Possible Utilization of Two-Phase Olive Pomace (TPOP) to Formulate Potential Functional Beverages: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals and Instruments
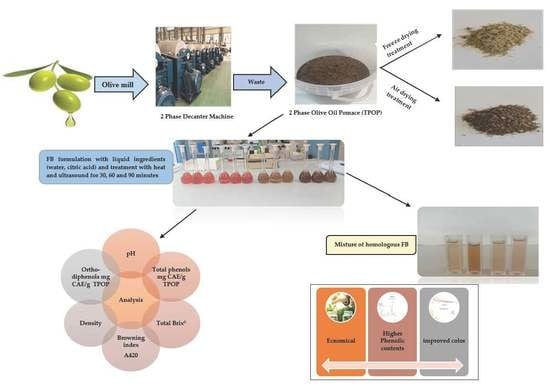
2.2. TPOP Treatment and Functional Beverages (FB) Preparation
2.3. Physicochemical Analysis
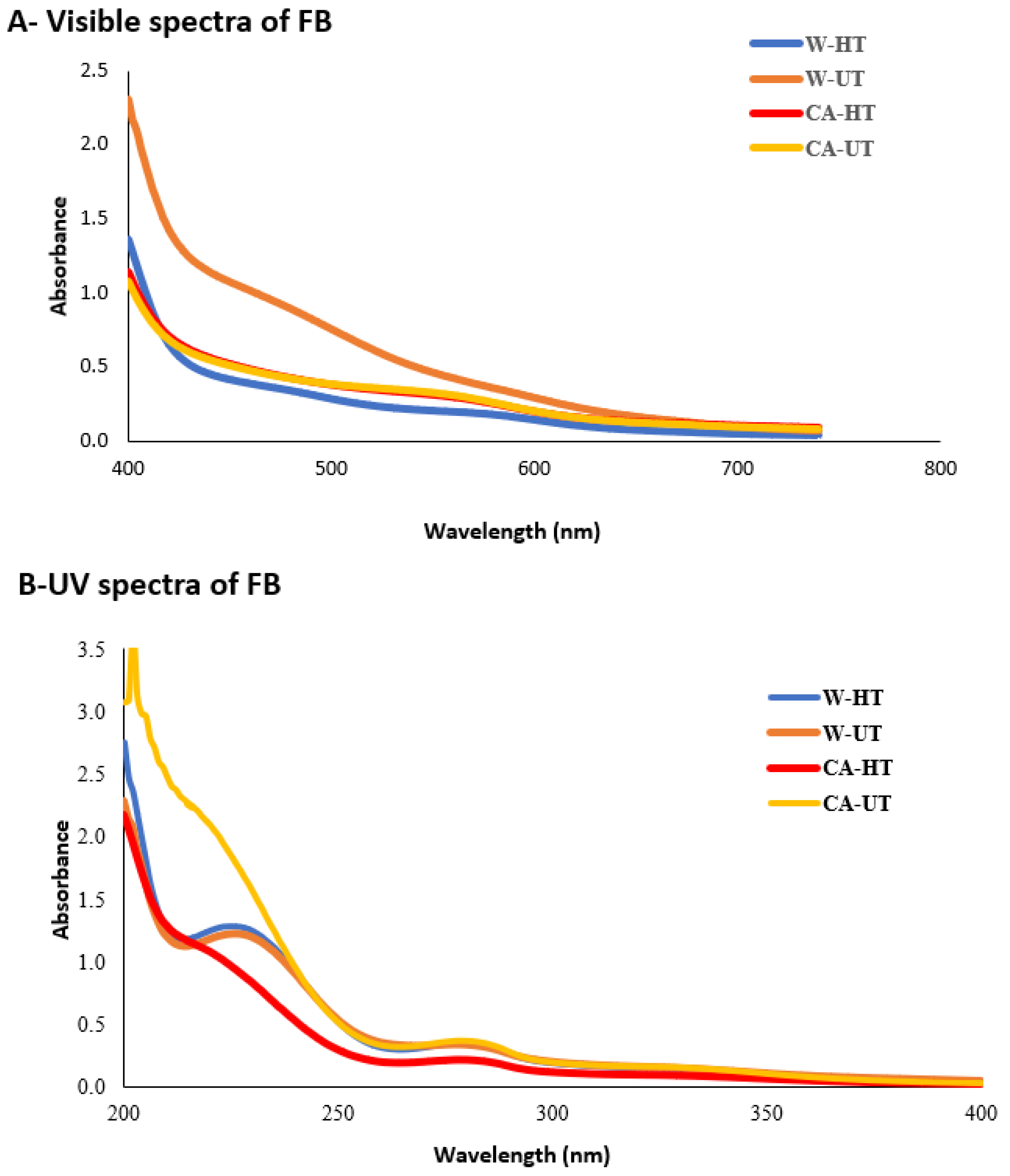
2.4. Browning Index and Spectra Determination
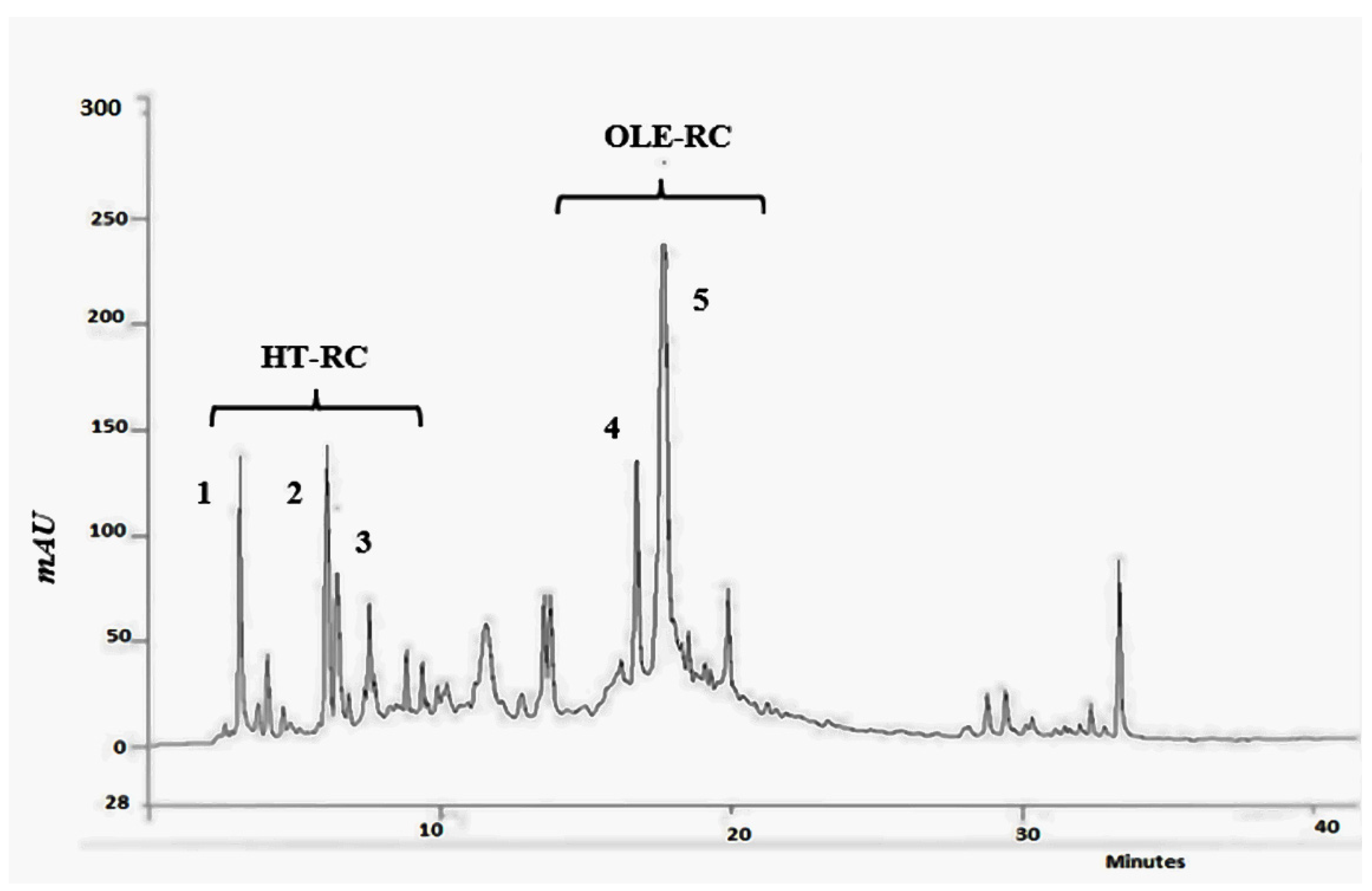
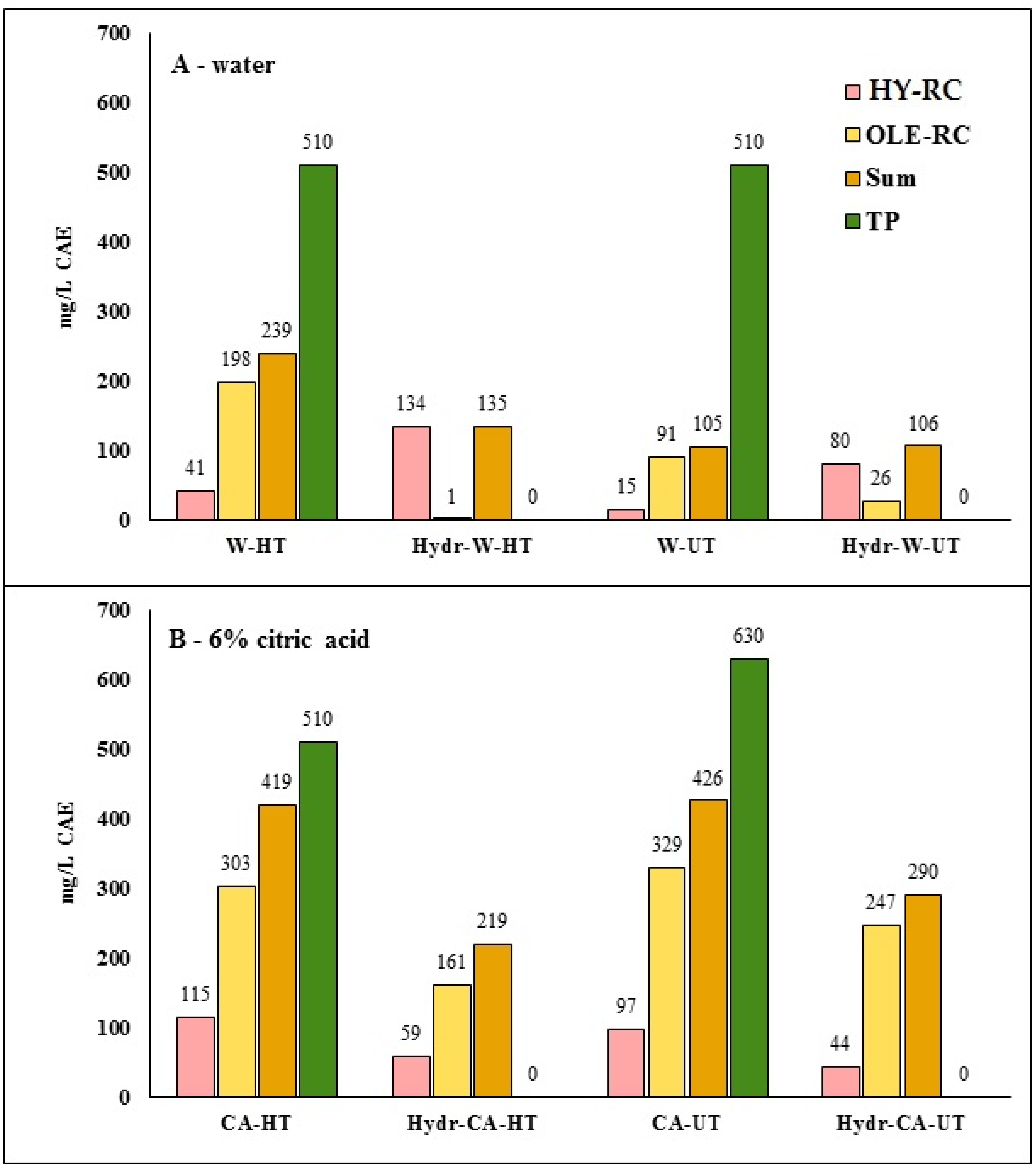
2.5. Phenolic Component
2.6. Antioxidant Activity
2.7. Statistical Analyses
3. Results
3.1. General Characteristics of Two-Phase Olive Pomace (TPOP) and Functional Beverages (FB)
3.2. Color Evaluation and UV-VIS Spectra
3.3. Phenolic Profile of FB
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, B.C.; González, P.A.M.; Oludemi, T.; Ayuda, D.B.; González, M.S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, F.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J. Novel botanical ingredients for beverages. Clin. Dermatol. 2009, 27, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F.; Ramos, C.L. Functional beverages from cereals. In Functional and Medicinal Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 351–379. [Google Scholar] [CrossRef]
- Cong, L.; Bremer, P.; Mirosa, M. Functional beverages in selected countries of Asia pacific region: A review. Beverages 2020, 6, 21. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rehman, A.; Usman, M.; Ali, A.; Ahmad, M.M.; Shehzad, Q.; Siddeeg, A. Influence of guar gum and chitosan enriched with lemon peel essential oil coatings on the quality of pears. Food Sci. Nutr. 2022, 10, 2443–2445. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food by-products as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Banias, G.; Achillas, C.; Vlachokostas, C.; Moussiopoulos, N.; Stefanou, M. Environmental impacts in the life cycle of olive oil: A literature review. J. Sci. Food Agric. 2017, 97, 1686–1697. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Pre-treatment of olive mill wastewaters at laboratory and mill scale and subsequent use in agriculture: Legislative framework and proposed soil quality indicators. Resour. Conserv. Recycl. 2012, 69, 82–89. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2012, 50, 596–601. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. New by-products rich in bioactive sustances from the olive oil mill processing. J. Sci. Food Agric. 2018, 98, 225–230. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery systems for hydroxytyrosol supplementation: State of the art. Colloids Interfaces 2020, 4, 25. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Int. Food Res. J. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Cardinali, A.; Linsalata, V.; Lattanzio, V.; Ferruzzi, M.G. Verbascosides from olive mill wastewater: Assessment of their bioaccessibility and intestinal uptake using an in vitro digestion/caco-2 model system. J. Food Sci. 2011, 76, H48–H54. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Yücetepe, A.; Kasapoglu, K.N.; Ozçelik, B. High-value compounds from olive oil processing waste. In Edible Oils, Extraction, Processing, and Applications; Chemat, S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 179–204. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and innovative approaches to obtain virgin olive oils with a higher level of bioactive constituents. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 179–215. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; Garcıa, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Moral, P.S.; Méndez, M.V.R. Production of pomace olive oil. Grasas Y Aceites 2006, 57, 47–55. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods. 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Foti, P.; Occhipinti, P.S.; Romeo, F.V.; Timpanaro, N.; Musumeci, T.; Randazzo, C.L.; Randazzo, C.; Caggia, C. Phenols recovered from olive mill wastewater as natural booster to fortify blood orange juice. Food Chem. 2022, 393, 133428. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Nag, A. Antioxidant activity of various phenol extracts of olive-oil mill wastewaters. Acta Aliment. 2009, 38, 77–86. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iftikhar, A.; Lopez, F. Antioxidant effect of traditional and new vinegars on functional oil/vinegar dressing-based formulations. Eur. Food Res. Technol. 2022, 248, 1573–1582. [Google Scholar] [CrossRef]
- Song, J.; Chen, X.; Song, Y.; Tia, W.; Liu, J. The olive fruit wine research on the fermentation process and aromatic ingredients. In International Conference on Machinery, Materials Engineering, Chemical Engineering and Biotechnology, 2nd ed.; Atlantis Press: Zingzhau, China, 2015; pp. 603–608. [Google Scholar]
- Yao, Q.; He, G.; Guo, X.; Hu, Y.; Shen, Y.; Gou, X. Antioxidant activity of olive wine, a by-product of olive mill wastewater. Pharm. Biol. 2016, 54, 2276–2281. [Google Scholar] [CrossRef][Green Version]
- De Leonardis, A.; Macciola, V.; Iorizzo, M.; Lombardi, S.J.; Lopez, F.; Marconi, E. Effective assay for olive vinegar production from olive oil mill wastewaters. Food Chem. 2018, 240, 437–440. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Masino, F.; Macciola, V.; MultiTech, G.; Antonelli, A.; Marconi, E. A study on acetification process to produce olive vinegar from oil mill wastewaters. Eur. Food Res. Technol. 2019, 245, 2123–2131. [Google Scholar] [CrossRef]
- Jeong, H.L.; Jin, W.J.; Kwang, D.M.; Kee, J.P. Effects of anti-browning agents on polyphenoloxidase activity and total phenolics as related to browning of fresh cut ‘Fuji’ apple. ASEAN Food J. 2008, 15, 79–87. [Google Scholar]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Trujillo, M.; Espartero, J.L.; Fernández-Bolaños, J. Isolation of a powerful antioxidant from Olea europaea fruit-mill waste: 3,4-Dihydroxyphenylglycol. LWT-Food Sci. Technol. 2009, 42, 483–490. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Macciola, V.; Boukhchina, S.; de La Torre Fornell, R.; De Leonardis, A. The negligible role of ellagic acid in preventing fat oxidation of Tunisian walnuts (Juglans regia L.). J. Food Meas. Charact. 2017, 11, 1406–1411. [Google Scholar] [CrossRef]
- Fernández-Prior, M.Á.; Fatuarte, J.C.P.; Oria, A.B.; Viera-Alcaide, I.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. New liquid source of antioxidant phenolic compounds in the olive oil industry: Alperujo water. Foods 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in large quantities of highly purified hydroxytyrosol from liquid—Solid waste of two-phase olive oil processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Gómez, E.; Guillén, R.; Jiménez, A.; Heredia, A.; Rodríguez, R. Total recovery of the waste of two-phase olive oil processing: Isolation of added-value compounds. J. Agric. Food Chem. 2004, 52, 5849–5855. [Google Scholar] [CrossRef]
- Regulation, E.C. No, 432, 2012. Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
- Hutchings, J.B. Evolution and human’s attraction and reaction to colour: Food and health. Color Res. Applic. 2021, 46, 140–145. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Mohamed Ahmed, I.A.; Babiker, E.E. Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. Int. J. Food Sci. Technol. 2017, 52, 2115–2125. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Dehghani, F.; Perego, P. The effect of citric acid on the phenolic contents of olive oil. Food Chem. 2009, 116, 617–623. [Google Scholar] [CrossRef]
- Yusmaman, W.M.; Gunlazuardi, J. The role of citric acid modifiers addition in the preparation of TiO2 nanoparticles with the solvothermal method. AIP Conf. Proc. 2020, 2243, 020033. [Google Scholar] [CrossRef]
- Rodríguez, G.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernandez-Bolanos, J. Effect of steam treatment of alperujo on the composition, enzymatic saccharification, and in vitro digestibility of alperujo. J. Agric. Food Chem. 2007, 55, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Macciola, V.; Cuomo, F.; De Leonardis, A. Importance of oleacin and oleocanthal on the oxidative stability of extra virgin olive oil measured by Rancimat. Riv. It. Sost. Grasse. 2020, 97, 21–29. [Google Scholar]
- Michel, T.; Termentzi, A.; Gikas, E.; Halabalaki, M.; Smith, A.B.; Skaltsounis, A.L. Oleacin and oleocanthal: Two olive oil bioactives in multiple chemical forms. Planta Medica 2012, 78, 43. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Chemical characterization and properties of a polymeric phenolic fraction obtained from olive oil waste. Food Res. Int. 2013, 54, 2122–2129. [Google Scholar] [CrossRef]
- Jang, J.H.; Moon, K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Jahromi, M.A.; Moein, M.R.; Etemadfard, H.; Zebarjad, Z. In vitro free radical scavenging effect and total phenolic and flavonoid contents of 30 iranian plant species. Trends in Pharm. Sci. 2016, 2, 229–238. [Google Scholar]

| N. | Tags | Treatments Description and Liquid Ingredients |
|---|---|---|
| 1 | W-HT30 | Heat treatment for 30 min in distilled water |
| 2 | W-HT60 | Heat treatment for 60 min in distilled water |
| 3 | W-HT90 | Heat treatment for 90 min in distilled water |
| 4 | W-HT | Mixture of 1, 2 and 3 samples |
| 5 | W-UT30 | Ultrasonic treatment for 30 min in distilled water |
| 6 | W-UT60 | Ultrasonic treatment for 60 min in distilled water |
| 7 | W-UT90 | Ultrasonic treatment for 90 min in distilled water |
| 8 | W-UT | Mixture of 5, 6 and 7 samples |
| 9 | CA-HT30 | Heat treatment (80 °C) for 30 min in 6% citric acid |
| 10 | CA-HT60 | Heat treatment (80 °C) for 60 min in 6% citric acid |
| 11 | CA-HT90 | Heat treatment (80 °C) for 90 min in 6% citric acid |
| 12 | CA-HT | Mixture of 9, 10 and 11 samples |
| 13 | CA-UT30 | Ultrasonic treatment (80 °C) for 30 min in 6% citric acid |
| 14 | CA-UT60 | Ultrasonic treatment (80 °C) for 60 min in 6% citric acid |
| 15 | CA-UT90 | Ultrasonic treatment (80 °C) for 90 min in 6% citric acid |
| 16 | CA-UT | Mixture of 12, 13 and 14 samples |
| 17 | FD-TPOP | Frieze dried two-phase olive pomace—repeated extraction with methanol: water 80% v/v |
| 18 | AD-TPOP | Air-dried (7 days) two-phase olive pomace—extraction with methanol: water 80% v/v |
| Parameters | Results |
|---|---|
| Humidity (%) | 63.4 ± 5.1 |
| Solid residue (%) | 36.5 ± 3.2 |
| Total oil (%) | 2.6 ± 0.4 |
| Total protein (N × 6.25) (%) | 1.9 ± 0.3 |
| Ash (%) | 1.5 ± 0.2 |
| pH | 5.86 ± 0.73 |
| N. | Samples | pH | Density | Total Soluble Solid °Brix | Total Phenols mg CAE/g TPOP | o-Diphenols mg CAE/g TPOP |
|---|---|---|---|---|---|---|
| 1 | FD-TPOP | 5.11 ± 0.13 a | Nd * | Nd * | 7.5 ± 0.3 a | 1.7 ± 0.2 a |
| 2 | AD-TPOP | 5.15 ± 0.11 a | Nd * | Nd * | 3.9 ± 0.2 b | 1.3 ± 0.2 a |
| 3 | W-HT30 | 5.10 ± 0.12 a | 0.998 ± 0.001 a | 0.7 ± 0.1 a | 2.0 ± 0.1 c | 2.5 ± 0.1 b |
| 4 | W-HT60 | 5.16 ± 0.11 a | 0.996 ± 0.001 a | 0.7 ± 0.1 a | 1.7 ± 0.1 c | 2.3 ± 0.1 b |
| 5 | W-HT90 | 5.10 ± 0.10 a | 0.996 ± 0.001 a | 0.6 ± 0.1 a | 1.9 ± 0.2 c | 2.5 ± 0.2 b |
| Mean (3–5) | 5.12 ± 0.09 | 0.997 ± 0.001 | 0.6 ± 0.0 | 1.9 ± 0.1 | 2.5 ± 0.2 | |
| 6 | W-HT | 5.11 ± 0.12 a | 0.996 ± 0.001 a | 0.7 ± 0.1 | 1.7 ± 0.2 c | 2.3 ± 0.2 b |
| 7 | W-UT30 | 5.12 ± 0.13 a | 0.996 ± 0.001 a | 0.6 ± 0.0 a | 1.7 ± 0.1 c | 2.0 ± 0.1 b |
| 8 | W-UT60 | 5.15 ± 0.10 a | 0.996 ± 0.001 a | 0.5 ± 0.0 a | 1.9 ± 0.1 c | 2.2 ± 0.1 b |
| 9 | W-UT90 | 5.14 ± 0.11 a | 0.995 ± 0.001 a | 0.5 ± 0.0 a | 1.8 ± 0.1 c | 2.1 ± 0.1 b |
| Mean (7–8) | 5.14 ± 0.02 | 0.996 ± 0.001 | 0.5 ± 0.0 | 1.8 ± 0.1 | 2.1 ± 0.1 | |
| 10 | W-UT | 5.14 ± 0.09 a | 0.995 ± 0.001 a | 0.6 ± 0.0 a | 1.7 ± 0.2 c | 2.0 ± 0.2 b |
| 11 | CA-HT30 | 1.98 ± 0.11 b | 1.022 ± 0.006b | 5.8 ± 0.4 b | 2.1 ± 0.2 c | 2.5 ± 0.2 b |
| 12 | CA-HT60 | 2.02 ± 0.10 b | 1.020 ± 0.008b | 5.6 ± 0.3 b | 2.2 ± 0.1 c | 2.5 ± 0.2 b |
| 13 | CA-HT90 | 1.99 ± 0.12 b | 1.016 ± 0.006b | 5.6 ± 0.2 b | 2.1 ± 0.2 c | 2.2 ± 0.1 b |
| Mean (11–13) | 1.99 ± 0.08 | 1.019 ± 0.005 | 5.7 ± 0.3 | 2.1 ± 0.1 | 2.4 ± 0.2 | |
| 14 | CA-HT | 1.99 ± 0.12 b | 1.020 ± 0.004 b | 5.9 ± 0.3 b | 2.1 ± 0.1 c | 2.3 ± 0.3 b |
| 15 | CA-UT30 | 1.96 ± 0.10 b | 1.020 ± 0.004 b | 5.5 ± 0.3 b | 2.0 ± 0.1 c | 2.3 ± 0.1 b |
| 16 | CA-UT60 | 1.96 ± 0.10 b | 1.020 ± 0.005 b | 5.6 ± 0.3 b | 1.9 ± 0.1 c | 2.5 ± 0.1 b |
| 17 | CA-UT90 | 1.93 ± 0.09 b | 1.032 ± 0.007 b | 5.5 ± 0.3 b | 2.1 ± 0.2 c | 2.6 ± 0.2 b |
| Mean (15–17) | 1.96 ± 0.02 | 1.024 ± 0.007 | 5.5 ± 0.3 | 2.0 ± 0.1 | 2.5 ± 0.2 | |
| 18 | CA-UT | 1.95 ± 0.08 b | 1.020 ± 0.005 b | 5.1 ± 0.4 b | 1.9 ± 0.2 c | 2.3 ± 0.2 b |
| Samples | Linear Equation | R2 | IC50 (mg/mL) |
|---|---|---|---|
| CA-HT | y = 1.85x ± 4.83 | 0.999 | 24.4 ± 1.5 a |
| CA-U | y = 3.36x ± 4.59 | 0.943 | 13.5 ± 1.2 b |
| W-HT | y = 5.43x ± 8.10 | 0.967 | 7.7 ± 1.6 c |
| W-U | y = 3.56x ± 18.33 | 0.983 | 8.9 ± 1.3 c |
| FD-TPOP | y = 1.70x ± 7.99 | 0.999 | 24.7 ± 3.2 a |
| AD-TPOP | y = 0.66x ± 9.40 | 0.915 | 61.1 ± 3.8 d |
| Ascorbic acid | y = 6.94x ± 3.42 | 0.996 | 6.7 ± 1.6 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leonardis, A.; Iftikhar, A.; Macciola, V. Possible Utilization of Two-Phase Olive Pomace (TPOP) to Formulate Potential Functional Beverages: A Preliminary Study. Beverages 2022, 8, 57. https://doi.org/10.3390/beverages8030057
De Leonardis A, Iftikhar A, Macciola V. Possible Utilization of Two-Phase Olive Pomace (TPOP) to Formulate Potential Functional Beverages: A Preliminary Study. Beverages. 2022; 8(3):57. https://doi.org/10.3390/beverages8030057
Chicago/Turabian StyleDe Leonardis, Antonella, Ayesha Iftikhar, and Vincenzo Macciola. 2022. "Possible Utilization of Two-Phase Olive Pomace (TPOP) to Formulate Potential Functional Beverages: A Preliminary Study" Beverages 8, no. 3: 57. https://doi.org/10.3390/beverages8030057
APA StyleDe Leonardis, A., Iftikhar, A., & Macciola, V. (2022). Possible Utilization of Two-Phase Olive Pomace (TPOP) to Formulate Potential Functional Beverages: A Preliminary Study. Beverages, 8(3), 57. https://doi.org/10.3390/beverages8030057