Functional Beverages in the 21st Century
Abstract
1. Introduction
2. The Role of Water in Human Life
- Intercellular (inside the cell);
- Extracellular (blood plasma, lymph);
- Transcellular (body water contained in the epithelial-lined spaces) [22].
3. Drinks for Athletes: Are We Sure about That?
- Sports and Performance Drinks
- Energy drinks
- Ready-to-drink teas
- Milk-based drinks
- Fortified fruit drinks
- Soy-based drinks
- Fortified water
4. Composition of Sports Drinks
5. Composition of Energy Drinks
- -
- -
- -
6. The Difference between Isotonic Sports Drinks and Energy Drinks
7. Pros and Cons of Sports and Energy Drinks
8. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, U.; Mumtaz, R.; Anwar, H.; Mumtaz, S.; Qamar, A.M. Water Quality Monitoring: From Conventional to Emerging Technologies. Water Sci. Technol. Water Supply 2020, 20, 28–45. [Google Scholar] [CrossRef]
- Calderon, R.L. The Epidemiology of Chemical Contaminants of Drinking Water. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2000, 38, S13–S20. [Google Scholar] [CrossRef]
- Starzak, M.; Mathlouthi, M. Cluster Composition of Liquid Water Derived from Laser-Raman Spectra and Molecular Simulation Data. Food Chem. 2003, 82, 3–22. [Google Scholar] [CrossRef]
- Bjerrum, N. Structure and Properties of Ice. Science 1952, 115, 385–390. [Google Scholar] [CrossRef]
- Smith, L. Chapter Twelve—Historical Perspectives on Water Purification. In Chemistry and Water; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 421–468. ISBN 978-0-12-809330-6. [Google Scholar]
- Cahoon, L.B. Chapter 15—Water Purification: Treatment of Microbial Contamination. In Advances in Water Purification Techniques; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 385–395. ISBN 978-0-12-814790-0. [Google Scholar]
- Owa, F.D. Water Pollution: Sources, Effects, Control and Management. Mediterr. J. Soc. Sci. 2013, 4, 65. [Google Scholar] [CrossRef]
- Pandey, S. Water Pollution and Health. Kathmandu Univ. Med. J. 2006, 4, 128–134. [Google Scholar]
- Revenga, C.; Mock, G. Dirty Water: Pollution Problems Persist. Pilot Anal. Glob. Ecosyst. Freshw. Syst. 2001, 6, ISBN 1-56973-460-7. [Google Scholar]
- Anthonj, C.; Diekkrüger, B.; Borgemeister, C. Thomas Kistemann Health Risk Perceptions and Local Knowledge of Water-Related Infectious Disease Exposure among Kenyan Wetland Communities. Int. J. Hyg. Environ. Health 2019, 222, 34–48. [Google Scholar] [CrossRef]
- Wen, X.; Chen, F.; Lin, Y.; Zhu, H.; Yuan, F.; Kuang, D.; Jia, Z.; Yuan, Z. Microbial Indicators and Their Use for Monitoring Drinking Water Quality—A Review. Sustainability 2020, 12, 2249. [Google Scholar] [CrossRef]
- Cha, Y.; Park, S.S.; Kim, K.; Byeon, M.; Stow, C.A. Water resources of the Black Sea Basin at high spatial and temporal. Water Resour. Res. 2014, 10, 5375–5377. [Google Scholar] [CrossRef]
- Linden, K.G.; Mohseni, M. Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment; Springer: Cham, Switzerland, 2019; Volume 67, ISBN 978-3-319-76881-6. [Google Scholar]
- Cidu, R.; Frau, F.; Tore, P. Drinking Water Quality: Comparing Inorganic Components in Bottled Water and Italian Tap Water. J. Food Compos. Anal. 2011, 24, 184–193. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Qu, Y.; Chen, H.; Zhou, X.; Hong, H.; Sun, H.; Lin, H.; Deng, W.; Wu, F. Using Simple and Easy Water Quality Parameters to Predict Trihalomethane Occurrence in Tap Water. Chemosphere 2022, 286, 131586. [Google Scholar] [CrossRef] [PubMed]
- Ober, J.; Karwot, J. Tap Water Quality: Seasonal User Surveys in Poland. Energies 2021, 14, 3841. [Google Scholar] [CrossRef]
- Sofi, M.H.; Gudi, R.; Karumuthil-Melethil, S.; Perez, N.; Johnson, B.M.; Vasu, C. PH of Drinking Water Influences the Composition of Gut Microbiome and Type 1 Diabetes Incidence. Diabetes 2014, 63, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Johnston, N. Potential Benefits of PH 8.8 Alkaline Drinking Water as an Adjunct in the Treatment of Reflux Disease. Ann. Otol. Rhinol. Laryngol. 2012, 121, 431–434. [Google Scholar] [CrossRef]
- Cabral, J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Rodríguez-Lázaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.J.; D’Agostino, M.; Santos, R.; Saiz, J.C.; Rzeżutka, A.; et al. Virus Hazards from Food, Water and Other Contaminated Environments. FEMS Microbiol. Rev. 2012, 36, 786–814. [Google Scholar] [CrossRef]
- Hikal, W.M. Parasitic Contamination of Drinking Water and Egyptian Standards for Parasites in Drinking Water. Open J. Ecol. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Schoeller, D.A. Changes in Total Body Water with Age. Am. J. Clin. Nutr. 1989, 50, 1176–1181. [Google Scholar] [CrossRef]
- Shimamoto, H.; Komiya, S. The Turnover of Body Water as an Indicator of Health. J. Physiol. Anthropol. Appl. Human Sci. 2000, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, P.M. Role of Water in Some Biological Processes. Microbiol. Rev. 1990, 54, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Gana, F.M. Sources and function of a water in the human body. Int. J. Adv. Sci. Res. 2022, 2, 22–27. [Google Scholar] [CrossRef]
- Kleiner, S.M. Water: An Essential but Overlooked Nutrient. J. Am. Diet. Assoc. 1999, 99, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Karppanen, H.; Karppanen, P.; Mervaala, E. Why and How to Implement Sodium, Potassium, Calcium, and Magnesium Changes in Food Items and Diets? J. Hum. Hypertens. 2005, 19, S10–S19. [Google Scholar] [CrossRef]
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Blanuša, M.; Orlić, Ž.C.; Orct, T.; Kostial, K. Comparison of Calcium, Magnesium, Sodium, Potassium, Zinc, and Creatinine Concentration in 24-h and Spot Urine Samples in Women. Clin. Chem. Lab. Med. 2009, 47, 216–221. [Google Scholar] [CrossRef]
- Wenk, C.; Kuhnt, M.; Kunz, P.; Steiner, G. Methodological Studies of the Estimation of Loss of Sodium, Potassium, Calcium and Magnesium through the Skin during a 10 Km Run. Z. Ernahrungswiss. 1993, 32, 301–307. [Google Scholar] [CrossRef]
- Sutehall, S.; Muniz-Pardos, B.; Bosch, A.N.; Di Gianfrancesco, A.; Pitsiladis, Y.P. Sports Drinks on the Edge of a New Era. Curr. Sports Med. Rep. 2018, 17, 112–116. [Google Scholar] [CrossRef]
- Jiménez, S.L.; Díaz-Lara, J.; Pareja-Galeano, H.; Del Coso, J. Caffeinated Drinks and Physical Performance in Sport: A Systematic Review. Nutrients 2021, 13, 2944. [Google Scholar] [CrossRef]
- Meenakshi Sharma Mineral Supplements, Multivitamins, and Energy Drinks Are Commonly Consumed. Soc. Sci. J. Adv. Res. 2021, 1, 10–14. [CrossRef]
- Placentino, U.; Sogari, G.; Viscecchia, R.; De Devitiis, B.; Monacis, L. The New Challenge of Sports Nutrition: Accepting Insect Food as Dietary Supplements in Professional Athletes. Foods 2021, 10, 1117. [Google Scholar] [CrossRef]
- Agnihotri, A.; Bhattacharya, S. “BodyArmor Versus Gatorade: A Proxy Cola War?”. In SAGE Business Cases; SAGE Publications Ltd.: London, UK, 2023. [Google Scholar] [CrossRef]
- Jäger, R.; Harris, R.C.; Purpura, M.; Francaux, M. Comparison of new forms of creatine in raising plasma creatine levels. J. Int. Soc. Sports Nutr. 2007, 4, 17. [Google Scholar] [CrossRef]
- Fields, S.K.; Macdonald, J.; Joseph, A.M.; Wold, L.E.; Collins, C.L.; Dawn Comstock, R. Consumption of Sports and Energy Drinks by High School Athletes in the United States: A Pilot Study. Beverages 2015, 1, 218–224. [Google Scholar] [CrossRef]
- Bonetti, D.L.; Hopkins, W.G. Effects of Hypotonic and Isotonic Sports Drinks on Endurance Performance and Physiology. Sportscience 2010, 14, 63–70. [Google Scholar]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The Hydrating Effects of Hypertonic, Isotonic and Hypotonic Sports Drinks and Waters on Central Hydration During Continuous Exercise: A Systematic Meta-Analysis and Perspective. Sport. Med. 2022, 52, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lagunas, V.; Ding, Z.; Bernard, J.R.; Wang, B.; Ivy, J.L. Added Protein Maintains Efficacy of a Low-Carbohydrate Sports Drink. J. Strength Cond. Res. 2010, 24, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Bonetti, D.L.; Hopkins, W.G. Unilateral Fluid Absorption and Effects on Peak Power after Ingestion of Commercially Available Hypotonic, Isotonic, and Hypertonic Sports Drinks. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 480–491. [Google Scholar] [CrossRef]
- Colakoglu, F.F.; Cayci, B.; Yaman, M.; Karacan, S.; Gonulateş, S.; Ipekoglu, G.; Er, F. The Effects of the Intake of an Isotonic Sports Drink before Orienteering Competitions on Skeletal Muscle Damage. J. Phys. Ther. Sci. 2016, 28, 3200–3204. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.; Świderski, F.; Kulik, K.; Waszkiewicz-Robak, B. Designing Functional Fruit-Based Recovery Drinks in Powder Form That Contain Electrolytes, Peptides, Carbohydrates and Prebiotic Fiber Taking into Account Each Component’s Osmolality. Molecules 2021, 26, 5607. [Google Scholar] [CrossRef] [PubMed]
- Leśniewicz, A.; Grzesiak, M.; Żyrnicki, W.; Borkowska-Burnecka, J. Mineral Composition and Nutritive Value of Isotonic and Energy Drinks. Biol. Trace Elem. Res. 2016, 170, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Bechke, E.E.; Zaplatosch, M.E.; Choi, J.Y.; Adams, W.M. Utility of an Isotonic Beverage on Hydration Status and Cardiovascular Alterations. Nutrients 2022, 14, 1286. [Google Scholar] [CrossRef]
- Tomczyk, M.; Zaguła, G.; Dżugan, M. A Simple Method of Enrichment of Honey Powder with Phytochemicals and Its Potential Application in Isotonic Drink Industry. LWT 2020, 125, 109204. [Google Scholar] [CrossRef]
- Styburski, D.; Dec, K.; Baranowska-Bosiacka, I.; Goschorska, M.; Hołowko, J.; Żwierełło, W.; Skórka-Majewicz, M.; Janda, K.; Rosengardt, A.; Gutowska, I. Can Functional Beverages Serve as a Substantial Source of Macroelements and Microelements in Human Nutrition?—Analysis of Selected Minerals in Energy and Isotonic Drinks. Biol. Trace Elem. Res. 2020, 197, 341–348. [Google Scholar] [CrossRef]
- Sidery, M.B.; Macdonald, I.A. The Effect of Meal Size on the Cardiovascular Responses to Food Ingestion. Br. J. Nutr. 1994, 71, 835–848. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A.; Tran, C.; Paquot, N. Fructose and Metabolic Diseases: New Findings, New Questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for Training and Competition. J. Sports Sci. 2011, 29 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef]
- Urdampilleta, A.; Gómez-Zorita, S.; Soriano, J.M.; Martínez-Sanz, J.M.; Medina, S.; Gil-Izquierdo, A. Hydration and Chemical Ingredients in Sport Drinks: Food Safety in the European Context. Nutr. Hosp. 2015, 31, 1889–1899. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Moseley, L.; Mainwaring, G.I.; Samuels, S.; Perry, S.; Mann, C.H. Exogenous Carbohydrate Oxidation during Ultraendurance Exercise. J. Appl. Physiol. 2006, 100, 1134–1141. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Thorburn, M.S.; Thorp, R.M.; Broadbent, S.; Shi, X. Effect of Graded Fructose Coingestion with Maltodextrin on Exogenous 14C-Fructose and 13C-Glucose Oxidation Efficiency and High-Intensity Cycling Performance. J. Appl. Physiol. 2008, 104, 1709–1719. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Rowlands, D.S. Fructose-Maltodextrin Ratio in a Carbohydrate-Electrolyte Solution Differentially Affects Exogenous Carbohydrate Oxidation Rate, Gut Comfort, and Performance. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 181–189. [Google Scholar] [CrossRef]
- Coombes, J.S.; Hamilton, K.L. The Effectiveness of Commercially Available Sports Drinks. Sport. Med. 2000, 29, 181–209. [Google Scholar] [CrossRef]
- Shirreffs, S.M. Hydration in Sport and Exercise: Water, Sports Drinks and Other Drinks. Nutr. Bull. 2009, 34, 374–379. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Halder, S.; Daw, S. Importance of sports drinks as a performance prerequisites. Senhri J. Multidiscip. Stud. 2020, 5, 2582–6840. [Google Scholar] [CrossRef]
- Jacob, S. Consumption Pattern of Nutritional Health Drinks and Energy Drinks among University Students in Ajman, UAE. Gulf Med. J. 2013, 2, 22–26. [Google Scholar]
- Visram, S.; Cheetham, M.; Riby, D.M.; Crossley, S.J.; Lake, A.A. Consumption of Energy Drinks by Children and Young People: A Rapid Review Examining Evidence of Physical Effects and Consumer Attitudes. BMJ Open 2016, 6, e010380. [Google Scholar] [CrossRef]
- Tahmassebi, J.F.; BaniHani, A. Impact of Soft Drinks to Health and Economy: A Critical Review. Eur. Arch. Paediatr. Dent. 2020, 21, 109–117. [Google Scholar] [CrossRef]
- Wardle, J.; Huon, G. An Experimental Investigation of the Influence of Health Information on Children’s Taste Preferences. Health Educ. Res. 2000, 15, 39–44. [Google Scholar] [CrossRef]
- Angeles-agdeppa, I.; Kurilich, A.C.; Harjani, Y.; Capanzana, M.V. The Effect of a Nutrient Fortified Oat Drink on Iron, Zinc, Vitamin A, and Vitamin C Status among Filipino Children. World Acad. Sci. Eng. Technol. 2012, 70, 1119–1127. [Google Scholar]
- Obuzor, G.U.; Ajaezi, N.E. Nutritional Content of Popular Malt Drinks Produced in Nigeria. Afr. J. Food Sci. 2010, 4, 585–590. [Google Scholar]
- Tynes, M.; Hepprich, M.; Timper, K. Regular Intake of Energy Drinks and Multivitamin Supplements Is Associated with Elevated Plasma Vitamin B6 Levels in Post-Bariatric Patients. Sci. Rep. 2021, 11, 17830. [Google Scholar] [CrossRef]
- Reid, J.L.; McCrory, C.; White, C.M.; Martineau, C.; Vanderkooy, P.; Fenton, N.; Hammond, D. Consumption of Caffeinated Energy Drinks Among Youth and Young Adults in Canada. Prev. Med. Rep. 2017, 5, 65–70. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy Beverages: Content and Safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef]
- Acheson, K.J.; Zahorska-Markiewicz, B.; Pittet, P.; Anantharaman, K.; Jéquier, E. Caffeine and Coffee: Their Influence on Metabolic Rate and Substrate Utilization in Normal Weight and Obese Individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Alford, C.; Cox, H.; Wescott, R. The Effects of Red Bull Energy Drink on Human Performance and Mood. Amino Acids 2001, 21, 139–150. [Google Scholar] [CrossRef]
- Grgic, J.; Mikulic, P. Caffeine Ingestion Acutely Enhances Muscular Strength and Power but Not Muscular Endurance in Resistance-Trained Men. Eur. J. Sport Sci. 2017, 17, 1029–1036. [Google Scholar] [CrossRef]
- Caine, J.J.; Geracioti, T.D. Taurine, Energy Drinks, and Neuroendocrine Effects. Cleve. Clin. J. Med. 2016, 83, 895–904. [Google Scholar] [CrossRef]
- Sawabe, Y.; Tagami, T.; Yamasaki, K. Determination of Taurine in Energy Drinks by HPLC Using a Pre-Column Derivative. J. Health Sci. 2008, 54, 661–664. [Google Scholar] [CrossRef]
- Lage-Yusty, M.A.; Villar-Blanco, L.; López-Hernandez, J. Evaluation of Caffeine, Vitamins and Taurine in Energy Drinks. J. Food Nutr. Res. 2019, 58, 107–114. [Google Scholar]
- Doerner, J.M.; Kuetting, D.L.; Luetkens, J.A.; Naehle, C.P.; Dabir, D.; Homsi, R.; Nadal, J.; Schild, H.H.; Thomas, D.K. Caffeine and Taurine Containing Energy Drink Increases Left Ventricular Contractility in Healthy Volunteers. Int. J. Cardiovasc. Imaging 2015, 31, 595–601. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Galan, B.S.M.; Santos, P.C.; Pritchett, K.; Pfrimer, K.; Ferriolli, E.; Papoti, M.; Marchini, J.S.; de Freitas, E.C. Taurine: A Potential Ergogenic Aid for Preventing Muscle Damage and Protein Catabolism and Decreasing Oxidative Stress Produced by Endurance Exercise. Front. Physiol. 2017, 8, 710. [Google Scholar] [CrossRef]
- Schimpl, F.C.; da Silva, J.F.; de Carvalho Gonçalves, J.F.; Mazzafera, P. Guarana: Revisiting a Highly Caffeinated Plant from the Amazon. J. Ethnopharmacol. 2013, 150, 14–31. [Google Scholar] [CrossRef]
- Moustakas, D.; Mezzio, M.; Rodriguez, B.R.; Constable, M.A.; Mulligan, M.E.; Voura, E.B. Guarana Provides Additional Stimulation over Caffeine Alone in the Planarian Model. PLoS ONE 2015, 10, e0123310. [Google Scholar] [CrossRef]
- Patrick, M.; Kim, H.A.; Oketch-Rabah, H.; Marles, R.J.; Roe, A.L.; Calderón, A.I. Safety of Guarana Seed as a Dietary Ingredient: A Review. J. Agric. Food Chem. 2019, 67, 11281–11287. [Google Scholar] [CrossRef]
- Rath, M. Energy Drinks: What Is All the Hype? The Dangers of Energy Drink Consumption. J. Am. Acad. Nurse Pract. 2012, 24, 70–76. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Ascione, A. Guarana and Physical Performance: A Myth or Reality? 2020. J. Hum. Sport Exerc. 2020, 15 (Proc3), S539–S551. [Google Scholar] [CrossRef]
- Yang, M.S.; Wu, M.Y. Chinese Ginseng. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 693–705. ISBN 978-0-12-802147-7. [Google Scholar]
- Qi, B.; Zhang, L.; Zhang, Z.; Ouyang, J.; Huang, H. Effects of Ginsenosides-Rb1 on Exercise-Induced Oxidative Stress in Forced Swimming Mice. Pharmacogn. Mag. 2014, 10, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-W.; Xiao, S.-Y.; Wang, J.; Hou, W.; Wang, Y.-P. Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites. Molecules 2019, 24, 2985. [Google Scholar] [CrossRef]
- Duchan, E.; Patel, N.D.; Feucht, C. Energy Drinks: A Review of Use and Safety for Athletes. Phys. Sportsmed. 2010, 38, 171–179. [Google Scholar] [CrossRef]
- Ballard, S.L.; Wellborn-Kim, J.J.; Clauson, K.A. Effects of Commercial Energy Drink Consumption on Athletic Performance and Body Composition. Phys. Sportsmed. 2010, 38, 107–117. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Veasey, R.; Watson, A.; Dodd, F.; Jones, E.; Maggini, S.; Haskell, C.F. Effects of High-Dose B Vitamin Complex with Vitamin C and Minerals on Subjective Mood and Performance in Healthy Males. Psychopharmacology 2010, 211, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodriguez, R.; Pallarés, J.G. Performance Outcomes and Unwanted Side Effects Associated with Energy Drinks. Nutr. Rev. 2014, 72 (Suppl. S1), 108–120. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Kennedy, D.O. Cognitive and Physiological Effects of an “Energy Drink”: An Evaluation of the Whole Drink and of Glucose, Caffeine and Herbal Flavouring Fractions. Psychopharmacology 2004, 176, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Mix, J.A.; Crews, W.D.J. An Examination of the Efficacy of Ginkgo Biloba Extract EGb761 on the Neuropsychologic Functioning of Cognitively Intact Older Adults. J. Altern. Complement. Med. 2000, 6, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Lohninger, A. Supplementation of L-Carnitine in Athletes: Does It Make Sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef]
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. L-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef]
- Pomeranz, J.L.; Munsell, C.R.; Harris, J.L. Energy Drinks: An Emerging Public Health Hazard for Youth. J. Public Health Policy 2013, 34, 254–271. [Google Scholar] [CrossRef]
- Road, S.; Kingdom, U. Energy Drinks: What’ s the Evidence? Food Res. Collab. 2017, 44, 1–13. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Winiarska-Mieczan, A.; Kwiecień, M.; Klebaniuk, R.; Danek-Majewska, A.; Kowalczuk-Vasilev, E.; Kwiatkowski, P. Spożywanie Napojów Energetyzujących Przez Młodzież Województwa Lubelskiego Consumption of Energy Drinks by Teenagers in Lublin Province. Probl. Hig. Epidemiol. 2018, 99, 140–145. [Google Scholar]
- Shearer, J.; Graham, T.E. Performance Effects and Metabolic Consequences of Caffeine and Caffeinated Energy Drink Consumption on Glucose Disposal. Nutr. Rev. 2014, 72, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Żukiewicz-Sobczak, W.; Sobczak, P.; Siłuch, M.; Weiner, M.; Pawłowicz-Sosnowska, E.T.; Wojtyła-Buciora, P.; Chmielewski, J.; Żeber-Dzikowska, I.; Gworek, B.; Zagórski, J. Analysis of the Chemical Composition of Energy Drinks for Contents of Stimulant and Filling Compounds. Przem. Chem. 2018, 97, 560–564. [Google Scholar] [CrossRef]
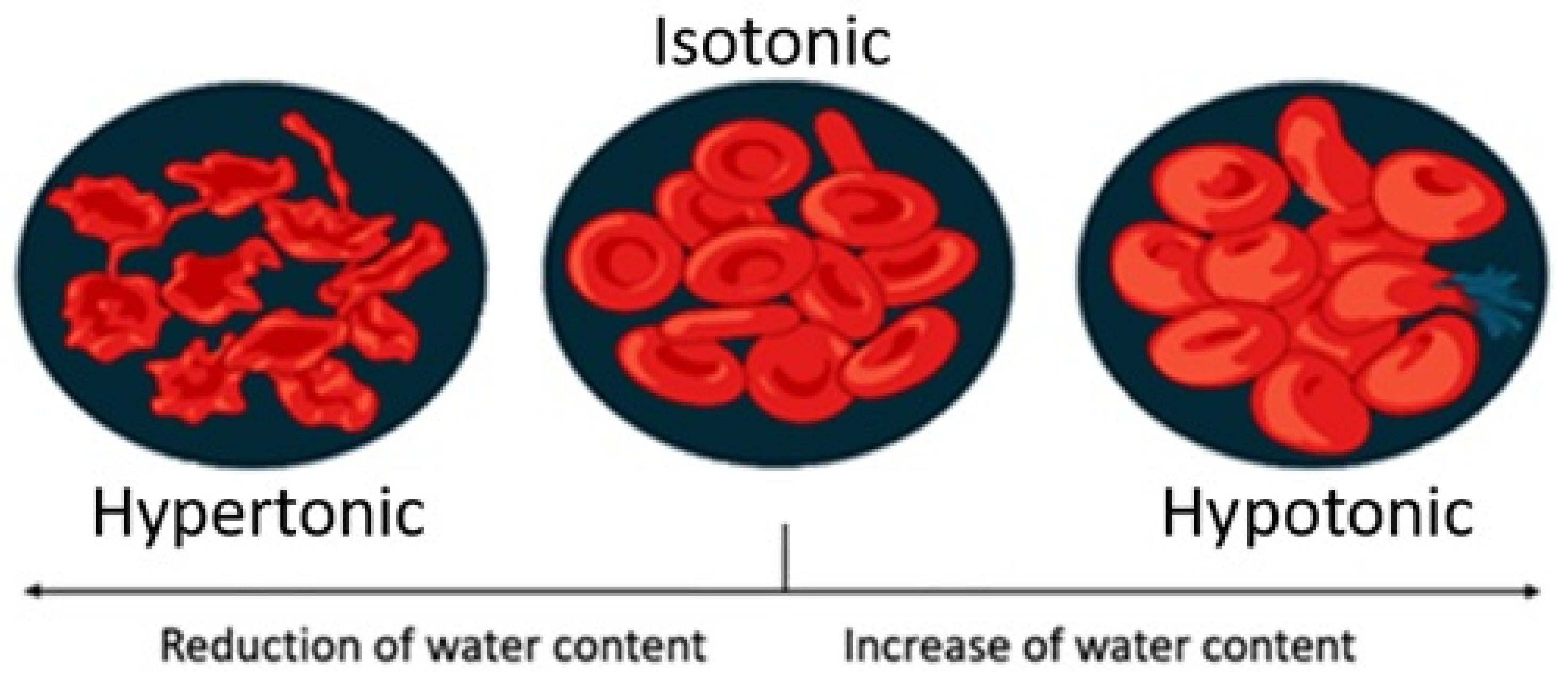
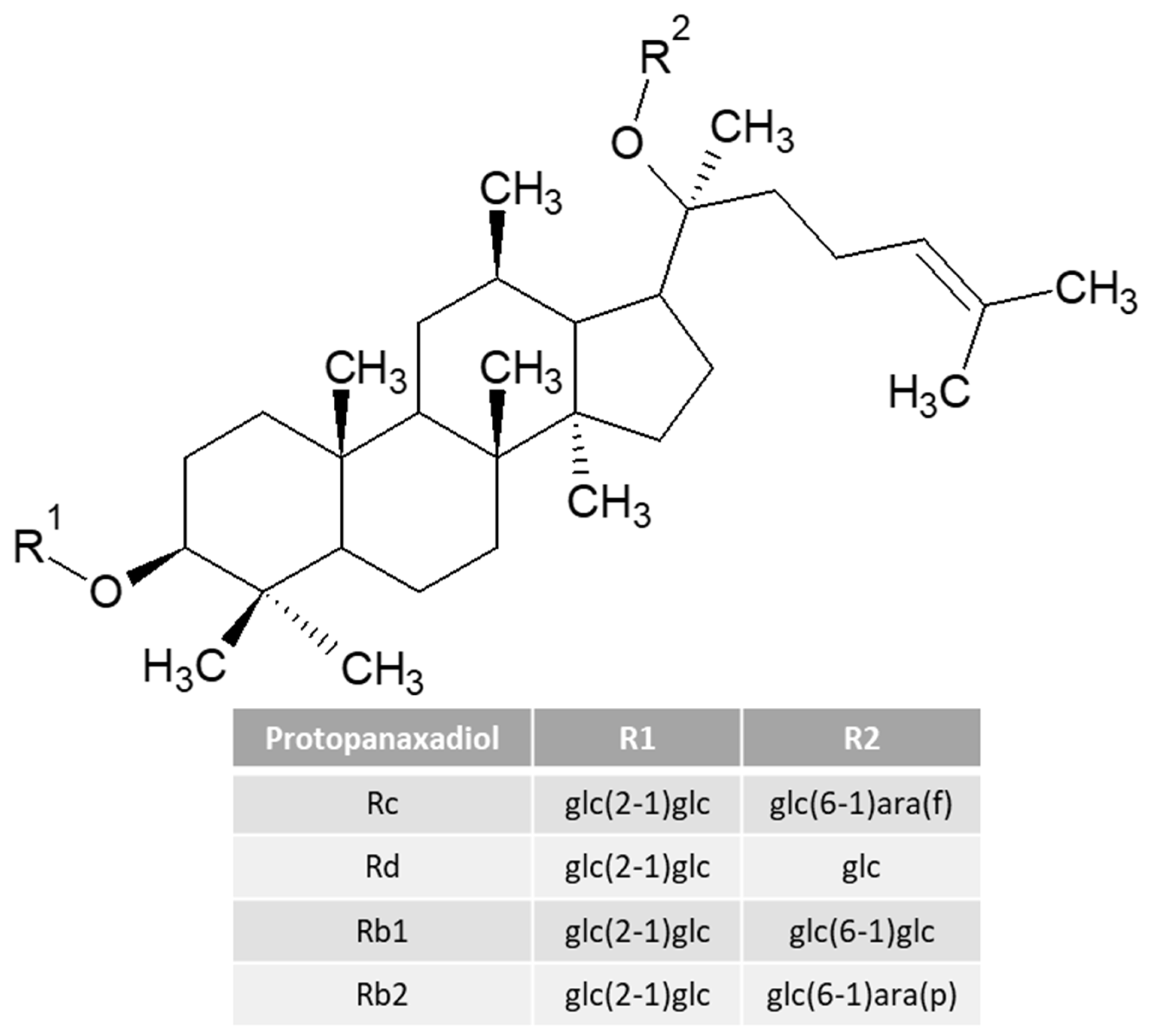
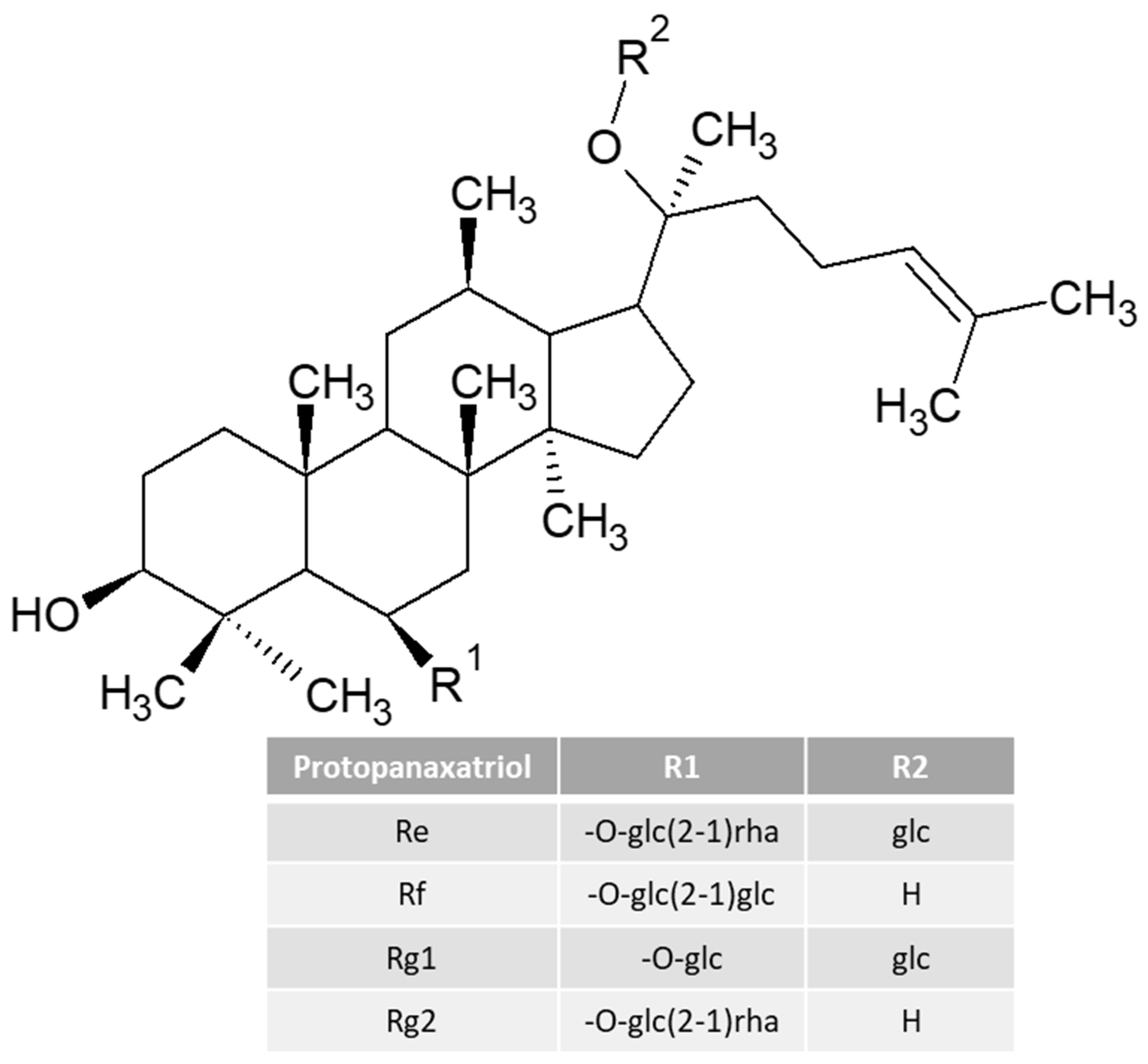

| Sport Beverages | Osmolarity | Beneficial Additional Ingredients | Amount of Carbohydrates Per 100 mL Beverage | Calories Per 100 mL | Example of a Drink | Recommendations for Consumption | References |
|---|---|---|---|---|---|---|---|
| Hypotonic drink | lower than body fluids | none (in fruit juices vitamins) | 0–6 g | 0–16 kcal | light solution of fruit juice in water, spring water, | before and during exercise | [38,39] |
| Isotonic drink | close to body fluids | vitamins, mineral salts | 6–10 g | 24–32 kcal | commercially available products | physical workers and pro- and hobbyist athletes, | [40,41] |
| Hypertonic drink | higher than body fluids | none (in fruit juices vitamins) | over 10 g | 40 kcal | cola, soft drinks, lemonade, fruit juice | after training for pro- and hobbyist athletes | [43,44,45] |
| Ingredient | 4 Move Zero (750 mL) | Powerade (500 mL) | Oshee (750 mL) | Crazy Wolf (500 mL) | Gatorade (591 mL) | Isostar (750 mL) |
|---|---|---|---|---|---|---|
| Carbohydrates (g) | 0.1 | 3.9 | 5.7 | 6.5 | 5.8 | 6.7 |
| Sodium (mg) | 20 | 50 | n.d. | 40 | 49 | 70 |
| Potassium (mg) | 13 | 12.5 | n.d. | n.d. | n.d. | n.d. |
| Calcium (mg) | 1.6 | 1.3 | n.d. | n.d. | n.d. | 32 |
| Magnesium (mg) | 0.7 | 0.6 | n.d. | n.d. | n.d. | 12 |
| Artificial sweeteners | Yes | Yes | Yes | Yes | No | No |
| Energy content (kJ) | 4.57 | 69 | 101 | 114 | 97 | 122 |
| Ingredient | Monster (500 mL) | Red Bull (250 mL) | Burn (250 mL) | Tiger (250 mL) | N-Gine (250 mL) | Max Force (250 mL) | Black (250 mL) |
|---|---|---|---|---|---|---|---|
| Carbohydrates (g) | 12 | 11 | 13.3 | 10.9 | 10.8 | 10.9 | 10.8 |
| Sodium (g) | 0.08 | 0.04 | 0.02 | 0.07 | 0.1 | 0.1 | 0.1 |
| Riboflavin (mg) | 0.7 | x | x | x | x | x | x |
| Pantothenic acid (mg) | x | 2 | 1.5 | 2 | 1.98 | 2 | 1.98 |
| Niacin (mg) | 8.5 | 8 | 6.5 | 7 | 7 | 6.4 | 7 |
| B6 (mg) | 0.8 | 2 | 0.3 | 1.4 | 1.4 | 0.56 | 1.4 |
| B12 (ug) | 2.5 | 2 | 0.38 | 0.5 | 0.5 | 0.5 | 0.5 |
| Taurine (mg) | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| Ginseng (mg) | 82 | x | x | x | x | x | x |
| L-carnitine (mg) | 42 | x | x | x | x | x | x |
| Caffeine (mg) | 32 | 32 | 32 | 32 | 32 | 30 | 32 |
| Energy content (kJ) | 203 | 192 | 242 | 197 | 196 | 198 | 195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugajski, M.; Buszewska-Forajta, M.; Buszewski, B. Functional Beverages in the 21st Century. Beverages 2023, 9, 27. https://doi.org/10.3390/beverages9010027
Sugajski M, Buszewska-Forajta M, Buszewski B. Functional Beverages in the 21st Century. Beverages. 2023; 9(1):27. https://doi.org/10.3390/beverages9010027
Chicago/Turabian StyleSugajski, Mateusz, Magdalena Buszewska-Forajta, and Bogusław Buszewski. 2023. "Functional Beverages in the 21st Century" Beverages 9, no. 1: 27. https://doi.org/10.3390/beverages9010027
APA StyleSugajski, M., Buszewska-Forajta, M., & Buszewski, B. (2023). Functional Beverages in the 21st Century. Beverages, 9(1), 27. https://doi.org/10.3390/beverages9010027








