Figs (Ficus carica L.) Used as Raw Material for Obtaining Alcoholic Fermented Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Fig as the Raw Material for the Preparation of an Alcoholic Fermented Beverage
2.2. Fig Processing for the Production of the Fermented Alcoholic Beverage
2.3. Analyses
2.3.1. Determination of Chemical Characteristics of Fig Fruits
2.3.2. Determination of the Chemical Characteristics of the Fermented Alcoholic Beverages from Figs
2.3.3. Sensory Analyses of Fermented Alcoholic Beverages from Figs
2.3.4. Statistical Analyses
3. Results
3.1. Chemical Parameters of the Figs Used to Produce Fermented Alcoholic Beverages
3.2. Fermentation Evolution and Parameters of the Alcoholic Beverages Resulting from Figs
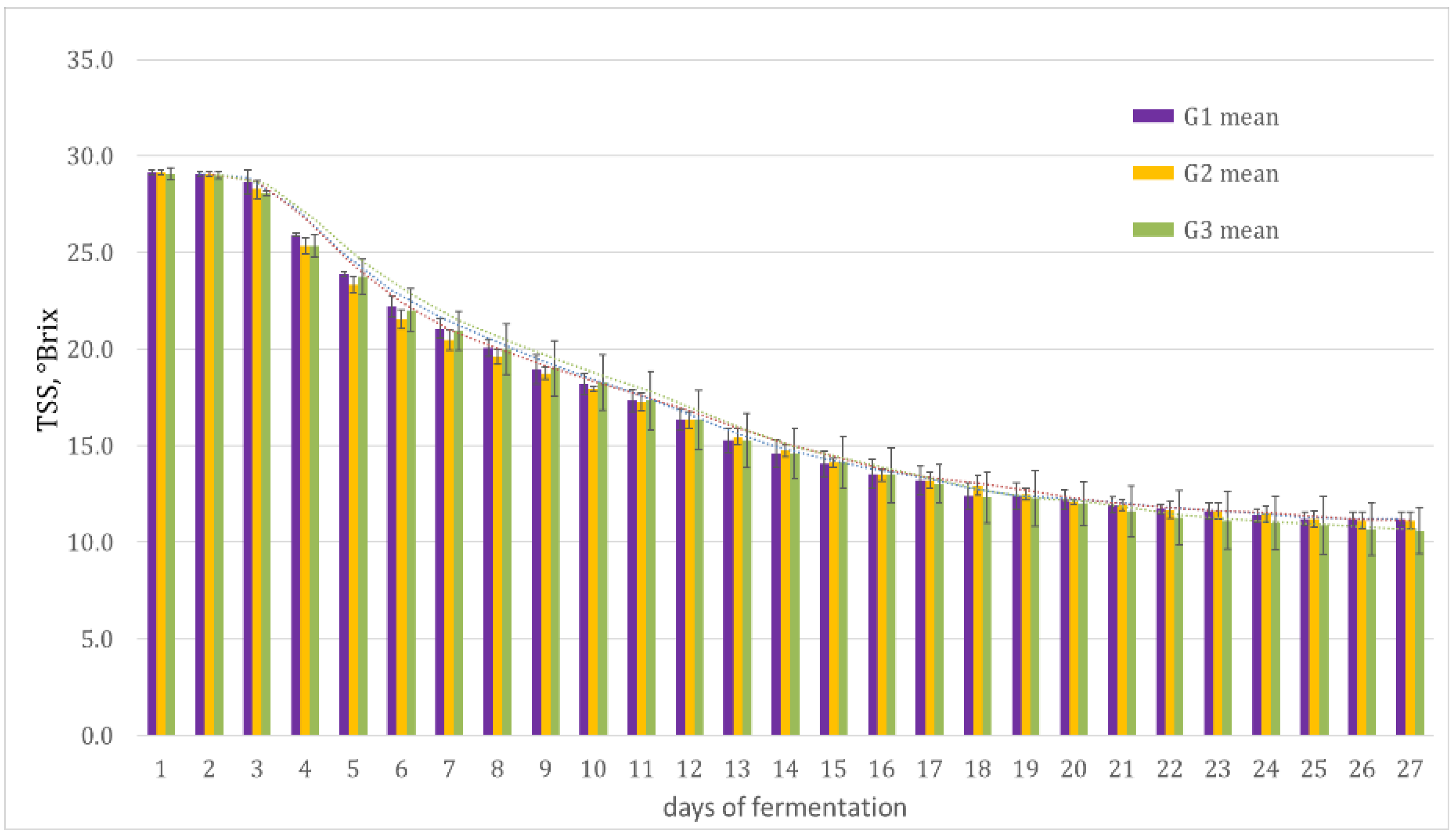
3.2.1. Evolution of the Sugar Content during the Fermentation to Produce Alcoholic Beverages from Figs
3.2.2. Chemical Parameters of the Alcoholic Beverages Resulting from Figs
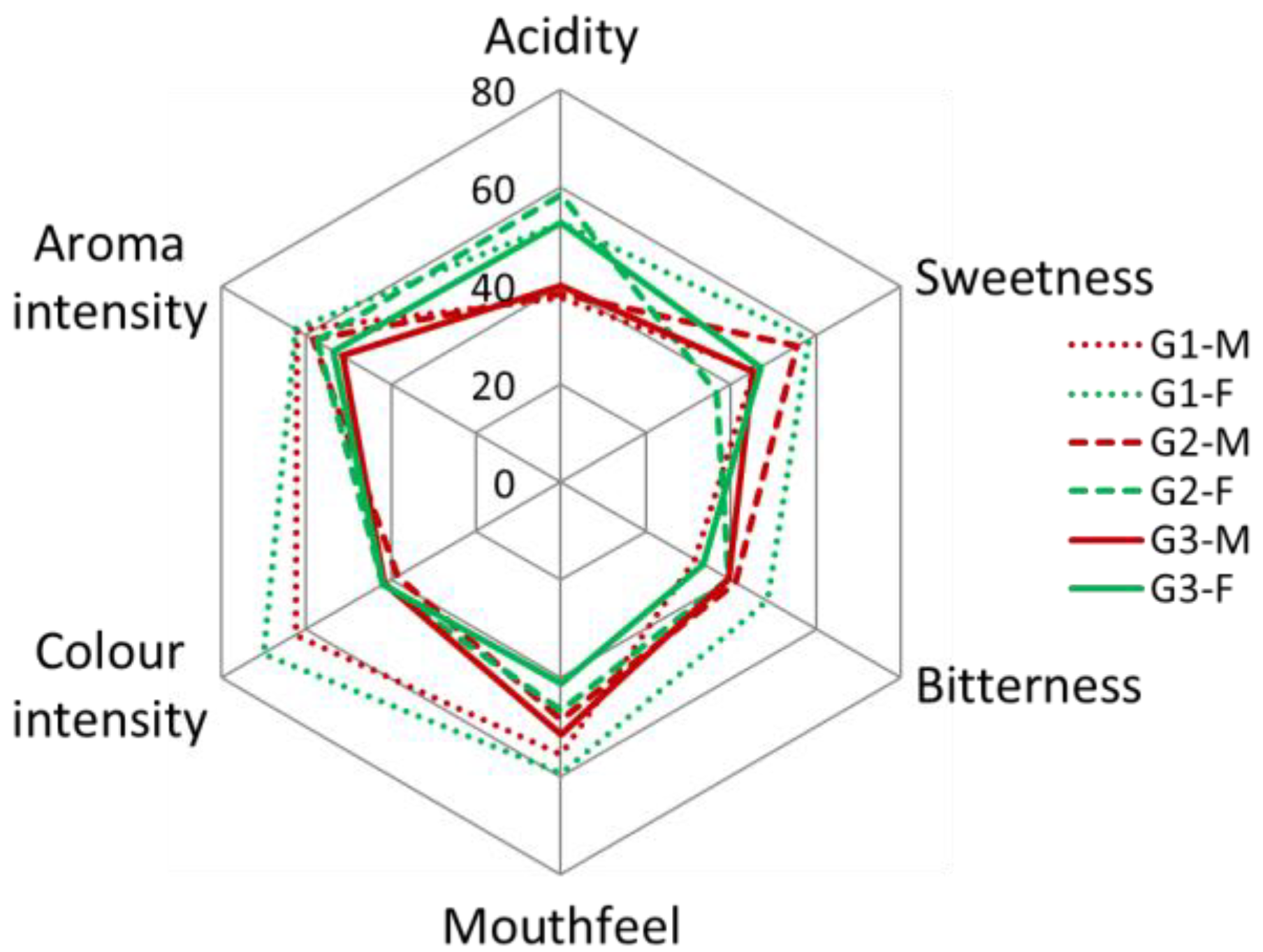
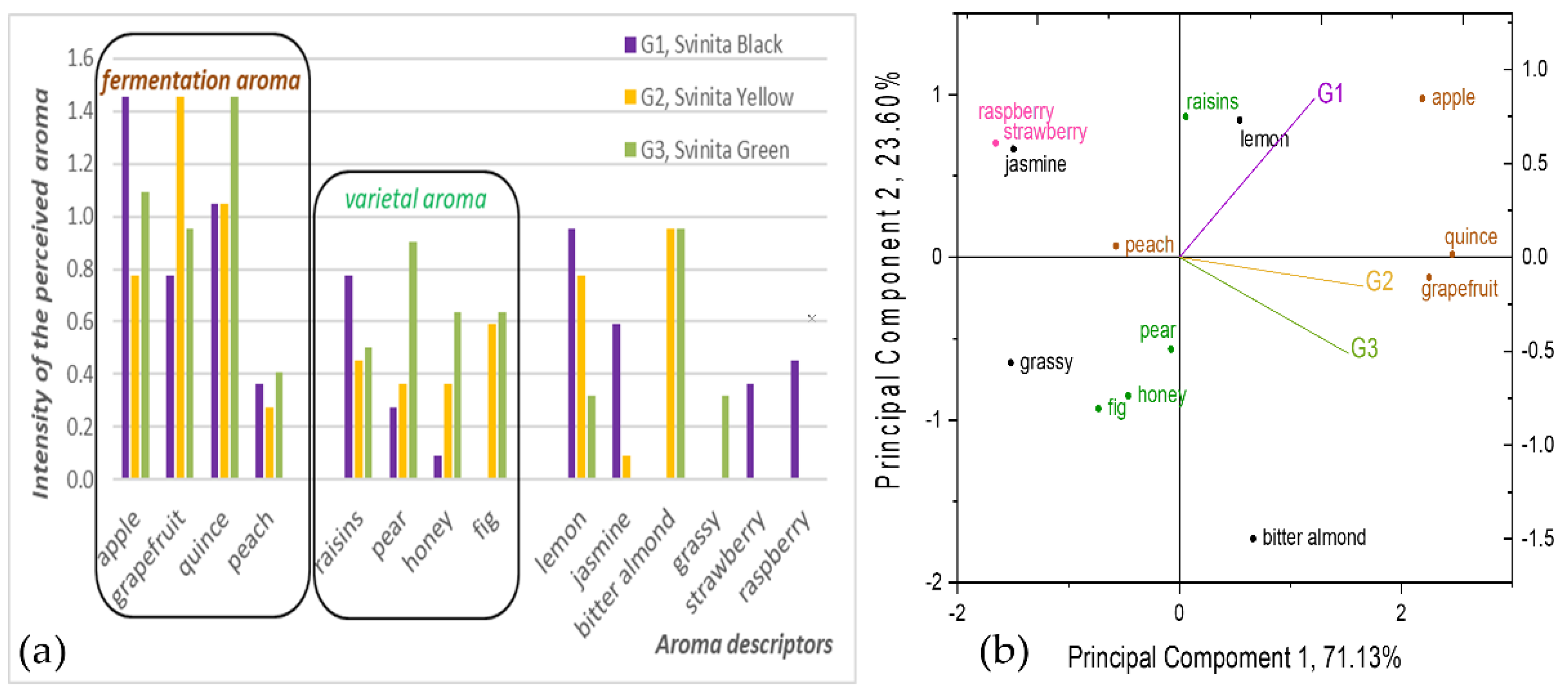
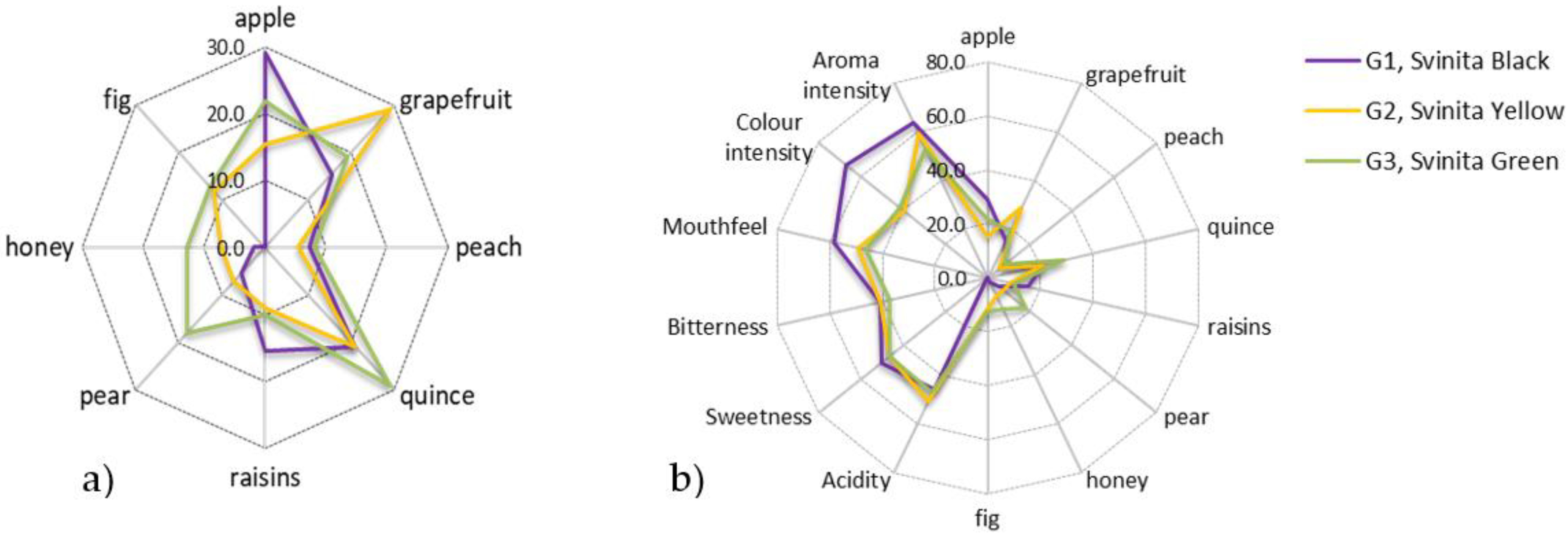
3.2.3. Sensory Features of Fermented Alcoholic Beverages from Figs
4. Discussion
4.1. Chemical Parameters of the Figs Used to Produce Fermented Alcoholic Beverages
4.2. Fermentation Evolution and Parameters of the Alcoholic Beverages Resulting from Figs
4.2.1. Evolution of the Sugar Content during the Fermentation to Produce Alcoholic Beverages from Figs
4.2.2. Chemical Parameters of the Alcoholic Beverages Resulting from Figs
4.2.3. Sensory Features of Fermented Alcoholic Beverages from Figs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veberic, R.; Mikulic-Petkovsek, M. Chapter 11—Phytochemical Composition of Common Fig (Ficus carica L.) Cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 235–255. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Cat, A.; Catal, M.; Erkan, M. First report of Alternaria alternata causing postharvest decay in fig (Ficus carica cv. Bursa Siyahi) fruit in Turkey. J. Biotechnol. 2018, 280, S84. [Google Scholar] [CrossRef]
- Martínez-Damián, M.T.; Cruz-Arvizu, O.; Cruz-Alvarez, O. Effect of modified atmosphere packaging on nutraceutical quality and overall appearance of figs stored at 1 °C. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2292–2305. [Google Scholar] [CrossRef]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Milan, Italy, 2009; p. 4. [Google Scholar]
- Sengun, I.Y. Microbiological and chemical properties of fig vinegar produced in Turkey. Afr. J. Microbiol. Res. 2013, 7, 2332–2338. [Google Scholar] [CrossRef]
- Khezri, S.; Dehgahan, P.; Mahmoudi, R.; Jafarlou, M. Fig Juice Fermented with Lactic Acid Bacteria as a Nutraceutical Product. Pharcaceutical Sci. 2016, 22, 260–266. [Google Scholar] [CrossRef]
- Wijayanty, E.D.; Setiawan, N.C.E.; Cristi, J.P. Effect of Lactic Acid Fermentation on Total Phenolic Content and Antioxidant Activity of Fig Fruit Juice (Ficus carica). Health Science International Conference. (HSIC 2017). Adv. Health Sci. Res. 2017, 2, 282–289. [Google Scholar] [CrossRef][Green Version]
- Jeong, M.R.; Cha, J.D.; Yun, S.I.; Han, J.H.; Lee, Y.E. Manufacturing of Wine with Korean Figs (Ficus carica L.) and Quality Improvement by Adding Fig Leaves. J. East Asian Soc. Diet. Life 2005, 15, 112–118. [Google Scholar]
- Kadam, N.U.; Upadhye, A.A.; Ghosh, J.S. Short communication: Fermentation and characterization of wine from dried Ficus carica (L.) using Saccharomyces cerevisiae NCIM 3282. Int. Food Res. J. 2011, 18, 1569–1571. [Google Scholar]
- Milicevici, B.; Ackar, D.; Babic, J.; Jozinovic, A.; Milicevici, R.; Oroz, M.; Subari, C.D. Impact of the fermentation process with immobilized yeast cells on the aroma profile and sensory quality of distillates produced from two fig (Ficus carica L.) cultivars. Poljoprivreda 2017, 23, 49–55. [Google Scholar] [CrossRef]
- Çalişkan, O.; Polat, A.A. Fruit characteristics of fig cultivars and genotypes grown in Turkey. Sci. Hortic. 2008, 115, 360–367. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Serradilla, M.J.; del Carmen Villalobos, M.; Ruiz-Moyano, S.; Martín, A. Influence of ripening stage on bioactive compounds and antioxidant activity in nine fig (Ficus carica L.) varieties grown in Extremadura, Spain. J. Food Compos. Anal. 2017, 64, 203–212. [Google Scholar] [CrossRef]
- Shi, Y.; Mon, A.M.; Fu, Y.; Zhang, Y.; Wang, C.; Yang, X.; Wang, Y. The genus Ficus (Moraceae) used in diet: Its plant diversity, distribution, traditional uses and ethnopharmacological importance. J. Ethnopharmacol. 2018, 226, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Vaya, J. Chapter 17 Fig, Carob, Pistachio, and Health. In Bioactive Foods in Promoting Health: Fruits and Vegetables, B Effects of Individual Vegetables on Health; Watson, R., Preedy, V., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 245–263. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Samaris, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef]
- Barolo, M.I.; Mostacero, N.R.; López, S.N. Ficus carica L. (Moraceae): An ancient source of food and health. Food Chem. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- dos Anjos Cruz, J.M.; Corrêa, R.F.; Lamarão, C.V.; Kinupp, V.F.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Ficus spp. fruits: Bioactive compounds and chemical, biological and pharmacological properties. Food Res. Int. 2022, 152, 110928. [Google Scholar] [CrossRef]
- Lianju, W.; Weibin, J.; Kai, M.; Zhifeng, L.; Yelin, W. The Production and Research of Fig (Ficus Carica L.) in China. Acta Hort. 2003, 605, 191–196. [Google Scholar] [CrossRef]
- Yildirim, H.K. Evaluation of colour parameters and antioxidant activities of fruit wines. Int. J. Food Sci. Nutr. 2006, 57, 47–63. [Google Scholar] [CrossRef]
- Dueñas, M.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Escribano-Bailón, T. Anthocyanin composition in fig (Ficus carica L.). J. Food Compos. Anal. 2008, 21, 107–115. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Harzallah, A.A.; Bhouri, M.; Amri, Z.; Soltana, H.; Hammami, M. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind. Crops Prod. 2016, 83, 255–267. [Google Scholar] [CrossRef]
- Liu, X.; Xu, S.; Wang, M.; Wang, L.; Liu, J. Effect of mixed fermentation with Pichia fermentans, Hanseniaspora uvarum and Wickeramomyces anomala on the quality of fig (Ficus carica L.) wines. J. Food Processing Preserv. 2020, 45, e15169. [Google Scholar] [CrossRef]
- Antoce, O.A.; Cojocaru, G.A. Evaluation by Flash GC Electronic Nose of the Effect of Combinations of Yeasts and Nutrients on the Aromatic Profiles of Feteasca Regala Wines after Two Years of Storage. Fermentation 2021, 7, 223. [Google Scholar] [CrossRef]
- Pratik, B.P. Preparation of Fig Wine (Ficus carica L.) Mahatma Phule Krishi Vidyapeeth, Rahuri. Bachelor’s Thesis, Mahatma Phule Agricultural University, Ahmednagar, India, 2010. [Google Scholar]
- Rodrigues-Solana, R.; Galengo, L.R.; Perez-Santin, E.; Romano, A. Production method and varietal source influence the volatile profiles of spirits prepared from fig fruits (Ficus carica L.). Eur. Food Res. Techology 2018, 244, 2213–2229. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No 1293/2005 of 5 August 2005 Amending Regulation (EEC) No 2676/90 Determining Community Methods for the Analysis of Wines. Off. J. Eur. Union 2005, 65, 219–222. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EC) No 606/2009 of 10 July 2009 laying down certain detailed rules for implementing Council Regulation (EC) No 479/2008 as regards the categories of grapevine products, oenological practices and the applicable restrictions. Off. J. Eur. Union 2009, 21, 266–324. [Google Scholar]
- Antoce, O.A.; Nămoloşanu, C.I. Method for Sensory Profile Construction for Defining and Evaluating Wine Tipicality. RO Patent No. 123129/2010, 29 June 2007. [Google Scholar]
- Ask the AWRI: Predicting Alcohol Levels. Grapegrower and Winemaker. 2016. Available online: https://www.awri.com.au/wp-content/uploads/2018/04/s1809.pdf (accessed on 25 July 2022).
- The Commission of the European Communities. Commission Regulation (EC) No 1308/2013 of the European Parliament and of the Council, of 17 December 2013, establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Off. J. Eur. Union 2013, 347, 671–854. [Google Scholar]



| Fig Physicochemical Parameters * | Fig Variety G1 | Fig Variety G2 | Fig Variety G3 |
|---|---|---|---|
| Skin colour | purple | yellow | green |
| Mass (g/fruit) | 20.44 ± 0.95 a | 47.49 ± 2.25 b | 33.39 ± 2.70 c |
| TSS (°Brix) | 13.95 ± 0.59 a | 15.07 ± 0.39 a | 13.98 ± 0.30 a |
| Acidity (malic acid g kg−1) | 2.22 ± 0.04 a | 1.19 ± 0.02 b | 1.05 ± 0.03 c |
| pH | 4.40 ± 0.02 a | 4.95 ± 0.04 b | 5.13 ± 0.03 c |
| Fig Beverage Physicochemical Parameter | Fig Variety G1 | Fig Variety G2 | Fig Variety G3 |
|---|---|---|---|
| alcohol degree (% v/v) | 18.00 ± 0.09 a | 17.36 ± 0.09 b | 17.48 ± 0.08 b |
| reducing sugar (g L−1 glucose + fructose) | 19.90 ± 0.91 a | 26.98 ± 1.80 b | 12.59 ± 0.43 c |
| total acidity (g L−1 tartaric acid and meq L−1) | 4.87 ± 0.11 a 64.93 ± 1.47 a | 5.06 ± 0.06 a 67.46 ± 0.80 a | 5.02 ± 0.08 a 66.93 ± 1.07 a |
| malic acid (g L−1 and meq L−1) | 0.34 ± 0.05 a 5.08 ± 0.75 a | 0.42 ± 0.02 a 6.27 ± 0.30 a | 0.43 ± 0.01 a 6.42 ± 0.15 a |
| lactic acid (g L−1 and meq L−1) | 0.23 ± 0.03 a 2.56 ± 0.33 a | 0.21 ± 0.03 a 2.33 ± 0.33 a | 0.17 ± 0.02 a 1.89 ± 0.22 a |
| volatile acidity (g L−1 acetic acid and meq L−1) | 0.55 ± 0.05 a 9.17 ± 0.83 a | 0.49 ± 0.04 a 8.17 ± 0.67 a | 0.53 ± 0.02 a 8.83 ± 0.33 a |
| pH | 3.41 ± 0.01 a | 3.37 ± 0.01 ab | 3.35 ± 0.01 b |
| free SO2 (mg L−1) | 4.07 ± 0.98 a | 4.94 ± 1.00 a | 3.27 ± 0.65 a |
| total SO2 (mg L−1) | 37.43 ± 1.14 a | 37.20 ± 3.70 a | 32.73 ± 3.72 a |
| K+ mg L−1 | 177.25 ± 18.74 a | 164.50 ± 5.07 a | 167.75 ± 16.00 a |
| Ca2+ mg L−1 | 51.58 ± 4.15 a | 57.10 ± 4.95 a | 53.25 ± 2.52 a |
| Density | 0.9929 ± 1.25 × 10−4 a | 0.9949 ± 6.58 × 10−4 b | 0.9912 ± 8.74 × 10−4 b |
| Fig Beverage Sensory Parameters | Fig Variety G1 | Fig Variety G2 | Fig Variety G3 |
|---|---|---|---|
| Acidity | 45.91 ± 4.49 a | 50.68 ± 3.95 a | 47.27 ± 3.72 a |
| Sweetness | 50.45 ± 4.36 a | 46.59 ± 4.31 a | 46.59 ± 3.97 a |
| Bitterness | 40.68 ± 4.28 a | 40.91 ± 3.77 a | 37.05 ± 2.86 a |
| Mouthfeel (extract) | 58.18 ± 2.36 a | 49.32 ± 3.21 ab | 46.14 ± 2.42 b |
| Colour intensity | 67.27 ± 2.67 a | 39.32 ± 3.30 b | 41.59 ± 3.46 b |
| Aroma intensity | 63.64 ± 4.06 a | 59.32 ± 3.87 a | 52.95 ± 3.69 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisescu, E.; Antoce, A.O. Figs (Ficus carica L.) Used as Raw Material for Obtaining Alcoholic Fermented Beverages. Beverages 2022, 8, 60. https://doi.org/10.3390/beverages8040060
Moisescu E, Antoce AO. Figs (Ficus carica L.) Used as Raw Material for Obtaining Alcoholic Fermented Beverages. Beverages. 2022; 8(4):60. https://doi.org/10.3390/beverages8040060
Chicago/Turabian StyleMoisescu, Emilia, and Arina Oana Antoce. 2022. "Figs (Ficus carica L.) Used as Raw Material for Obtaining Alcoholic Fermented Beverages" Beverages 8, no. 4: 60. https://doi.org/10.3390/beverages8040060
APA StyleMoisescu, E., & Antoce, A. O. (2022). Figs (Ficus carica L.) Used as Raw Material for Obtaining Alcoholic Fermented Beverages. Beverages, 8(4), 60. https://doi.org/10.3390/beverages8040060






