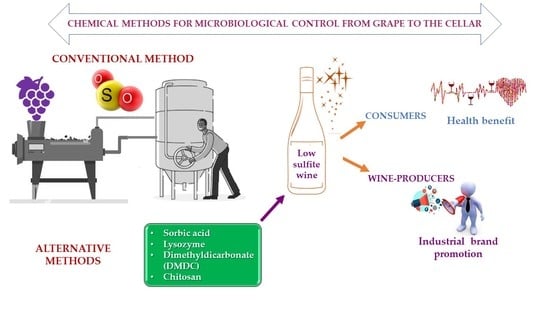
Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications
Abstract
1. Introduction
2. Sulfur Dioxide
2.1. Antiseptic Activity of SO2
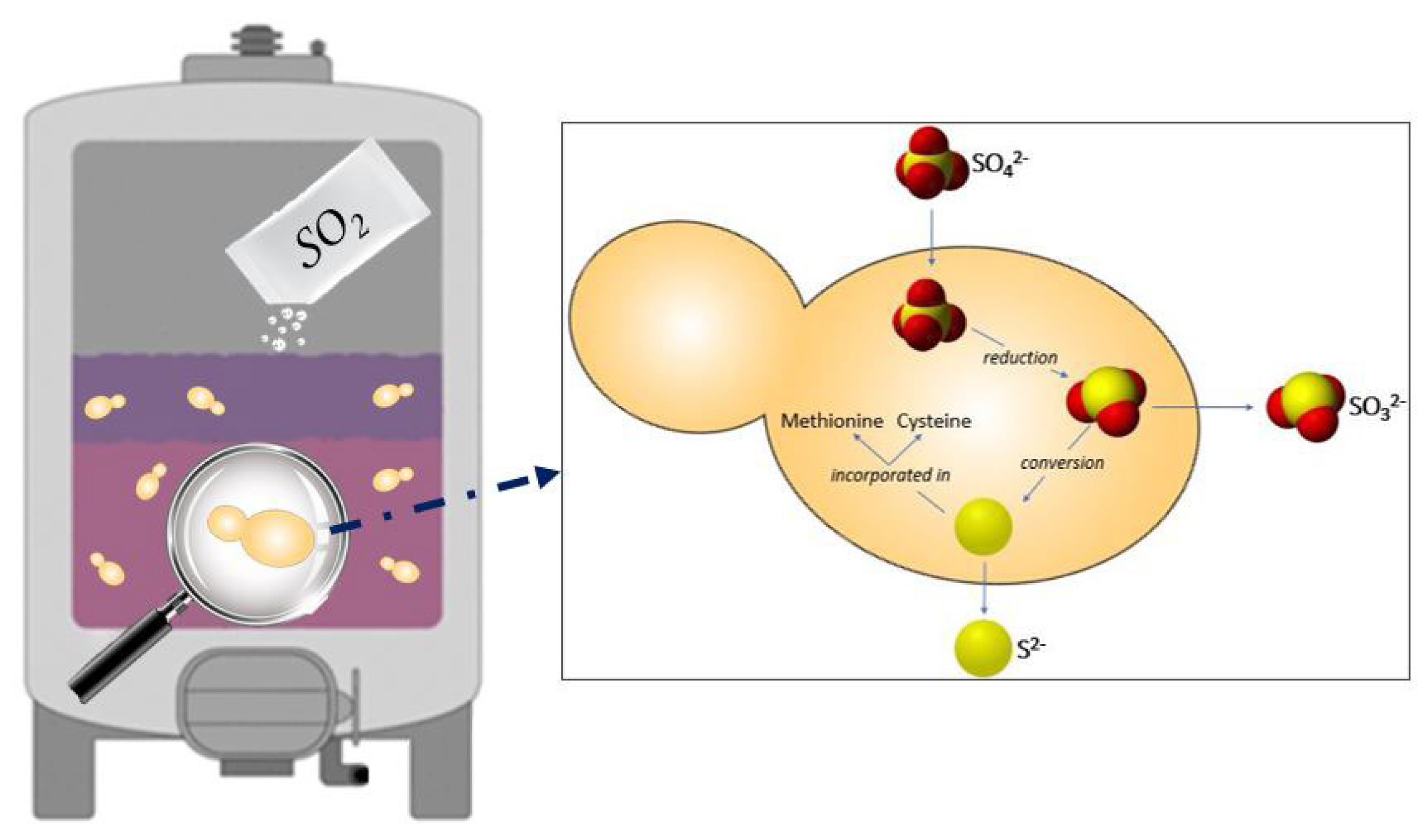
2.1.1. Activity against Yeasts
- -
- Metabolic pathways involved in the production of sulfur compounds, such as the amino acids methionine and cysteine [37];
- -
- Sulfite detoxification by the membrane efflux [37];
- -
- Production of SO2-binding molecules, such as acetaldehyde [23];
- -
- The cell entry into a VBNC state [38]. This physiological state is described as a protection strategy in which the cells can wait for more favorable conditions. In wine, the VBNC state allows spoilage yeasts and bacteria to survive throughout the wine fermentation process and into the wine bottle. In wine conditions, the presence of chemical stressors such as SO2 has been shown to induce a VBNC state in S. cerevisiae and other yeast species, such as Brettanomyces/Dekkera bruxellensis, and reactivate later, when conditions become more favorable [39]. The removal of SO2 from the wine environment can be obtained by increasing the pH in order to shift the chemical equilibrium of SO2 with a decrease of the concentration in molecular SO2, favoring the exit from the VBNC state [40].
2.1.2. Activity against Bacteria
2.2. Technological Activities of SO2
2.3. Negative Effects of Sulfur Dioxide
3. Alternative Methods to SO2
3.1. Sorbic Acid
3.2. Lysozyme
3.3. Dimethyl Dicarbonate
3.4. Chitosan
- The addition of 100 g/hL is authorized to prevent hazing in wine, and to reduce the concentration of heavy metals (Fe, Pb, Cd, Cu). Different studies confirmed the clarifying action of chitosan and the prevention of protein haze phenomena, mainly in white wine, with the use at the permitted doses [78,79,80]. Similar results were found also in matrices different from the wine, such as beer [81] or fruit juices [82,83]. Chitosan also has an action of sorption of heavy metals, such as iron and copper, avoiding the formation of hazing phenomena in wine. A reduction of 70% of iron and 30% of copper is observed at a dose of 1000 mg/L [84], whereas other authors reported the ability to reduce the content of iron, lead, and cadmium in wine by adding doses of this polysaccharide ranging from 200 to 2000 mg/L [85].
- Doses of 500 g/hL are allowed to reduce any contamination by ochratoxin A (OTA), a mycotoxin which can be present in wine at a maximum dose of 2 μg/L (EC 2005), produced by fungi of the genera, Aspergillus and Penicillium, classified in group 2B as a “possible human carcinogen” by the International Agency for Research on Cancer [86]. Different studies demonstrated the ability of chitosan, at doses of about 4000–5000 mg/L, to remove a large percentage of OTA in wine [87,88].
- Amounts of 10 g/hL can be used to reduce the concentration of unwanted microorganisms, especially Brettanomyces spp. Details regarding the antimicrobial activity will be reported below (see Section 3.4.1, Section 3.4.2, Section 3.4.3).
3.4.1. Antiseptic Activity
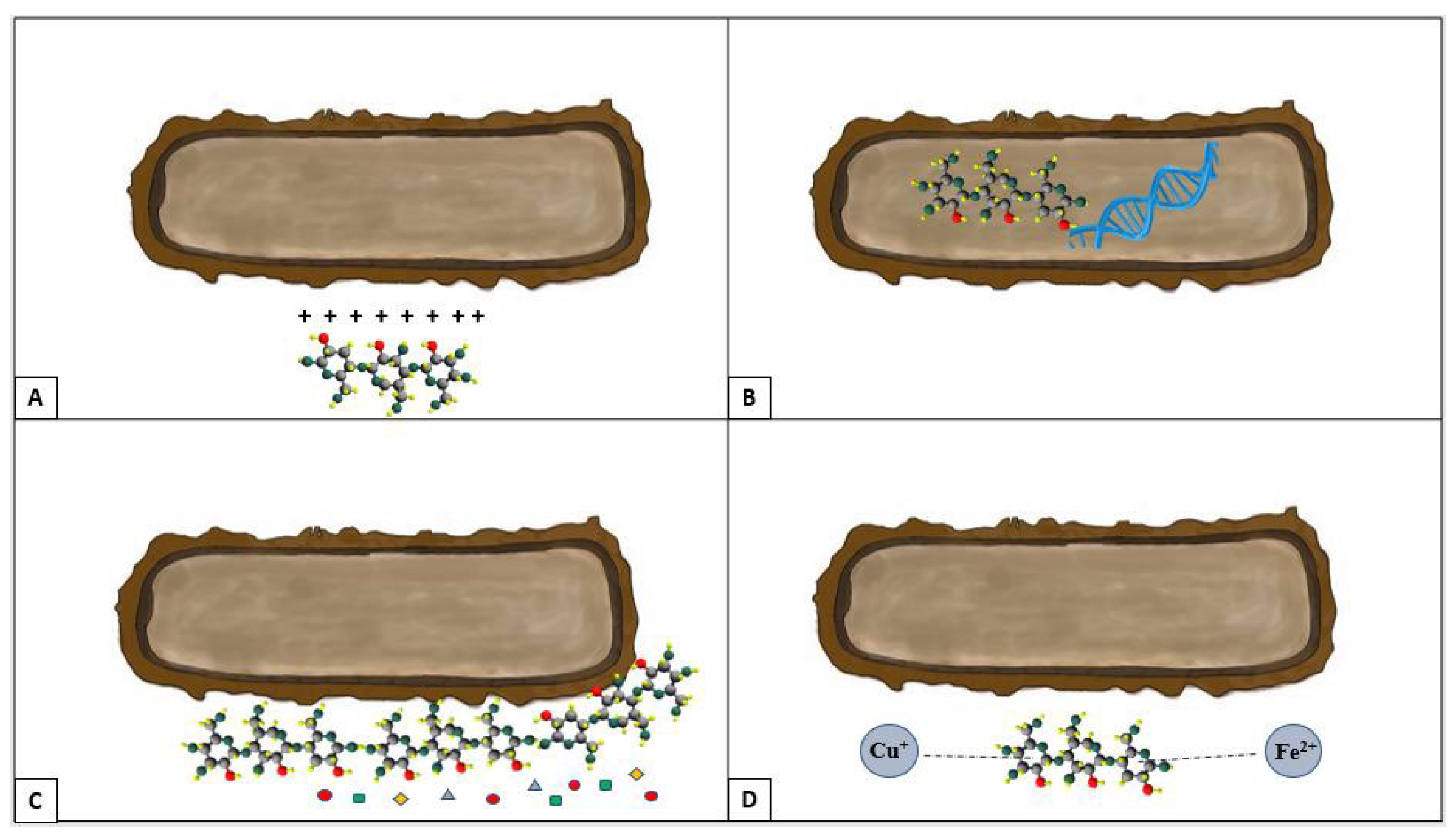
3.4.2. Activity against Yeasts
3.4.3. Antibacterial Activity
3.4.4. Sources of Chitosan
3.5. Other Substances
3.5.1. Phenolic Compounds
3.5.2. Colloidal Silver Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mas, A.; Portillo, M.C. Strategies for microbiological control of the alcoholic fer-mentation in wines by exploiting the microbial terroir complexity: A mini-review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, E.; Ruiz-Rico, M.; Guillamón, J.M.; Pérez-Esteve, É.; Barat, J.M. Improved antimicrobial activity of immobilised essential oil components against representative spoilage wine microorganisms. Food Control 2018, 94, 177–186. [Google Scholar] [CrossRef]
- Capece, A.; Pietrafesa, R.; Siesto, G.; Romano, P. Biotechnological Approach Based on Selected Saccharomyces cerevisiae Starters for Reducing the Use of Sulfur Dioxide in Wine. Microorganisms 2020, 8, 738. [Google Scholar] [CrossRef]
- Avramova, M.; Vallet-Courbin, A.; Maupeu, J.; Masneuf-Pomarède, I.; Albertin, W. Molecular diagnosis of Brettanomyces bruxellensis’ sulfur dioxide sensitivity through genotype specific method. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 1. [Google Scholar]
- Quiròs, C.; Herrero, M.; Garcìa, L.A.; Dìaz, M. Effects of SO2 on lactic acid bacteria physiology when used as a preservative compound in malolactic fermentation. J. Brew. Distill. 2011, 118, 89–96. [Google Scholar]
- Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Garijo, P.; González-Arenzana, L.; Gutíerrez, A.R.; López-Alfaro, I.; Santamaría, P. Application of colloidal silver versus sulfur dioxide during vinification and storage of Tempranillo red wines. Aust. J. Grape Wine Res. 2014, 20, 51–61. [Google Scholar] [CrossRef]
- Du Toit, W.J.; Pretorius, I.S.; Lonvaud-Funel, A. The effect of sulphur dioxide and oxygen on the viability and culturability of a strain of Acetobacter pasteurianus and a strain of Brettanomyces bruxellensis isolated from wine. J. Appl. Microbiol. 2005, 98, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Santos, M.; Nunes, C.; Saraiva, J.; Coimbra, M. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in wines: Rational use and possible alternatives. In Red Wine Technology; Morata, A., Ed.; Academic Press: New York, NY, USA, 2019; pp. 309–321. [Google Scholar]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Peng, Z.; Duncan, B.; Pocock, K.F.; Sefton, M.A. The effect of ascorbic acid on oxidative browning of white wines and model wines. Aust. J. Grape Wine Res. 1998, 4, 127–135. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Valášek, P.; Mlček, J.; Fišera, M.; Fišerová, L.; Sochor, J.; Baroň, M.; Juríková, T. Effect of various sulphur dioxide additions on amount of dissolved oxygen, total antioxidant capacity and sensory properties of white wines. Mitt. Klosterneubg. 2014, 64, 193–200. [Google Scholar]
- King, A.D.; Ponting, J.D.; Sanshuck, D.W.; Jackson, R.; Mihara, K. Factors Affecting Death of Yeast by Sulfur Dioxide. J. Food Prot. 1981, 44, 92–97. [Google Scholar] [CrossRef]
- Howe, P.A.; Worobo, R.; Sacks, G.L. Conventional Measurements of Sulfur Dioxide (SO2) in Red Wine Overestimate SO2 Antimicrobial Activity. Am. J. Enol. Vitic. 2018, 69, 210–220. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2016; pp. 140–148. [Google Scholar]
- Noble, J.; Sanchez, I.; Blondin, B. Identification of new Saccharomyces cerevisiae variants of the MET2 and SKP2 genes controlling the sulfur assimilation pathway and the production of undesirable sulfur compounds during alcoholic fermentation. Microb. Cell. Factories 2015, 14, 68. [Google Scholar] [CrossRef]
- Zara, G.; Nardi, T. Yeast Metabolism and Its Exploitation in Emerging Winemaking Trends: From Sulfite Tolerance to Sulfite Reduction. Fermentation 2021, 7, 57. [Google Scholar] [CrossRef]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Cuijvers, K.; Van Den Heuvel, S.; Varela, C.; Rullo, M.; Solomon, M.; Schmidt, S.; Borneman, A. Alterations in Yeast Species Composition of Uninoculated Wine Ferments by the Addition of Sulphur Dioxide. Fermentation 2020, 6, 62. [Google Scholar] [CrossRef]
- Divol, B.; Miot-Sertier, C.; Lonvaud-Funel, A. Genetic characterization of strains of Saccharomyces cerevisiae responsible for ‘refermentation’ in Botrytis-affected wines. J. Appl. Microbiol. 2006, 100, 516–526. [Google Scholar] [CrossRef]
- Chandra, M.; Oro, I.; Ferreira-Dias, S.; Malfeito-Ferreira, M. Effect of ethanol, sulfur dioxide and glucose on the growth of wine spoilage yeasts using response surface methodology. PLoS ONE 2015, 10, e0128702. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.D. Resistance of yeast species to benzoic and sorbic acids and to sulfur dioxide. J. Food Prot. 1985, 48, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Oelofse, A.; Pretorius, I.S.; Du Toit, M. Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. S. Afr. J. Enol. Vitic. 2008, 29, 128–144. [Google Scholar] [CrossRef]
- Schifferdecker, A.J.; Dashko, S.; Ishchuk, O.P.; Piskur, J. The wine and beer yeast Dekkera bruxellensis. Yeast 2014, 31, 323–332. [Google Scholar] [CrossRef]
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef][Green Version]
- Stratford, M.; Rose, A.H. Transport of sulphur dioxide by Saccharomyces cerevisiae. J. Gen. Microbiol. 1986, 132, 1–6. [Google Scholar] [CrossRef]
- Borst-Pauwels, G.W.F.H. Ion transport in yeast. Biochim. Biophys. Acta 1981, 650, 88–127. [Google Scholar] [CrossRef]
- Gunnison, A.F. Sulphite toxicity: A critical review of in vitro and in vivo data. Food Chem. Toxicol. 1981, 19, 667–682. [Google Scholar] [CrossRef]
- Cole, R.D. Sulfitolysis. In Methods in Enzymology; Hirs, C.H.W., Ed.; Academic Press: New York, NY, USA; London, UK, 1967; Volume XI Enzyme Structure, pp. 206–208. [Google Scholar]
- Benoit Divol, B.; du Toit, M.; Duckitt, E. Surviving in the presence of sulphur dioxide: Strategies developed by wine yeasts. Appl. Microbiol. Biotechnol. 2012, 95, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bakalinsky, A.T. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 2000, 16, 881–888. [Google Scholar] [CrossRef]
- Morgan, S.C.; Haggerty, J.J.; Johnston, B.; Jiranek, V.; Durall, D.M. Response to Sulfur Dioxide Addition by Two Commercial Saccharomyces cerevisiae Strains. Fermentation 2019, 5, 69. [Google Scholar] [CrossRef]
- Agnolucci, M.; Rea, F.; Sbrana, C.; Cristani, C.; Fracassetti, D.; Tirelli, A.; Nuti, M. Sulphur dioxide affects culturability and volatile phenol production by Brettanomyces/Dekkera bruxellensis. Int. J. Food Microbiol. 2010, 143, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Serpaggi, V.; Remize, F.; Recorbet, G.; Gaudot-Dumas, E.; Sequeira-LeGrand, A.; Alexandre, H. Characterization of the “viable but nonculturable” (VBNC) state in the wine spoilage yeast Brettanomyces. Food Microbiol. 2012, 30, 438–447. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid bacteria: Physiology and carbon sources oxidation. Indian J. Microbiol. 2013, 4, 377–384. [Google Scholar] [CrossRef]
- Millet, V.; Lonvaud-Funel, A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000, 30, 136–141. [Google Scholar] [CrossRef]
- Onetto, C.A.; Costello, P.J.; Kolouchova, R.; Jordans, C.; McCarthy, J.; Schmidt, S.A. Analysis of Transcriptomic Response to SO2 by Oenococcus oeni Growing in Continuous Culture. Microbiol. Spectr. 2021, 9, e01154-21. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols, and oxygen in a wine-model system: Central role of iron and copper. Am. J. Enol. Vitic. 2007, 58, 53–60. [Google Scholar]
- Li, H.; Wang, H.; Yuan, C.; Wang, S. Wine Chemistry; Scientific Publishing Company: Beijing, China, 2005. [Google Scholar]
- Lester, M.R. Sulfite sensitivity: Significance in human health. J. Am. Coll. Nutr. 1995, 14, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Vally, H.; Thompson, P.J. Role of sulfite additives in wine induced asthma: Single dose and cumulative dose studies. Thorax 2001, 56, 763–769. [Google Scholar] [CrossRef]
- Stead, D. The effect of hydroxycinnamic acids and potassium sorbate on the growth of 11 strains of spoilage yeasts. J. Appl. Bacteriol. 1995, 78, 82–87. [Google Scholar] [CrossRef]
- Delfini, C.; Cersosimo, M.; Del Prete, V.; Strano, M.; Gaetano, G.; Pagliara, A.; Ambrò, S. Resistance Screening Essay of Wine Lactic Acid Bacteria on Lysozyme: Efficacy of Lysozyme in Unclarified Grape Musts. J. Agric. Food Chem. 2004, 52, 1861–1866. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Bağder Elmaci, S.; Gülgör, G.; Tokatli, M.; Erten, H.; İşci, A.; Özçelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 2015, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.; del Monaco, S.; Cerrutti, P.; Galvagno, M. Selective antimicrobial activity of chitosan on beer spoilage bacteria and brewing yeasts. Biotechnol. Lett. 2004, 26, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, Z.; Pazourek, J.; Havel, J. Simultaneous determination of trans-resveratrol and sorbic acid in wine by capillary zone electrophoresis. Electrophoresis 2002, 23, 263–267. [Google Scholar] [CrossRef]
- Steels, H.; James, S.A.; Roberts, I.N.; Stratford, M. Sorbic acid resistance: The inoculum effect. Yeast 2000, 16, 1173–1183. [Google Scholar] [CrossRef]
- Silva, P.; Cardoso, H.; Gerós, H. Studies on the Wine Spoilage Capacity of Brettanomyces/Dekkera spp. Am. J. Enol. Vitic. 2004, 55, 1. [Google Scholar]
- Du Toit, M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons from nature’s own arsenal—A review. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef][Green Version]
- Liburdi, K.; Benucci, I.; Esti, M. Lysozyme in Wine: An Overview of Current and Future Applications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1062–1073. [Google Scholar] [CrossRef]
- Penas, E.; Di Lorenzo, C.; Uberti, F.; Restani, P. Allergenic Proteins in Enology: A Review on Technological Applications and Safety Aspects. Molecules 2015, 20, 13144–13164. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Veneri, G.; Zapparoli, G. Evaluating the Efficacy of Lysozyme Against Lactic Acid Bacteria Under Different Winemaking Scenarios. S. Afr. J. Enol. Vitic. 2010, 31, 99–105. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.C.; Zhang, G.; Krentz, S.; Darius, S.; Power, J.; Lagarde, G. Inhibition of spoilage lactic acid bacteria by lysozyme during wine alcoholic fermentation. Aust. J. Grape Wine Res. 2002, 8, 76–83. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Villa, A.; Henschke, P.A. The chemical and sensorial effects of lysozyme addition to red and white wines over six months’ cellar storage. Aust. J. Grape Wine Res. 2004, 10, 143–150. [Google Scholar] [CrossRef]
- Weber, P.; Kratzin, H.; Brockow, K.; Ring, J.; Steinhart, H.; Paschke, A. Lysozyme in wine: A risk evaluation for consumers allergic to hen’s egg. Mol. Nutr. Food Res. 2009, 53, 1469–1477. [Google Scholar] [CrossRef]
- Sonni, F.; Bastante, M.J.C.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- Nieto-Rojo, R.; Luquin, A.; Ancin-Azpilicueta, C. Improvement of wine aromatic quality using mixtures of lysozyme and dimethyl dicarbonate, with low SO2 concentration. Food Addit. Contam. 2015, 32, 1965–1975. [Google Scholar]
- Renouf, V.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethlydicarbonate to prevent Brettanomyces bruxellensis growth in wine. Food Control 2008, 19, 208–216. [Google Scholar] [CrossRef]
- Delfini, C.; Gaia, P.; Schellino, R.; Strano, M.; Pagliara, A.; Ambrò, S. Fermentability of Grape Must after Inhibition with Dimethyl Dicarbonate (DMDC). J. Agric. Food Chem. 2002, 50, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Daudt, C.E.; Ough, C.S. Action of dimethyldicarbonate on various yeasts. Am. J. Enol. Vitic. 1980, 31, 21–23. [Google Scholar]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Porter, L.J.; Ough, C.S. The effects of ethanol, temperature, and dimethyl dicarbonate on viability of Saccharomyces cerevisiae Montrachet No. 522 in wine. Am. J. Enol. Vitic. 1982, 33, 222–225. [Google Scholar]
- Zuehlke, J.M.; Glawe, D.A.; Edwards, C.G. Efficacy of Dimethyl Dicarbonate Against Yeasts Associated with Washington State Grapes and Wines. J. Food Process. Preserv. 2015, 39, 1016–1026. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Eder, R. Application of chitosan and chitin-glucan for the protection of must and wine. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwert. 2012, 62, 108–116. [Google Scholar]
- Chagas, R.; Monteiro, S.; Ferreira, R.B. Assessment of potential effects of common fining agents used for white wine protein stabilization. Am. J. Enol. Vitic. 2012, 63, 574–578. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Gassara, F.; Antzak, C.; Ajila, C.M.; Sarma, S.J.; Brar, S.K.; Verma, M. Chitin and chitosan as natural flocculants for beer clarification. J. Food Eng. 2015, 166, 80–85. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Clarification of fruit juice with chitosan. Process Biochem. 2004, 39, 2229–2232. [Google Scholar] [CrossRef]
- Rungsardthong, V.; Wongvuttanakul, N.; Kongpien, N.; Chotiwaranon, P. Application of fungal chitosan for clarification of apple juice. Process Biochem. 2006, 41, 589–593. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Riponi, C. Efficacy of chitosan in inhibiting the oxidation of (+)-catechin in white wine model solutions. J. Agric. Food Chem. 2014, 62, 9868–9875. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. Eur. Food Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- El Khoury, A.E.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Kurtbay, H.M.; Bekçi, Z.; Merdivan, M.; Yurdakoç, K. Reduction of ochratoxin a levels in red wine by bentonite, modified bentonites, and chitosan. J. Agric. Food Chem. 2008, 56, 2541–2545. [Google Scholar] [CrossRef]
- Quintela, S.; Villarán, M.C.; López De Armentia, I.; Elejalde, E. Ochratoxin a removal from red wine by several oenological fining agents: Bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit. Contam. 2012, 29, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.; Li, C.; Lee, C.; Chen, H. Influence of micronized chitosan on antioxidative activities in grape juice. Food Sci. Nutr. 2013, 4, 224–228. [Google Scholar] [CrossRef]
- Castro, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thétiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Castro Marín, A.; Colangelo, D.; Lambri, M.; Riponi, C.; Chinnici, F. Relevance and perspectives of the use of chitosan in winemaking: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3450–3464. [Google Scholar] [CrossRef] [PubMed]
- Scansani, S.; Rauhut, D.; Brezina, S.; Semmler, H.; Benito, S. The Impact of Chitosan on the Chemical Composition of Wines Fermented with Schizosaccharomyces pombe and Saccharomyces cerevisiae. Foods 2020, 9, 1423. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.; Galvao, K.N.; Jeong, K.C. Underlying Mechanism of Antimicrobial Activity of Chitosan Microparticles and Implications for the Treatment of Infectious Diseases. PLoS ONE 2014, 9, 92723. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.R.; Hardwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Ralston, G.B.; Tracey, M.V.; Wrench, P.M. The inhibition of fermentation in baker’s yeast by chitosan. Biochim. Biophys. Acta 1964, 93, 652–655. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Gómez-Rivas, L.; Escudero-Abarca, B.I.; Aguilar-Uscanga, M.G.; Hayward-Jones, P.M.; Mendoza, P.; Ramírez, M. Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J. Ind. Microbiol. Biotechnol. 2004, 31, 16–22. [Google Scholar] [PubMed]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Boorsma, A.; Delneri, D.; Brul, S.; Oliver, S.G.; Klis, F.M. Cellular processes and pathways that protect Saccharomyces cerevisiae cells against the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2007, 6, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Lopez-Llorca, L. Omics for investigating chitosan as an antifungal and gene modulator. J. Fungi 2016, 2, 11. [Google Scholar] [CrossRef]
- Picariello, L.; Rinaldi, A.; Blaiotta, G.; Moio, L.; Pirozzi, P.; Gambuti, A. Effectiveness of chitosan as an alternative to sulfites in red wine production. Eur. Food Res. Technol. 2020, 246, 1795–1804. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef]
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of a fungal-origin chitosan preparation on Brettanomyces bruxellensis in the context of wine aging. Wine Stud. 2014, 3, 13–15. [Google Scholar] [CrossRef]
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. Oeno One 2016, 50, 49–56. [Google Scholar] [CrossRef]
- Portugal, C.; Sáenz, Y.; Rojo-Bezares, B.; Zarazaga, M.; Torres, C.; Cacho, J.; Ruiz-Larrea, F. Brettanomyces susceptibility to antimicrobial agents used in winemaking: In vitro and practical approaches. Eur. Food Res. Technol. 2014, 238, 641–652. [Google Scholar] [CrossRef]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2015, 118, 123–131. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, D.; Costa, E.M.; Silva, S.; Pintado, M.M.; Couto, J.A. The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J. Chitin Chitosan Sci. 2013, 1, 240–245. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Pittman, S.J.; McAlpine, C.A. Movements of marine fish and decapod crustaceans: Process, theory and application. Adv. Mar. Biol. 2003, 44, 205–294. [Google Scholar]
- Gillett, R. Global study of shrimp fisheries. FAO Fish. Tech. Pap. 2008, 475, 331. [Google Scholar]
- Faber, M.A.; Pascal, M.; El Kharbouchi, O.; Sabato, V.; Hagendorens, M.M.; Decuyper, I.I.; Bridts, C.H.; Ebo, D.G. Shellfish allergens: Tropomyosin and beyond. Allergy 2016, 72, 842–848. [Google Scholar] [CrossRef]
- Chitosan Market Size, Share and Industry Analysis Report by Source (Shrimps, Prawns, Crabs, Lobsters, Fungi) and End-User (Water Treatment, Cosmetics & Toiletries, Food & Beverage, Healthcare/Medical, Agrochemical, Biotechnology), Regional Outlook, Growth Potential, Competitive Market Share & Forecast, 2021–2027. Available online: https://www.gminsights.com/industry-analysis/chitosan-market (accessed on 12 August 2022).
- Chitin and Chitosan Derivates World Market Report. Available online: https://www.strategyr.com/market-report-chitin-and-chitosan-derivatives-forecasts-global-industry-analysts-inc.asp (accessed on 12 August 2022).
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Nijdam, D.; Rood, T.; Westhoek, H. The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 2012, 37, 760–770. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- MacLeod, M.; Leinonen, I.; Wall, E.; Houdijk, J.G.M.; Eory, V.; Burns, J.B.; Vosough Ahmadi, B.; Gomez-Barbero, M. Impact of Animal Breeding on GHG Emissions and Farm Economics; (EUR 29844 EN ed.); European Commission: Brussels, Belgium, 2019. [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Jucker, C.; Lupi, D.; Moore, C.D.; Leonardi, M.G.; Savoldelli, S. Nutrient recapture from insect farm waste: Bioconversion with Hermetia illucens (L.) (Diptera: Stratiomyidae). Sustainability 2020, 12, 362. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.M.; Caniani, D.; Masi, S.; Falabella, P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectol. 2022, 75, 75–82. [Google Scholar]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects 2022, 13, 41. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of the chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Food Allergies. Available online: https://www.fda.gov/food/food-labeling-nutrition/food-allergies (accessed on 12 August 2022).
- de Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Vanzo, A.; Nemanic, J. Effect of red wine maceration techniques on oligomeric and polymeric proanthocyanidins in wine, cv. Blaufränkisch. Vitis 2002, 41, 47–51. [Google Scholar]
- Sacchi, K.L.; Visón, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–205. [Google Scholar]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; Bartolomé, B.; Martínez-Rodríguez, A.; Pueyo, E.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control 2008, 19, 835–841. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols form grape. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, I.G. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Johnston, M.D.; Hanlon, G.W.; Denyer, S.P.; Lambert, R.J.W. Membrane damage to bacteria caused by single and combined biocides. J. Appl. Microbiol. 2003, 94, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stivala, M.G.; Villecco, M.B.; Enriz, D.; Fernandez, P.A. Effect of phenolic compounds on viability of wine spoilage lactic acid bacteria. A structure-activity relationship study. Am. J. Enol. Vitic. 2017, 68, 228–233. [Google Scholar] [CrossRef]
- Esparza, I.; Martínez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.; Martín-Álvarez, P.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef]
- Alberto, M.R.; Farías, M.E.; Manca de Nadra, M.C. Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J. Agric. Food Chem. 2001, 49, 4359–4363. [Google Scholar] [CrossRef]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Moreno-Rojas, J.M.; Zafrilla, P.; Cayuela, J.M.; Mulero, J.; Puertas, B.; Guerrero, R.F.; Piñeiro, Z.; Giron, F.; et al. Efficacy of olive oil mill extract in replacing sulfur dioxide in wine model. LWT Food Sci. Technol. 2015, 61, 117–123. [Google Scholar] [CrossRef]
- EFSA, Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage. Pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033–2078. [Google Scholar]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; García-Romero, E.; Huertas-Nebreda, B.; Gómez-Alonso, S. Colloidal silver complex as an alternative to sulphur dioxide in winemaking. Food Control 2012, 23, 73–81. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Crespo, J.; López-de-Luzuriaga, J.M.; Olmos, M.E.; Monge, M.; Rodríguez-Álfaro, M.P.; Martín-Alvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. Novel biocompatible silver nanoparticles for controlling the growth of lactic acid bacteria and acetic acid bacteria in wines. Food Control 2015, 50, 613–619. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Monge, M.; Miralles, B.; Armentia, G.; Cueva, C.; Crespo, J.; Luzuriaga, J.M.L.; Olmos, M.E.; Bartolomé, B.; Llano, D.G.; et al. Some new findings on the potential use of biocompatible silver nanoparticles in winemaking. Innov. Food Sci. Emerg. Technol. 2019, 51, 64–72. [Google Scholar] [CrossRef]
| Process Phase | Times of SO2 Addition | SO2 Action |
|---|---|---|
| Grape and Must | Before the start of alcoholic fermentation |
|
| Wine | Filtration, decanting, and aging |
|
| Before bottling |
|
| Compound | Chemical Structure | Admitted Amount | Winemaking Stage | Antimicrobial Activity |
|---|---|---|---|---|
| Sorbic acid | 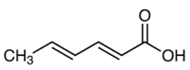 | 200 mg/L | Wine storage of sweet wines | Yeasts (S. cerevisiae, Candida spp.) in association with SO2 [7,49] |
| Lysozyme * | 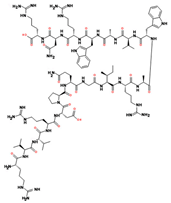 | 500 mg/L (considered as cumulative, taking into account any additions to the must) |
| Gram-positive bacteria (not active against Gram-negative bacteria and the yeast cell) [50] |
| Dimethyl dicarbonate (DMDC) | 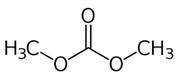 | 200 mg/L (with no residues in the marketed wine) | Prior to bottling in wine with sugar content ≥5 g/L | Yeasts (Zygosaccharomyces bailii, Zygoascus hellenicus, and Lachancea thermotolerans) [51] |
| Chitosan |  | 10 g/hL |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, F.; Siesto, G.; Pietrafesa, R.; Romano, P.; Salvia, R.; Scieuzo, C.; Falabella, P.; Capece, A. Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications. Beverages 2022, 8, 58. https://doi.org/10.3390/beverages8030058
Tedesco F, Siesto G, Pietrafesa R, Romano P, Salvia R, Scieuzo C, Falabella P, Capece A. Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications. Beverages. 2022; 8(3):58. https://doi.org/10.3390/beverages8030058
Chicago/Turabian StyleTedesco, Francesco, Gabriella Siesto, Rocchina Pietrafesa, Patrizia Romano, Rosanna Salvia, Carmen Scieuzo, Patrizia Falabella, and Angela Capece. 2022. "Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications" Beverages 8, no. 3: 58. https://doi.org/10.3390/beverages8030058
APA StyleTedesco, F., Siesto, G., Pietrafesa, R., Romano, P., Salvia, R., Scieuzo, C., Falabella, P., & Capece, A. (2022). Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications. Beverages, 8(3), 58. https://doi.org/10.3390/beverages8030058