Abstract
Recent social, economic, and technological evolutions have impacted consumption habits. The new consumer is more rational, more connected and demanding with products, more concerned with the management of the family budget, with the health, origin, and sustainability of food. The food industry over the last few years has shown remarkable technological and scientific evolution, with an impact on the development and innovation of new products using non-thermal processing. Non-thermal processing technologies involve methods by which fruit juices receive microbiological inactivation and enzymatic denaturation with or without the direct application of low heat, thereby lessening the adverse effects on the nutritional, bioactive, and flavor compounds of the treated fruit juices, extending their shelf-life. The recognition of the nutritional and protective values of fruit juices and fermented fruit beverages is evident and is attributed to the presence of different bioactive compounds, protecting against chronic and metabolic diseases. Fermentation maintains the fruit's safety, nutrition, and shelf life and the development of new products. This review aims to summarize the chemical and sensory characteristics of fruit juices and fermented fruit drinks, the fermentation process, its benefits, and its effects.
1. Introduction
The current trend in the food industry is to develop new products that present quality and food safety characteristics, responding to the needs and preferences of consumers. When adopting a more sustainable lifestyle, consumers are increasingly demanding, giving preference to natural, healthier, innovative, and tastier products with nutraceutical and sustainable characteristics and with a minimum amount of chemical preservatives and/or processing technologies [1,2]. As such, the sustainable development of the functional food market is increasingly evident, in which fruit juices and fermented fruit beverages begin to occupy a prominent place [3]. According to Corbo et al. [4], in 2008, the food industry presented an expected growth of between 2–3%, with the functional food market presenting an expectation of approximately 10%. This fact could be explained partially by the growing number of lactose-intolerant consumers, dairy allergies, the preference for products with low cholesterol content, and the growing trend of vegetarianism, which leads consumers to avoid the consumption of dairy drinks [5,6,7]. Also, the tendency of consumers to avoid highly sugary products is not insignificant in the valorization of functional food and beverages [8]. Several studies have been carried out highlighting the beneficial health effects of integrating fruit into the human diet as a supply of dietary nutrients and bioactive compounds, including dietary fiber, vitamins, minerals, polyphenols, flavonoids, monoterpenes, and bioactive peptides [8,9,10,11]. Thus, the intake of fruit has a notable effect on the prevention of signs of aging, cardiovascular diseases, cataracts [12], and strokes [13], presenting anti-inflammatory, anticancer, antidiabetic, and neuroprotective properties [11,14,15]. In addition, fruit juices are considered alternative food products, being developed as probiotic substrates in recent years as an alternative to dairy products. Because they are well accepted by consumers and have a high nutritional value with positive health effects, fruit juices are ideal vehicles for probiotics [16,17].
Considering the extremely perishable nature of the fruits, finding new technologies for conservation and processing has become a focus for innovation in the food industry. Processing fruit to obtain juices or nectars is a means to transform perishable products into storable and marketable products, adding economic value to the fruit, avoiding waste, and minimizing losses that may occur during the marketing of the fresh product [18]. Fermentation is one of the oldest food processing technologies. As early as the Paleolithic and Neolithic eras, foods such as bread and wine were consumed [3]. Without the need for highly developed technologies, fermentation occurs spontaneously and naturally through the action of microorganisms present in or added to the substrates. Probiotics are non-pathogenic live microorganisms, and their selection is extremely important in the fruit fermentation process due to the biochemical changes they promote in the initial product [10]. Fermentation consists of the action of microorganisms on the raw material in a biotransformation process in which carbohydrates are transformed into organic acids or alcohols [19,20]. There are numerous fermentation techniques and microorganisms used in this process [21]. However, while there is diversification, four main fermentation processes can be considered: acetic, alkaline, alcoholic, and lactic fermentation [22]. The microorganisms involved in fermentation can be bacteria, yeasts, and molds [10]. Sometimes the microbial species involved in fermentation are not known and may even belong to multiple species. In fermented fruit beverages, the microorganisms used are mainly lactic acid bacteria (LAB), such as Lactobacillus plantarum, Lactobacillus brevis, Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus casei, Leuconostoc mesenteroides, Leuconostoc citreum, Leuconostoc fallax, Leuconostoc kimchi, Pediococcus pentosaceus, Pediococcus acidilactici, Weissella confusa, Weissella cibaria, but also yeast, and acetic acid bacteria [8,10,13].
Thus, changes in the nutritional value, sensory characteristics, and microbial safety of the product are a consequence of the fermentation process. This is a technology that, according to several authors [3,10,23], has great advantages due to the improvement of food taste, food safety, and shelf life, with an increase in its nutritional properties by the production of interesting active principles. This review highlights the chemical and sensory characteristics of fruit juices and fermented fruit drinks, the fermentation process, its benefits, and its effects. The acceptance of fermented beverages by consumers will also be considered.
2. Fruit Juices
2.1. The Chemical Composition of Fruit
It is recognized that fruit consumption constitutes a large contribution of macronutrients, micronutrients, phytochemicals, and structural carbohydrates [15,24,25] (Figure 1), resulting in health benefits. According to dietary guidelines, fruit intake decreases excessive oxidative stress, preventing chronic and metabolic diseases while also acting on energy intake [26].
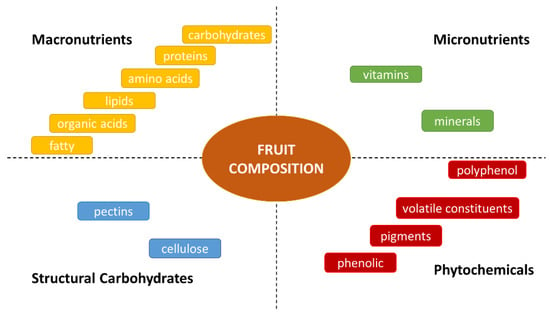
Figure 1.
Fruit composition.
Indeed, fruits are recognized as fundamental sources of vitamins, minerals, dietary fiber, and antioxidants. Their nutritional value and sensory characteristics depend on species, variety, cultivation (conventional or biological), soil, climatic conditions, storage, transport, and shelf life. Currently, there is a tendency to combine different fruits to increase both the flavor and the contribution of nutritional qualities [27,28].
Fruits are important sources of vitamins and minerals, mainly vitamin C and the B complex, and precursors of vitamin A, as well as providing antioxidants [10,28].
Minerals are essential in human health as they affect the development of bones and teeth, in addition to being related to electrolyte and water balance, metabolic catalysts, oxygen binding, and hormonal functions [29]. Fruits can contain significant amounts of important minerals such as: potassium, particularly bananas, blackcurrants, and blackberries; magnesium, of which the highest content is recorded in blackberries; and iron, where the strawberry stands out. However, they are low in sodium and selenium. It is also observed that berries as a whole are an important source of minerals, of which the main minerals found are phosphorus, potassium, calcium, magnesium, and iron (Table 1).

Table 1.
Mineral and vitamin composition of different fresh fruits.
Without the ability to synthesize vitamins, these minerals are essential for the proper functioning of the human body due to their antioxidant potential [30]. Ascorbic acid, or vitamin C, exists mainly in red fruits, such as strawberries, cherries, red raspberries, black raspberries, blackberries, cranberries, and blueberries, with a higher incidence in black currants, oranges, and papayas, which also register considerable levels of vitamin C. Vitamin A is not found abundantly in fruits, with a few exceptions, such as mangos, papayas, melons, and even watermelons. Vitamin B6 (riboflavin) is not present in large amounts in fruits, but appears in appreciable amounts in blueberries, cherries, strawberries, cranberries, and plums (Table 1).
The benefits of a diet rich in dietary fiber have long been known [34,35], namely in physiological responses to satiety, gastrointestinal tract physiology [35], lower risk of colorectal cancer, lower total and LDL cholesterol, and cardiovascular disease [36]. The term dietary fiber consists of polysaccharides (cellulose, hemicellulose, pectins, gums, mucilages) and lignin [27,34,37]. The fiber content in the fruit ranges from 1 to 3.17 g/100 g FW, with pears and figs showing the highest amounts, 3.1 and 2.9 g/100 g FW, respectively [31]. The red fruits were recorded to possess lower fiber contents, where the cranberries present the highest fiber content (35.7 mg/100 g FW), followed by the raspberries (5.8–6.5 mg/100 g FW) and the blackberries (4.5–5.3 7 mg/100 g FW) [27,31,32].
Glucose, sucrose, and fructose are the main sugars in the fruits, and although there are significant variations in their amount, according to Septembre–Malaterre et al. [10], the number of sugars in the fruit can vary between 5 and 22% of fresh weight, with citrus fruits among those with the lowest percentage of sugars and bananas with the highest. Mikulic–Petkovsek et al. [38] determined that fructose and glucose are the main sugars present in red fruits; not detecting sucrose in blackberry and raspberry fruits.
Phenolic compounds are one of the major classes of secondary plant metabolites and are among the most abundant natural antioxidants in the diet. Fruit is one of the foods richest in polyphenols, contributing to about half of the total nutritional intake [39]. They are associated with the prevention of numerous pathologies associated with oxidative stress, acting as antioxidants, also exhibiting antibacterial, antitumor, antimalarial, and antiviral characteristics, among others [15,40]. The phenolic potential of fruits depends on many factors, of which genetic attributes, maturity stage, and growing conditions are of primary importance [15,41].
About 8000 different plant phenolic structures are known [42], which are divided into major families such as phenolic acids, flavonoids, and stilbenes [10,43]. In red fruits, most of the phenolics present belong fundamentally to two classes: phenolic acids and anthocyanins, although each species has its profile [15]. For example, blueberries are rich in quercetin and caffeic acid (31.0–83.0 and 2.0–27.35 mg/kg fresh weight, respectively) [44,45,46], while lingonberries are rich in p-coumaric and ferulic acid (37.6–251.1 and 16.2–221.7, respectively) [47,48].
Among the flavonoids, flavanols and proanthocyanidins are the most present in the human diet. In fruits, catechins are represented with high content in apricots and cherries [49,50]. Proanthocyanidins are particularly abundant in cranberries (418.8 mg/100 g fresh weight), blueberries (179.8–331.9 mg/100 g fresh weight), plums (215.9–256.6 mg/100 g fresh weight), apples (69.6–141 mg/100 g fresh weight), blackcurrants (147.8 mg/100 g fresh weight), and strawberries (145 mg/100 g fresh weight) [51]. Anthocyanins are also abundant in fruits, found mainly in the fruit skin. Anthocyanin content is related to the increasing color intensity as the fruit ripens [52,53]. Grapes are the main dietary source of anthocyanins. The monomeric anthocyanins in grape skin extracts were mainly malvidin, particularly the malvidin-3-glucoside (1.40–7.09 mg/g of skin and 0.62–6.09 mg/g of skin, respectively) [54].
Lignans are found in relatively low concentrations in various fruits, having a positive impact on the prevention of heart disease, mamma cancer, and osteoporosis [55]. The highest content of lignan is observed in pears (15.56 mg/100 g food), apricots (11.57 mg/100 g food), grapefruits (7.44 mg/100 g food), peaches (6.83 mg/100 g food), and strawberries (6.2 mg/100 g food) [56].
Stilbenes are rarely present in human food. Trans-resveratrol can be found in grape skins with well-known beneficial health effects [57,58], namely in the prevention of human cardiovascular diseases. The highest concentration of this phenolic compound was found in grape skin, with a higher concentration in the red compared to the white varieties [59].
The chemical composition of the fruit affects the sensory characteristics of the juice. According to Francis and Newton [60], aroma results from complex interactions of numerous chemical compounds. Essentially, the cultivar [61,62,63], agricultural practices (conventional vs. organic) [64,65], post-harvest treatments, and the different techniques used to extend the shelf life of fruit and fruit juices [66,67], lead to variations in their sensory characteristics. Several techniques can be used to preserve the shelf life of this type of product, including thermal and non-thermal processing methods. However, their use should prevent the loss of the sensory properties of the juice or limited effectiveness of the treatment, since, in the search for the development of differentiating products, the mixed fruit juices are an option responding to the consumer demand for new flavors with added nutritional value, better sensory characteristics, and more striking colors [68,69].
2.2. Juice Composition vs. Processing Technologies
The consumer demand for fruit juices is growing as they are a naturally rich source of bioactive compounds, however, their susceptibility to spoilage limits the shelf-life [70]. For this reason, the food industry is constantly searching for new processing technologies to extend the shelf life with a low impact on the fruit juice quality, as the consumers are now more conscious of health and diet [71,72]. To extend the shelf life of fruit juices, the most commonly used preservation process is thermal processing (pasteurization and sterilization). For example, apple juice is treated by HTST at 77 to 88 °C for 25 to 30 s [73] and orange juice by HTST at 90 to 95 °C for 15 to 30 s [74]. However, this process may promote undesirable quality changes in the juice composition and the sensory and nutritional values of the fruit juice [75].
For example, Vegara et al. [76] evaluated the influence of pomegranate juice pasteurized on anthocyanin stability and verified that the application of thermal treatments (65 and 90 °C for 30 or 5 s) diminished the percentage of anthocyanins in the polymeric form but increased the monomeric anthocyanins. Also, Aguilar-Rosas et al. [77] studied the high-temperature short time (HTST) pasteurization process (90 °C; 30 s) of apple juice and observed a decrease in the concentration of the total phenolic compounds (~32%), compared to the untreated juice.
Mena et al. [78] analyzed pomegranate juice before and after low-, mild- and high-temperature pasteurization (LTP, MTP, HTP, at 65, 80, and 95 °C, respectively, for periods of 30 or 60 s, and observed that the total anthocyanin concentration was different among thermally processed and untreated pomegranate juices, the lowest concentration being determinate in the control (untreated pomegranate juices), while the highest concentration of anthocyanins was found in the juice treated at 95 °C for 30 s.
Consequently, as consumers want fruit juices not only with extended shelf life but also with enhanced quality characteristics, researchers are looking for innovative non-conventional technologies such as high-pressure (HP), ultrasound (US), pulsed electric fields (PEF), ultraviolet-C radiation (UV-C), low-pressure plasma (LPP) and Ohmic heating (OH) (Table 2) to achieve the consumer demand for fruit juice with an extended shelf life, better quality, and an improved nutritional profile [72]. Recent studies reported a positive impact of non-thermal processing on juice quality [79,80]. Optimized non-thermal processing enhanced the content of the bioactive compounds in fruit juices and consequently their beneficial health effects [72,81].

Table 2.
Thermal and non-thermal processing technologies of fruit juices.
For example, Linhares et al. [82] compared the composition, stability, and bioactive compounds of juices produced with different processing technologies, thermal technologies such as high-temperature short time (HTST), ultrahigh temperature (UHT), and non-thermal technologies such as high power ultrasound (US), UV-pulsed-light and low-pressure plasma (LPP). These authors showed that all the juices produced with non-thermal processes increased the sugar content (glucose and fructose), and the amino acid betaine, except for the juices produced by the combination of the ultrasound process followed by low-pressure plasma (US.LPP). On the other hand, the juices produced by HTST and UHT showed higher concentrations of fatty acids and phenolic compounds. Also, the effects of ultrasound treatments on the quality of grapefruit juice were studied by Aadil et al. [83], and it was observed that all grapefruit juice samples were sonicated for 30, 60, and 90 min leading to an improvement in the total phenolic, flavonoids, and flavonol. These outcomes suggested that this non-thermal processing technology might be well applied at an industrial scale for the processing of grapefruit juice. Also, UV-C technology has been demonstrated to obtain microbiologically safe fruit juices with a low negative impact on final product quality [84].
Another example of non-thermal technology is the application of high-pressure (HP) and high hydrostatic pressure (HHP) processing on acid fruit juices. This technology is effective in the inactivation of microorganisms (meeting the Food and Drug Administration requirement of a 5-log reduction) and denaturation of diverse enzymes [85], without loss of vitamins, pigments, and compounds related to sensory characteristics [86]. High-pressure (HP) processing is preferred to thermal processes in terms of holding phenolic compounds. HP and HHP treatment at moderate temperature is described to have an insignificant effect on the anthocyanin concentration of diverse red fruit juices, as well as the flavor, taste, and color changes being minimal [87]. However, these authors also showed that the bioactive content of red fruit reduced with the intensity of the treatment in terms of processing time and pressure level. Varela-Santos et al. [88] evaluate the effect of HHP processing (350–550 MPa for 30, 90, and 150 s) on the concentration of anthocyanins, phenolic compounds, and color of pomegranate juice during 35 days of storage at 4 °C. These authors showed that HHP juice processing has a perceptible effect on the total color difference (ΔE) between untreated and treated samples, and the highest color difference was observed at day 35 of storage for 550 MPa during the 90 s. These results showed clearly that the color stability of pomegranate juice is dependent on the processing conditions. Orange juice showed an increase in flavanone after HPP processing (400 MPa, 40 °C, 1 min), compared to the untreated juice [75]. Also, Sánchez-Moreno et al. [89] and Oms-Oliu et al. [90] observed in orange juice treated with HP (400 MPa/40 °C/1 min) an enhancement in the concentration of naringenin by 20% and the concentration of hesperetin by 40%, compared with the untreated orange juice and the preservation of the orange juice sensory characteristics.
In addition, pulsed electrical field (PEF) processing, which applies short bursts of high voltage electricity for microorganism inactivation, has been successful in a variety of liquid products with relatively low viscosity and electrical conductivity such as orange juice and cranberry juice [91]. PEF has a high potential for microorganism inactivation and enzyme denaturation, extending the shelf life and preserving the nutritional, vitamin, aroma, and sensory characteristics due to the very short processing time and low processing temperature. Blueberry juice processed by HP (600 MPa/42 °C/5 min) and processed by PEF (36 kV/cm, 100 μs) stored refrigerated at 4 °C for 56 days, showed a 50% of ascorbic acid reduction in both unprocessed blueberry juices and in the PEF-treated juices at the end of the refrigeration time. However, HPP-treated blueberry juice better maintained the ascorbic acid content during the storage time with a reduction of 31%, and the anthocyanins in the blueberry juice treated with HP were also better preserved. Sánchez-Moreno et al. [89] considered that the PEF treatment did not modify flavanone content, but in general, the pasteurization process led to a diminished naringenin content (16.04%), with no modification in hesperetin. They also observed that even though the losses in total vitamin C were <9%, treatments with the higher temperatures (HPT) (90 °C/1 min), tend to show a greater reduction in the concentration of both forms of vitamin C. HP treatment (400 MPa/40 °C/1 min) led to an increase in carotenoid release (53.88%) and vitamin A value (38.74%). PEF treatment did not modify individual or total carotenoid content. Traditional thermal treatments did not have any effect on the total carotenoid content or on the vitamin A value. In apple juice, the treatment with PEF decreased the concentration of total phenolic compounds (~15%) compared to the untreated juice, however, this decrease was lower than that observed with thermal pasteurization, which decreased the phenolic compounds by 32% [77]. In summary, according to Sánchez-Moreno et al. [89], HP and PEF technologies were more effective than HPT treatment in preserving the bioactive compounds of orange juice. Likewise, Agcam et al. [92] showed that the total phenolic concentration of orange juice was enhanced after the PEF and thermal pasteurization treatments. Orange juice processed by PEF contained higher phenolic compound concentrations than those processed by the heat. The orange juice treated with PEF had more stable flavonoids and phenolic acids than those treated with thermal pasteurization. The PEF-treated samples had higher sensory scores than the heat-treated samples. Therefore, these authors suggested that the application of PEF processing to orange juice seems to be a promising alternative to thermal pasteurization to obtain an extended shelf life and better preservation of phenolic compounds and should be taken into consideration for industrial-scale production.
In recent times, cold plasma was considered suitable for use with fruit juices [93,94]. Therefore, cold plasma is accepted as a potential, novel, non-thermal technology for the quality improvement of fruit juices, and numerous research works have studied the application of cold plasma in fruit juices [1,81,95,96,97,98]. The treatment is performed under milder temperatures (< 70 °C), which contributes to the preservation of sensory characteristics and the maintenance of bioactive compounds in fruit juices [99]. Bursać Kovačević et al. [96], using a cold atmospheric gas-phase plasma in pomegranate juice, observed an increase in the concentration of anthocyanin between 21% and 35% compared to the untreated juice, which confirms that the cold plasma has a positive impact on anthocyanin stability. More recently, de Castro et al. [81] studied the application of cold plasma excitation frequency (200, 420, 583, 698, and 960 Hz) in the juice physicochemical properties. These authors concluded that after the application of this non-thermal treatment the content of ascorbic acid was increased by increasing the plasma excitation frequency. According to these authors, cold plasma application could be an interesting method to enhance the nutritional quality of fruit juices. It was also observed in diverse fruit juices, for example, strawberry juice, blackcurrant juice, and raspberry juice, that anthocyanins are stable to HP treatments, such as the application of cold plasma excitation frequency [79,80].
Several research works have been conducted on different fruit juices using ohmic heating which is also known as electrical resistance heating, such as the inactivation of microorganisms [100,101,102] and enzymes, for example, pectin methylesterase (EC.3.1.1.11) also called pectinesterase [103,104] and polyphenoloxidase, for minimizing enzymatic browning [105]. In orange juice treated with ohmic heating around 96% of the pectin methylesterase activity was reduced as observed by Demirdöven et al. [103]. In fruit juices, the use of ohmic heating to inactivate enzymes does not affect the juice flavor [106]. Hashemi et al. [107] compared different ohmic heating treatments (150, 200, and 250 V; 120 s; 99.4 °C) with the conventional heating process (90 °C; 15 min) for the inactivation of microorganisms in blended citrus juice (sweet lemon and orange). These researchers showed that the inactivation rate of pathogenic bacteria using ohmic heating increased by the increase of voltage from 150 to 250 V. Also, Darvishi et al. [108] studied the influence of ohmic heating on the concentration of black mulberry juice in comparison to the traditional heating treatment. Using ohmic heating the phenolic concentration of the juice was 3–4.5 times greater than if using traditional heating treatment.
3. Fruit Juice Fermented Beverage
The production of fermented beverages using fruits other than grapes, such as oranges, mangos, raspberries, pineapples, apples, pears, apricots, peaches, cherries, bananas, and papayas increased in the last years. The explanations for this are the usage of fruit with a lower quality standard for natural eating, overproduction, and the development of fermented beverages with flavors and aromas typical of the fruits utilized. According to Lopez et al. [126], fruit wines are undiluted alcoholic beverages, produced with fruits other than grapes, which are tastier, more nutritious, and lighter alcoholic drinks as they retain the maximum amounts of the nutrients existing in the fruits. Fruit fermented beverages go through a period of fermentation and aging, and generally have an alcohol percentage between 5% and 13%, and 2–3% of sugars [127]. Fruit fermented beverages are characterized by peculiar aromatic notes, phenolic composition, antioxidants, alcohol content, and other parameters.
3.1. Alcoolic, Acetic and Lactic Fermentation
3.1.1. Alcoholic Fermentation
Many fruits are consumed fresh, but it is not unusual that large quantities are wasted during harvest, due to climate fluctuations and improper handling (inadequate storage, transport, and microbial infections). The food industry tries hard to extend the shelf life of fruits so that they can be consumed all over the world, year-round [128]. Therefore, the exploitation of ripe fruits or their juices in the production of fermented beverages is an attractive way of utilizing surplus and over-ripe fruits. Fermentation also helps to enhance the nutritional value of beverages, allowing: (i) the preservation through acidification/alcohol production; (ii) the alteration of chemical nature and sensory properties of fruit; (iii) the improvement in the efficacy of some bioactive constituents; and (iv) the enhancement of nutritional value of foods and beverages [129,130].
Wine and cider, obtained from grape and apple juice fermentation, respectively, are well-known drinks, mainly fermented by yeasts, and during the process alcohol, esters, aldehydes, terpenes, and acids are produced [131]. Besides wine and cider, many other fermented fruit juices are made from fruits [132]. Today, an increasing number of other fruits are available for the production of fermented fruit juices [129]. Table 3 lists some examples of fermented fruit juices, indicating the first published works in which they were mentioned.

Table 3.
Examples of some fermented fruit juices. The first published works in which they were mentioned are also referenced.
The microorganism used to ferment the fruit juices and produce the fermented fruit beverages is usually a strain of S. cerevisiae species [129] or S. cerevisiae var. bayanus. The latter was used by Mena et al. [144] to produce a pomegranate juice fermented beverage.
Non-Saccharomyces yeasts, such as Hanseniaspora uvarum, Hanseniaspora opuntiae, Hanseniaspora occidentalis, Pichia kudriavzevii, and Torulaspora delbrueckii have been selected as candidates for an orange juice fermented beverage with higher volatile compounds concentration, odor active values, and sensory evaluation scores [145]. More recently, Kluyveromyces marxianus, Zygosaccharomyces rouxii, and Pichia kluyveri, studied by Gschaedler et al. [146] showed an increase in ethanol production in cider, when nutrients were added, obtaining more than 80 g/L of ethanol and showing that these yeasts have potential in the fermentation of apple juice. The use of non-Saccharomyces yeasts in co-inoculation (Torulaspora delbrueckii, Lachancea thermotolerans, and Saccharomyces cerevisiae) on apple mash fermentation was also studied for the production of Pálinka, a Hungarian fruit spirit [147]. According to the results obtained by Fejzullahu et al. [148], single and mixed cultures showed similar characteristics during mash fermentation and mixed cultures revealed a significantly higher concentration of volatile compounds and pleasant sensory attributes, compared to those exhibited by the pure culture of S. cerevisiae.
The presence of Saccharomyces and non-Saccharomyces yeast in fermented fruit juices also makes these drinks potential vehicles for probiotic microorganisms. S. boulardii, demonstrated above as able to ferment pomegranate juices [144], has the potential to inhibit pathogen growth [149]. S. boulardii can also degrade pathogen toxins, modulate intestinal microbiota, preserve normal intestinal physiology, and increase secretory IgA (sIgA) levels [150].
3.1.2. Lactic Acid Fermentation
Lactic acid fermentation (LAB) has been one of the oldest techniques to extend the shelf life of perishable foods [13] and is a valuable substitute for the bio-preservation of fruits and fruit juices. Single-fruit, blended smoothies, or fruit juices that can be fermented by LAB are healthy alternatives to promote fruit consumption [151,152]. Moreover, the scientific interest in the strategy of lactic acid fermented juices and the study of their probiotic properties have increased in the last years [150].
Lactic acid fermented foods and drinks have been produced for thousands of years due to their healthy features, and are well accepted by consumers [130]. Lactic acid fermentation leads to the synthesis of organic acids, carbon dioxide, ethanol, diacetyl, hydrogen peroxide, fatty acids, phenyl-lactic acid, and bacteriocins, all with antagonistic proprieties [153,154].
The fermented drinks may be obtained by “spontaneous” fermentation, by autochthonous lactic acid bacteria present in the raw material. The most usual strains are Enterococcus spp., Fructobacillus spp., Lactobacillus spp., Leuconostoc spp., Pediococcus spp., and Weissella spp. under anaerobic conditions [150], and moderate temperature (25–35 °C). Controlled fermentation using Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, Lactobacillus gasseri, and Lactobacillus acidophilus, are safer, easier to replicate, more reliable, and provide standardization and constant quality of the final products [130,152,154]. Among the desirable technological traits, lactic acid bacteria starter cultures for fruit-based drinks should grow rapidly, ferment diverse carbohydrate substrates, acidify the juice, even at low pH values and temperature, and should also tolerate and/or metabolize phenolic compounds [130]. Additionally, LAB enhances nutrient bioavailability and guarantees complete fermentation. Most of the starter cultures can synthesize antimicrobial compounds foodborne pathogens and spoilage microorganisms [130]. Regarding sensory attributes, LAB is expected to synthesize aroma and flavor compounds, or their precursors, such as acetic acid, esters, ketones, alcohols, and terpenes, producing fermented juices with pleasant flavors and without off-flavors. Also, LAB may increase the antioxidant activity of the matrix, and be able to produce or release bioactive compounds such as peptides, vitamins, amino acids, and phenolics [8,152,154,155].
Some examples of fruit juices fermented with LAB are red dragon fruit (Hylocereus polyrhizus) beverages where a total of 21 isolates of LAB were isolated and characterized. They belonged to the genus of Enterococcus, namely: Enterococcus faecalis or Enterococcus durans [156]; fermented noni (Morinda citrifolia L.) fruit juice, is considered one of the health-promoting beverages, fermented by Lactobacillus plantarum SK15 [157]; and blueberry juices fermented by lactic acid bacteria isolated from fruit environment. Lactobacillus plantarum LSJ-TY-HYB-T9 and LSJ-TY-HYB-T7, and Lactobacillus fermentum LSJ-TY-HYB-C22 and LSJ-TY-HYB-L16 were possible candidates to produce fermented fruit juices, including blueberry juice [158]. Functional lactic acid bacteria can thus be used to produce fruit juices with reduced sugar levels, which is expected to be beneficial for human health [159].
3.1.3. Acetic Acid Fermentation
Acetic Acid Bacteria (AAB) are mostly known for their use in the production of vinegar, vitamin C, and cellulose [160]. AAB is responsible for the production of vinegar, including fruit vinegar. The production of fruit vinegar is a way of making use of fruit by-products, frequently employed by the food industry since extra or second quality fruit can be used without compromising the quality of the final product [161]. For producing vinegar, a two-stage fermentation process is needed. The first stage is the conversion of sugars into ethanol by yeasts. Usually, this biological process is done by Saccharomyces species. The second stage is the oxidation of ethanol by acetic acid bacteria (Acetobacter and Gluconobacter species). Nevertheless, the focus of this review is the fruit juices and not the vinegar, a culinary condiment. According to the literature, in juices or juice-like products, acetic acid fermentation is only found in kombucha.
Kombucha is a fermented beverage that sensorially resembles carbonated cider, presenting a sweet, and slightly sour flavor [162]. It is obtained from sweetened black or green tea, via a symbiotic relationship named SCOBY (symbiotic culture of bacteria and yeast, a consortium of acetic acid bacteria, lactic acid bacteria, and osmophilic yeasts) [163]. The SCOBY association, also known as “tea fungus”, in the form of a cellulosic biofilm, transforms the sugar and tea components into bioactive compounds with probiotic effects. The main bacteria in “tea fungus” are AAB, mainly species from the genera Acetobacter (Acetobacter aceti, Acetobacter pasteurianus, Acetobacter nitrogenifigens), Gluconacetobacter (Gluconacetobacter sp A4, Gluconacetobacter sacchari, Gluconacetobacter oxydans), and Komagataeibacter (Komagataeibacter xylinus, Komagataeibacter kombuchae) [164]. AAB is part of the relatively stable bacterial community in kombucha and is responsible for the oxidation of ethanol which leads to the production of acetic acid. Other secondary metabolites such as gluconic acid (from glucose), glucuronic acid (from glucose; detoxifying properties), and D-saccharic acid-1,4-lactone (from glucose; radical-scavenging; some Gluconacetobacter species) are also produced [164]. The main yeasts found in kombucha are Saccharomyces sp., Zygosaccharomyces kombuchaensis, Torulopsis sp., Pichia spp., Brettanomyces sp. [165], and Zygosaccharomyces bailii [166]. Several lactic acid bacteria have also been isolated [165]. After fermentation, the kombucha chemicals include the following: sugars; tea polyphenols; organic acids; fiber; ethanol; amino acids; essential elements (Cu, Fe, Mn, Ni, and Zn); several vitamins such as vitamin C, and B vitamins; carbon dioxide; antibiotic substances; and hydrolytic enzymes [167,168].
Recently, alternative raw materials have been suggested for the production of kombucha, for example, fruit or vegetable juices and cocktails, plant infusions, or milk [162]. For fruit-based drinks, there is reference to Salak kombucha in the literature. Salak or “snake fruit” is a fruit growing in a palm from the Arecaceae family in Indonesia. Salak kombucha is obtained following 14 days of “tea fungus” fermentation of the salak juice [169]. The same procedure can be applied to other fruits, producing delicious colorful drinks.
4. Sensory Characteristics and Consumer Acceptance of Fruit Juice and Fermented Fruit Beverages
The parameters that define the quality of a drink are positive attributes such as the following: color and overall appearance; taste properties, including flavor, mouth persistence, and aftertaste; olfactory properties, such as aroma, odor, orthonasal and retronasal; and tactile properties, such as mouth feel, body, and absence of contaminants (odors and strange flavors). Among the negative attributes are discoloration, foaming, sedimentation, gas, unpleasant smell (particularly of ketone or vinegar), bitterness, and astringency.
The fruit juice thermal treatments lead to substantial modifications of the final product qualities. Although microbial and chemical safety is prevalent during fruit juice processing, the sensory attributes are also important. The attributes such as color or tactile properties are very important for both the first acceptance and regular purchasing of the products. Therefore, the sensory quality of fruit juices plays an important role in consumer satisfaction. Thermal treatments are the most commonly used in fruit juice processing, but they tend to induce negative changes to the nutritional and sensory characteristics of the juices [170]. The pasteurized samples were significantly less desirable in terms of odor, color, cloudiness, acidity, overall flavor, and overall likeness. Therefore, emerging non-thermal processes are applied to maintain the quality, however, to reduce the color degradation and browning in fruit juice, the optimization of the processing parameters is necessary. For example, HP treatment is a non-thermal treatment that has been described to inactivate microorganisms through membrane disruption and it preserves nutritional value with a reduced effect on fruit juices quality and sensory characteristics as lower molecular weight compounds, such as volatile compounds, pigments, and some vitamins are not changed, since covalent bonds are not affected by pressure [171]. Also, Aguilar-Rosas et al. [77] showed that PEF processing (4 μs, 35 kV/cm, and 1200 pps) maintained the volatile compounds responsible for apple juice flavor and color more than heat treatment, with 7% and 8.4% reduction of hexanal and hexyl acetate, respectively, unlike heat treatment which eliminated these compounds. Sensory analysis showed that the taste and flavor of fruit juices processed by PEF were preferred to thermally processed juices. The researchers Khandpur and Gogate [172] evaluated the quality of ultrasound orange juice concerning taste, smell, and mouth sensation and found that the ultrasound-treated juice was the most acceptable for consumers and was identical to the fresh unprocessed fruit juices. The positive effect of the ultrasound treatment is attributed to the removal of oxygen. After non-thermal treatments, juices showed the lowest variation in hedonic scores, if compared to the untreated juice [173].
Fermented fruit juices may have high acidity, making them unpleasant for consumers. Muhialdin et al. [174] added another approach that was followed by dragon fruit juices. Freeze-dried fruit juice (FDFJ) was added to fresh juice at different ratios, to reduce the acidity. A total of five samples at the mixture ratios 1:9, 2:8, 3:7, 4:6, and 0:10, were tasted by 65 random consumers (37 males and 18 females). The results showed a high preference among the random consumers for the sample containing FDFJ and fresh juice at a ratio of 1:9. However, the mixing ratio of 1:9 did not show a significant difference from the fresh juice.
The suitability of a mixture of juices from jicama, winter melon, and carrot as a raw medium to produce probiotic juice by strains of Lactobacillus plantarum and Lactobacillus acidophilus, was studied by Do and Fan [175]. Besides other parameters, their sensory acceptability was also investigated. Three versions of the fermented vegetable juice mixtures were served to judges. The judges indicated that they liked the color and texture of juices in all formulations and acceptance of the probiotic vegetable juice mixtures was in the range of five to eight on a nine-point hedonic scale. Moreover, the sensory quality was improved positively by sucrose addition or adding tropical fruit juices or multi-fruit juice (10% v/v).
The applicability of different LAB. strains (Lactobacillus plantarum (WJ-LP), L. rhamnosus (WJ-LR), L. casei (WJ-LC), L. brevis (WJ-LB), and Pediococcus pentosaceus (WJ-PP)) was evaluated recently in watermelon juice [176]. The sensory quality of fermented juices was evaluated by tasters that rated the overall liking using a nine-point hedonic scale This scale was also used to evaluate other sensory attributes such as appearance, aroma, sweetness, flavor, consistency, acidity, and color. Then, using the check-all-that-apply (CATA) test tasters checked all applicable sensory terms that described the sample from a list provided. At the end of the work, it was found that the lab strains significantly imprinted flavor characteristics which cause tasters to prefer L. brevis and P. pentosaceus fermented juices. These juices were the least penalized, had a higher purchasing power, and were associated with ‘watermelon flavor’, ‘natural taste’, ‘sweet’, and ‘watermelon color’ terms.
Due to their nutritional benefits, fermented beverages have become an influential player in the beverage industry. Kasron et al. [177] identified consumer acceptance and willingness to pay for fermented drinks in Malaysia. The field survey conducted showed that 54% of respondents knew about functional foods and 55% of these were aware of functional foods based on fruits. The survey also found that 30% of respondents had taken fermented drinks before. However, health issues are not the only reason why people consume fruit juices and their derivatives. Skąpska et al. [178], when studying “the development and consumer acceptance of functional fruit-herbal beverages”, found that the main motivation for purchasing was their sensory acceptance, even if the consumers were informed of their potential health benefits. In their study, Skąpska et al. [178] also established that Aronia beverages were the most accepted and could find buyers when introduced to the market. The other beverages (made with rugosa rose, acerola, sea buckthorn, or cranberry) were poorly or not accepted by the majority of the tasters, despite information on the pro-health effects of the products. The beverages were rated slightly higher by women than men and by people aged 25–34. Fermented beverages using selected lactic acid bacteria in the fermentation of various fruit juices, resulted in some cases in fruit beverages with enhanced nutritional and sensorial characteristics.
In addition, consumer paired preference tests were performed by Cordelle et al. [179] to establish the influence of gender and age on preferences for orange juice, and additionally to determine the reproducibility of consumer-liking patterns over repeated evaluation with 917 consumers. The outcomes of this research showed that neither gender nor age significantly affects consumer preferences for orange juice.
5. Final Remarks
Several studies have shown that fruit juices and fermented fruit beverages are commercially promising products. With great demand and acceptance by consumers, they are used not only as healthy drinks but also as therapeutic products, given their nutraceutical properties.
Moreover, due to the perishable nature of fruits, new technologies for conservation and processing are a demand in the food industry. Processing the fruits to obtain juices, nectars, or even fermented beverages is a valuable way to transform unpreserved products into storable products, adding economic value, avoiding waste, and minimizing losses that may occur during the marketing of the fresh products.
With consumer demand for new, healthy, and functional products produced sustainably, and the development of the technologies involved, it is expected that fermented beverages will position themselves in a prominent place in the functional food market. Nevertheless, it is important to consider the consumer sensory acceptability of this kind of juice as most of them, after fermentation, may gain some sensory attributes less pleasant to the consumers, such as excessive acidity. However, the sensory characteristics of fermented juices are highly dependent on the microorganisms used in the fermentation processes along with the selected processing techniques.
Author Contributions
T.P., A.V. and F.C. contribute equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds FCT Portuguese Foundation for Science and Technology-Portugal and COMPETE under the projects UIDB/00616/2020, UIDP/00616/2020, and UIDB/04033/2020.
Acknowledgments
The authors would like to thank the Chemistry Research Centre-Vila Real (CQVR) and CITAB/Inov4Agro Center for the Research and Technology of Agro-Environmental and Biological Sciences/Institute for Innovation, Capacity Building, and Sustainability of Agri-Food Production for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dasan, B.G.; Boyaci, I.H. Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol. 2018, 11, 334–343. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhao, Z.; Wu, X.; Lin, W.; Qin, Y.; Chen, H.; Wan, Y.; Zhou, C.; Bu, T.; Chen, H.; et al. A Review on Fruit and Vegetable Fermented Beverage-Benefits of Microbes and Beneficial Effects. Food Rev. Int. 2022, 38, 1–38. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the microbial composition of water kefir from multiple sources. FEMS Microbiol. Lett. 2013, 348, 79–85. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and Non-Dairy Probiotic Beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Gomes-Rochette, N.F.; Da Silveira Vasconcelos, M.; Nabavi, S.M.; Mota, E.F.; Nunes-Pinheiro, D.C.; Daglia, M.; De Melo, D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016, 17, 986–993. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-dried Jaboticaba Peel (Myrciaria Jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Tseng, Y.; Liu, C.; Liang, C. Anti-oxidant and Anti-aging Activities of Fermented Vegetable-Fruit Drink. J. Food Nutr. Res. 2021, 9, 240–250. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits—A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of Natural Active Compounds, Enzymes, and Probiotics for Fruit Juice Fortification, Preservation, and Processing: An Overview. J. Funct. Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Horácková, Š.; Rokytová, K.; Bialasová, K.; Klojdová, I.; Sluková, M. Fruit Juices with Probiotics–New Type of Functional Foods. Czech J. Food Sci. 2018, 36, 284–288. [Google Scholar] [CrossRef]
- Paula, F.J.A.; Guiné, R.P.F.; Cruz-Lopes, L.; Duarte, A.C.; Fragata, A.O.S.; Reis, M.A.L. Effects of pre- and post-parvest factors on the selected elements contents in fruit juices. Czech J. Food Sci. 2015, 33, 384–391. [Google Scholar] [CrossRef]
- Yunita, D.; Dodd, C.E.R. Microbial Community Dynamics of a Blue-veined Raw Milk Cheese from the United Kingdom. J. Dairy Sci. 2018, 101, 4923–4935. [Google Scholar] [CrossRef]
- Pawar, S.V.; Rathod, V.K. Role of Ultrasound in Assisted Fermentation Technologies for Process Enhancements. Prep. Biochem. Biotechnol. 2020, 50, 627–634. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeria, M.E.; Pandiellaa, S.S.; Canterob, D.; Webba, C. Cereal-based fermented foods and beverages. Int. Food Res. J. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Fermentations in world food processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Int. Food Res. J. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and Vegetable Intake and Body Mass Index in a Large Sample of Middle-Aged Australian Men and Women. Nutrients 2014, 6, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; MoustaidMoussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.; Da Silva, T.L.; Lima, L.C.O.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Sinha, P.S.; Rosen, H.N. Clinical Pharmacology of Bisphosphonates. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: London, UK, 2020; pp. 579–589. ISBN 9780128140826. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1-57881-072-8. [Google Scholar]
- USDA-ARS (US Department of Agriculture, Agricultural Research Service). USDA Nutrient Database for Standard Reference, Release 25, Software 1.2.2, from the Nutrient Data Laboratory. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 20 December 2021).
- Hakala, M.; Lapvetelainen, A.; Houpalahti Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compos. Anal. 2003, 16, 67–80. [Google Scholar] [CrossRef]
- Djordjević, B.; Šavikin, K.; Zdunić, G.; Janković, T.; Vulić, T.; Pljevljakušić, D.; Oparnica, C. Biochemical properties of the fresh and frozen black currants and juices. J. Med. Food. 2013, 16, 73–81. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary fibre intake in Australia. Paper II: Comparative examination of food sources of fibre among high and low fibre consumers. Nutrients 2018, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst. 2001, 93, 525–533. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 10. [Google Scholar] [CrossRef] [PubMed]
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, E.S.; Osagie, A.U. Antioxidant properties of methanolic extracts of some Nigerian plants on nutritionally-stressed rats. Niger. J. Basic Appl. Sci. 2012, 20, 7–20. [Google Scholar]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I. The role of phenolic compounds in the fight against cancer—A review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Panickar, K.S.; Anderson, R.A. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 8181–8207. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–378. [Google Scholar]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Ulrih, N.P.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Pilat, B.; Zadernowski, R.; Czaplicki, S.; Jez, M. Cold storage, freezing and lyophilisation and its effect on transformations of ˙phenolic compounds in lingonberry (Vaccinium vitis-idaea L.). Pol. J. Nat. Sci. 2018, 33, 101–113. [Google Scholar]
- D'Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Hara, Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacol. Res. 2011, 64, 100–104. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A. Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. OENO ONE 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M'hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.I.; Mateos, R.; Garcıa-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Jaros, D.; Thamke, I.; Raddatz, H.; Rohm, H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009, 229, 51–61. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Santos, R.; Pereira, R.; Câmara, J.S. Differential volatile organic compounds signatures of apple juices from Madeira Island according to variety and geographical origin. Microchem. J. 2019, 150, 104094. [Google Scholar] [CrossRef]
- Estrada-Beltran, A.; Salas-Salazar, N.A.; Parra-Quezada, R.A.; Gonzalez-Franco, A.C.; Soto-Caballero, M.C.; Rodriguez-Roque, M.J.; Flores-Cordova, M.A.; Chavez-Martinez, A. Effect of conventional and organic fertilizers on volatile compounds of raspberry fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 862–870. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, M.F.; Cosme, F.; Anjos, R. Influence of cultivar and conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus). J. Sci. Food Agric. 2018, 98, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on the phytochemical composition of 'Kweli' and 'Tulameen' raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.; Silva, P.; Medina, S.; Câmara, J.S. Differentiation of fresh and processed fruit juices using volatile composition. Molecules 2019, 24, 974. [Google Scholar] [CrossRef]
- Kebede, B.; Ting, V.; Eyres, G.; Oey, I. Volatile changes during storage of shelf stable apple juice: Integrating GC-MS fingerprinting and chemometrics. Foods 2020, 9, 165. [Google Scholar] [CrossRef]
- Sobhana, A.; Mathew, J.; Ambili Appukutan, A.; Mredhula Raghavan, C. Blending of cashew apple juice with fruit juices and spices for improving nutritional quality and palatability. Acta Hortic. 2015, 1080, 369–375. [Google Scholar] [CrossRef]
- Curi, P.N.; Almeida, A.B.D.; Tavares, B.D.S.; Nunes, C.A.; Pio, R.; Pasqual, M.; Souza, V.R.D. Optimization of tropical fruit juice based on sensory and nutritional characteristics. Food Sci. Technol. 2017, 37, 308–314. [Google Scholar] [CrossRef]
- Buzrul, S.; Hami, A.; Largeteau, A.; Demazeau, G. Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. Int. J. Food Microbiol. 2008, 124, 275–278. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.-W.; Zhang, Z.-H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.; Rabelo, M.C.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1H qNMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef]
- Moyer, J.C.; Aitken, H.C. Apple juice. In Fruit and Vegetable Juice Processing Technology; Nelson, P.E., Tressler, D.K., Eds.; AVI: Westport, CT, USA, 1980; pp. 212–267. [Google Scholar]
- Braddock, R.J. Single-strength orange juice and concentrates. In Handbook of Citrus By-Products and Processing Technology; Braddock, R.J., Ed.; Wiley: New York, NY, USA, 1999; pp. 53–83. [Google Scholar]
- Plaza, L.; Sanchez-Moreno, C.; Elez-Martinez, P.; Ancos, B.; Martin-Belloso, O.; Cano, M.P. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur. Food Res. Technol. 2006, 223, 487–493. [Google Scholar] [CrossRef]
- Vegara, S.; Mena, P.; Martí, N.; Saura, D.; Valero, M. Approaches to understanding the contribution of anthocyanins to the antioxidant capacity of pasteurized pomegranate juices. Food Chem. 2013, 141, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rosas, S.F.; Ballinas-Casarrubias, M.L.; Nevarez-Moorillon, G.V.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef]
- de Jesus, A.L.T.; Cristianini, M.; dos Santos, N.M.; Maróstica Júnior, M.R. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açai (Euterpe oleracea Martius) pulp. Food Res. Int. 2020, 130, 108856. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, A.L.T.; Leite, T.S.; Cristianini, M. High isostatic pressure and thermal processing of açai fruit (Euterpe oleracea Martius): Effect on pulp color and inactivation of peroxidase and polyphenol oxidase. Food Res. Int. 2018, 105, 853–862. [Google Scholar] [CrossRef] [PubMed]
- de Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef]
- Linhares, M.F.D.; Alves Filho, E.G.; Silva, L.M.A.; Fonteles, T.V.; Wurlitzer, N.J.; Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Thermal and non-thermal processing effect on açai juice composition. Food Res. Int. 2020, 136, 109506. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Shah, A.K.N.N.; Shamsudin, R.; Abdul Rahman, R.; Adzahan, N.M. Fruit Juice Production Using Ultraviolet Pasteurization: A Review. Beverages 2016, 2, 22. [Google Scholar] [CrossRef]
- Basak, S.; Ramaswamy, H.S.; Simpson, B.K. High pressure inactivation of pectin methyl esterase in orange juice using combination treatments. J. Food Biochem. 2001, 25, 509–552. [Google Scholar] [CrossRef]
- Fernández-García, A.; Butz, P.; Bognar, A.; Tauscher, B. Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange–lemon–carrot juice product after high pressure treatment and storage in different packaging. Eur. Food Res. Technol. 2001, 213, 290–296. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Elez-Martinez, P.; Martin-Belloso, O. Stability of health related compounds in plant foods through the application of non thermal processes. Trends Food Sci. Technol. 2012, 23, 111–123. [Google Scholar] [CrossRef]
- Jin, Z.T.; Zhang, Q.H. Pulsed electric field inactivation of microorganisms and preservation of quality of cranberry juice. J. Food Process. Preserv. 1999, 23, 481–497. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Akdemir Evrendilek, G. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurization. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef]
- Shi, X.M.; Zhang, G.J.; Wu, X.L.; Li, Y.X.; Ma, Y.; Shao, X.J. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans. Plasma Sci. 2011, 39, 1591–1597. [Google Scholar] [CrossRef]
- Surowsky, B.; Frohling, A.; Gottschalk, N.; Schluter, O.; Knor, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Paixão, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.N.; Rodrigues, S. Cold plasma effects on functional compounds of siriguela juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Park, I.-K.; Ha, J.-W.; Kang, D.-H. Investigation of optimum ohmic heating conditions for inactivation of Escherichia coli O157: H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes in apple juice. BMC Microbiol. 2017, 17, 117. [Google Scholar] [CrossRef]
- Kim, N.; Ryang, J.; Lee, B.; Kim, C.; Rhee, M. Continuous ohmic heating of commercially processed apple juice using five sequential electric fields results in rapid inactivation of Alicyclobacillus acidoterrestris spores. Int. J. Food Microbiol. 2017, 246, 80–84. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kang, D.-H. Comparison of pH effects on ohmic heating and conventional heating for inactivation of Escherichia coli O157: H7, Salmonella enterica Serovar Typhimurium and Listeria monocytogene s in orange juice. LWT-Food Sci. Technol. 2015, 64, 860–866. [Google Scholar] [CrossRef]
- Demirdöven, A.; Baysal, T. Optimization of Ohmic Heating Applications for Pectin Methylesterase Inactivation in Orange Juice. J. Food Sci. Technol. 2014, 51, 1817–1826. [Google Scholar] [CrossRef]
- Funcia, E.S.; Gut, J.A.W.; Sastry, S.K. Effect of Electric Field on Pectinesterase Inactivation during Orange Juice Pasteurization by Ohmic Heating. Food Bioprocess Technol. 2020, 13, 1206–1214. [Google Scholar] [CrossRef]
- Makroo, H.A.; Saxena, J.; Rastogi, N.K.; Srivastava, B. Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: Effects of the treatment on pH, lycopene, total phenolic content, and color of the juice. J. Food Processing Preserv. 2017, 41, e13271. [Google Scholar] [CrossRef]
- Elzubier, A.S.; Thomas, C.S.Y.; Sergie, S.Y.; Chin, N.L.; Ibrahim, O.M. The effect of buoyancy force in computational fluid dynamics simulation of a two-dimensional continuous ohmic heating process. Am. J. Appl. Sci. 2009, 6, 1902–1908. [Google Scholar] [CrossRef][Green Version]
- Hashemi, S.M.B.; Roohi, R. Ohmic heating of blended citrus juice: Numerical modeling of process and bacterial inactivation kinetics. Innov. Food Sci. Emerg. Technol. 2019, 52, 313–324. [Google Scholar] [CrossRef]
- Darvishi, H.; Salami, P.; Fadavi, A.; Saba, M.K. Processing kinetics, quality and thermodynamic evaluation of mulberry juice concentration process using Ohmic heating. Food Bioprod. Processing 2020, 123, 102–110. [Google Scholar] [CrossRef]
- Leizerson, S.; Shimoni, E. Effect of Ultrahigh-temperature Continuous Ohmic Heating Treatment on Fresh Orange Juice. J. Agric. Food Chem. 2005, 53, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Coates, G.A. Effect of Thermal Pasteurization on Valencia Orange Juice Color and Pigments. LWT Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Yu, L.J. Emerging Preservation Methods for Fruit Juices and Beverages. In Food Additive; InTech.: Rijeka, Croatia, 2012. [Google Scholar]
- Min, S.; Jin, Z.T.; Min, S.K.; Yeom, H.; Zhang, Q.H. Commercial-scale pulsed electric field processing of orange juice. J. Food Sci. 2003, 68, 1265–1271. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S.; Kim, E.; Kim, Y.-N.; Lee, J.; Lee, D.-U. Effects of Pulsed Electric Field and Thermal Treatments on Microbial Reduction, Volatile Composition, and Sensory Properties of Orange Juice, and Their Characterization by a Principal Component Analysis. Appl. Sci. 2021, 11, 186. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Jin, Z.T.; Ruhlman, K.T.; Qiu, X.; Zhang, Q.H.; Richter, E.R. Microbial safety and shelf-life of apple juice and cider processed by bench and pilot scale PEF systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 77–86. [Google Scholar] [CrossRef]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Effect of high hydrostatic pressure treatment on post processing antioxidant activity of fresh Navel orange juice. Food Chem. 2005, 91, 495–503. [Google Scholar] [CrossRef]
- Deliza, R.; Rosenthal, A.; Abadio, F.B.D.; Silva, C.H.; Castillo, C. Application of High Pressure Technology in the Fruit Juice Processing: Benefits Perceived by Consumers. J. Food Eng. 2005, 67, 241–246. [Google Scholar] [CrossRef]
- Torres, B.; Tiwari, B.K.; Patras, A.; Cullen, P.J.; Brunton, N.; ODonnell, C.P. Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 93–97. [Google Scholar] [CrossRef]
- Donsi, G.; Ferrari, G.; Di Matteo, M. High-pressure stabilization of orange juice: Evaluation of the effects of process conditions. Ital. J. Food Sci. 1996, 8, 99–106. [Google Scholar]
- Novotna, P.; Valentova, H.; Strohalm, J.; Kyhos, K.; Landfeld, A.; Houska, M. Sensory evaluation of high pressure treated apple juice during its storage. Czech J. Food Sci. 1999, 17, 196–198. [Google Scholar]
- Lambert, Y.; Demazeau, G.; Largeteau, A.; Bouvier, J.-M. Changes in aromatic volatile composition of strawberry after high pressure treatment. Food Chem. 1999, 67, 7–16. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Orsolani, L.; Martínez-Yépez, A.; Tapia, M.S. Microbiological and sensory quality of sonicated calcium-added orange juice. LWT-Food Sci. Technol. 2010, 43, 808–813. [Google Scholar] [CrossRef]
- Pala, C.U.; Toklucu, A.K. Microbial, physicochemical and sensory properties of UV-C processed orange juice and its microbial stability during refrigerated storage. LWT Food Sci. Technol. 2013, 50, 426–431. [Google Scholar] [CrossRef]
- Pala, C.U.; Toklucu, A.K. Effect of UV-C on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Ishita, C.; Athmaselvi, K.A. Changes in pH and colour of watermelon juice during ohmic heating. Int. Food Res. J. 2017, 24, 741–746. [Google Scholar]
- Lopez, A.C.A.E.; Andrade, S.H.; Amorim, R.P.; Duarte, J.C.; Ferreira, W. New Alcoholic Fermented Beverages—Potentials and Challenges. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 577–603. [Google Scholar] [CrossRef]
- Swami, S.B.; Thakor, N.J.; Divate, A.D. Fruit wine production: A review. J. Food Res. Technol. 2014, 2, 93–100. [Google Scholar]
- Barrett, D.M.; Lloyd, B. Advanced preservation methods and nutrient retention in fruits and vegetables. J. Sci. Food Agric. 2012, 92, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Wines from fruits other than grapes: Current status and future prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, L.G.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Int. Food Res. J. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Harding, G. A Wine Miscellany; Carkson Potter Publ.: New York, NY, USA, 2005. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terphenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic acid fermentation of apricot juice by mono- and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 2017, 27, 547–554. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Hu, J.; Zhao, G. Fermentation kinetics of different sugars by Apple wine Yeast Saccharomyces cerevisiae. J. Inst. Brew. 2004, 110, 340–346. [Google Scholar] [CrossRef]
- Robinson, J. The Oxford Companion to Wine, 3rd ed.; Oxford University Press: New York, NY, USA, 2006; p. 840. [Google Scholar]
- Selli, S.; Kürkçüoğlu, M.; Kafkas, E.; Cabaroglu, T.; Demirci, B.; Başer, K.H.C.; Canbas, A. Volatile flavour components of mandarin wine obtained from clementines (Citrus reticula Blanco) extracted by solid-phase microextraction. Flavour Fragr. J. 2004, 19, 413–416. [Google Scholar] [CrossRef]
- Morton, J.F. Jaboticabas. In Fruits of Warm Climates; Morton, J.F., Ed.; Creative Resource Systems: Winterville, NC, USA, 1987; pp. 371–374. [Google Scholar]
- Heatherbell, D.A.; Struebi, P.; Eschenbruch, R.; Withy, L.M. A New Fruit Wine from Kiwifruit: A Wine of Unusual Composition and Riesling Sylvaner Character. Am. J. Enol. Vitic. 1980, 31, 114–121. [Google Scholar]
- Lin, F. Florence Lin’s Chinese regional cookbook. In Hawthorn Books; Dutton Adult: New York, NY, USA, 1975; p. 39. ISBN 978-0-8015-2674-9. [Google Scholar]
- Adaikan, P.; Ganesan, A.A. Mechanism of the Oxytoxic activity of Comosus proteinases. J. Pharm. Biol. 2004, 42, 646–655. [Google Scholar] [CrossRef]
- Duarte, F.W.; Dragone, G.; Dias, R.D. Fermentative behavior of Saccharomyces strains during microvinification of raspberry juice (Rubus idaeus L.). Int. J. Food Microbiol. 2010, 143, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.W.; Dias, R.D.; Oliveira, M.J. Raspberry (Rubusidaeus L.) wine: Yeast selection, sensory evaluation, and instrumental analysis of volatile and other compounds. Food Res. Int. 2010, 43, 2303–2314. [Google Scholar] [CrossRef]
- Mena, P.; Gil-Izquierdo, A.; Moreno, D.A.; Martí, N.; García-Viguera, C. Assessment of the melatonin production in pomegranate wines. LWT-Food Sci. Technol. 2012, 47, 13–18. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of non-Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, F.F.; Kirchmayr, M.; Arellano-Plaza, M. Use of non-Saccharomyces yeasts in cider fermentation: Importance of the nutrients addition to obtain an efficient fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef] [PubMed]
- László, Z.; Hodúr, C.; Pálinka, J.C. Hungarian Distilled Fruit. Traditional Foods. In Integrating Food Science and Engineering Knowledge into the Food Chain; Kristbergsson, K., Oliveira, J., Eds.; Springer: Boston, MA, USA, 2016; Volume 10. [Google Scholar] [CrossRef]
- Fejzullahu, F.; Kiss, Z.; Kun-Farkas, G.; Kun, S. Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit. Foods 2021, 10, 1336. [Google Scholar] [CrossRef]
- Kunyeit, L.; Anu-Appaiah, K.A.; Rao, R.P. Application of probiotic yeasts on candida species associated infection. J. Fungi 2020, 6, 189. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Vilela, A. Consumer’s acceptability and health consciousness of probiotic and prebiotic of non-dairy products. Int. Food Res. J. 2022, 151, 110842. [Google Scholar] [CrossRef]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; M’hir, S.; Hamdi, M. Microbiological, biochemical, and functional aspects of fermented vegetable and fruit beverages. J. Chem. 2020, 2020, 5790432. [Google Scholar] [CrossRef]
- Ong, Y.Y.; Tan, W.S.; Rosfarizan, M.; Chan, E.S.; Tey, B.T. Isolation and identification of lactic acid bacteria from fermented red dragon fruit juices. J. Food Sci. 2012, 77, M560-4. [Google Scholar] [CrossRef]
- Saelee, M.; Sivamaruthi, B.S.; Sirilun, S.; Kesika, P.; Peerajan, S.; Chaiyasut, C. Effect of Green Tea Extract during Lactic Acid Bacteria Mediated Fermentation of Morinda citrifolia Linn. (Noni) Fruit Juice. Pak. J. Biol. Sci. 2019, 22, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Matsumoto, Y.; Nishida, S.; Sekimizu, K. Decreased sugar concentration in vegetable and fruit juices by growth of functional lactic acid bacteria. Drug Discov. Ther. 2017, 11, 30–34. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Emiljanowicz, k.E.; Malinowska-Pańczyk, E. Kombucha from alternative raw materials—The review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Vina, I.; Semjonovs, P.; Linde, R.; Deniņa, I. Current evidence on physiological activity and expected health effects of kombucha fermented beverage. J. Med. Food 2014, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Zubaidah, E.; Apriyadi, T.E.; Kalsum, U.; Widyastuti, E.; Estiasih, T.; Srianta, I.; Blanc, P.J. In vivo evaluation of snake fruit kombucha as hyperglycemia therapeutic agent. Int. Food Res. J. 2018, 25, 453–457. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Cadwallader, K.R.; Feng, H.; Martin, S.E. Sonication in combination with heat and low pressure as an alternative pasteurization treatment – Effect on Escherichia coli K12 inactivation and quality of apple cider. Ultrason. Sonochem. 2013, 20, 1131–1138. [Google Scholar] [CrossRef]
- Kasikci, B.M.; Bagdatlioglu, N. High hydrostatic pressure treatment of fruit, fruit products and fruit juices: A review on phenolic compounds. J. Food Health Sci. 2016, 2, 27–39. [Google Scholar]
- Khandpur, P.; Gogate, P.R. Understanding the effect of novel approaches based on ultrasound on sensory profile of orange juice. Ultrason. Sonochem. 2015, 27, 87–95. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Chandran, S. Effects of Thermal and Non-thermal Processing on Phenolic Compounds, Antioxidant Activity and Sensory Attributes of Chokanan Mango (Mangifera indica L.) Juice. Food Bioprocess Technol. 2015, 8, 2256–2267. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Zarei, M.; Hussin, A.S.M. Effects of metabolite changes during lacto-fermentation on the biological activity and consumer acceptability for dragon fruit juice. LWT-Food Sci. Technol. 2020, 121, 108992. [Google Scholar] [CrossRef]
- Do, T.; Fan, L. Probiotic Viability, Qualitative Characteristics, and Sensory Acceptability of Vegetable Juice Mixture Fermented with Lactobacillus Strains. Food Sci. Nutr. 2019, 10, 412–427. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Devaere, J.; Schouteten, J.J.; Gellynck, X.; de Winne, A.; Matemu, A.O.; Raes, K. Effect of lactic acid fermentation of watermelon juice on its sensory acceptability and volatile compounds. Food Chem. 2021, 358, 129809. [Google Scholar] [CrossRef]
- Kasron, N.; Abdul Manan, M.; Mat Azmin, M.N.H.; Saari, N.A.; Abd Latip, M. Consumer Acceptance of Fermented Drinks in Malaysia. Malays. J. Soc. Sci. Humanit. 2021, 6, 306–314. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Szczepańska, J.; Danielczuk, J.; Zawada, K. The Development and Consumer Acceptance of Functional Fruit-Herbal Beverages. Foods 2020, 9, 1819. [Google Scholar] [CrossRef]
- Cordelle, S.; Lange, C.; Schlich, P. On the consistency of liking scores: Insights from a study including 917 consumers from 10 to 80 years old. Food Qual. Prefer. 2004, 15, 831–841. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).









