants 2020, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Recovery of added-value compounds from orange and spinach processing residues: Green extraction of phenolic compounds and evaluation of antioxidant activity. Antioxidants 2021, 10, 1800. [Google Scholar] [CrossRef] [PubMed]
- M’hiri, N.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Proc. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- M’hiri, N.; Irina, I.; Cédric, P.; Ghoul, M.; Boudhrioua, N. Antioxidants of Maltease orange peel: Comparative investigation of the efficiency of four extraction methods. J. Appl. Pharmaceut. Sci. 2017, 7, 126–135. [Google Scholar]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. A comparative evaluation of aqueous natural organic acid media for the efficient recovery of flavonoids from red grape (Vitis vinifera) pomace. Waste Biomass Valorization 2015, 6, 391–400. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Kinetic modelling for flavanol extraction from red grape (Vitis vinifera L.) pomace using aqueous organic acid solutions. Inter. Food Res. J. 2014, 21, 1919–1924. [Google Scholar]
- Pappas, V.M.; Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Pressurized liquid extraction of polyphenols and anthocyanins from saffron processing waste with aqueous organic acid solutions: Comparison with stirred-tank and ultrasound-assisted techniques. Sustainability 2021, 13, 12578. [Google Scholar] [CrossRef]
- Van den Bruinhorst, A.; Kouris, P.; Timmer, J.; de Croon, M.; Kroon, M. Exploring orange peel treatment with deep eutectic solvents and diluted organic acids. Nat. Prod. Chem. Res. 2016, 4, 1–5. [Google Scholar]
- Moreno, M.T.; Rodríguez Mellado, J.M. Spectrophotometric and electrochemical assessment of the antioxidant capacity of aqueous and ethanolic extracts of citrus flavedos. Oxygen 2022, 2, 99–108. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).