Abstract
Winemaking is a process that generates a large volume of solid waste biomass, which is currently under extensive investigation as a bioresource of precious polyphenolic compounds. These substances are retrieved from vinification side streams principally by deploying solid–liquid extraction methods. In this frame, the present investigation had as objective the development of an alternative, green extraction process for polyphenols, through integration of ultrasonication as a pretreatment stage, and subsequent extraction with aqueous β-cyclodextrin. Polyphenol recovery from red grape pomace (RGP) was shown to be significantly enhanced by ultrasonication pretreatment, and the use of β-cyclodextrin effectively boosted the aqueous extraction. Under optimized conditions, established by response surface methodology, the maximum yield in total polyphenols was 57.47 mg GAE g−1 dm, at 80 °C, requiring a barrier of 10.95 kJ mol−1. The extract produced was significantly enriched in catechin and quercetin, compared to the aqueous extract, exhibiting also increased antiradical activity. These findings highlighted the value of the process developed for targeted recovery of certain polyphenols and the preparation of task-specific extracts.
1. Introduction
Globally, the agricultural activity related to food production has as a result the generation of wastes, which, according to FAO, in U.S.A. and in China only may exceed 47 million tons [1]. A large proportion of fruit, vegetable and cereal waste derives from industrial processing, and it is mainly composed of leaves, peels, roots, seeds and stems [2]. The improper handling and disposal of such materials, which are rich in organic load, may cause serious environmental pollution, and therefore their management and processing is of undisputed importance. On the other hand, the ongoing research on food waste valorization strategies integrated into a biorefinery concept has revealed the enormous potential of food side streams as bioresources of a vast variety of precious phytochemicals [3].
The wine industry has a crucial position in the agro-industrial sector, as grapes are one of the most important fruit crops worldwide [4]. The grape production was estimated around 77.8 metric tons in 2018 [5] and during vinification, approximately 25% of the grape mass results in grape marc. It has been estimated that the production of 6 L of wine is accompanied by approximately 1 kg of grape marc, which thus accounts for worldwide production of 10.5–13.1 million tons, on an annual basis. Grape marc consists principally of stems, skins, and seeds, which are the residues of grape crushing and juicing (pressing) steps. This bio-waste is particularly rich in multifunctional polyphenols, which belong to various classes, and they have been demonstrated to exhibit a range of biological activities [6].
The effective recovery of polyphenols from grape pomace has been a significant issue, and a wide diversity of methodologies have been developed for this purpose [7]. However, in compliance with the Green Chemistry principles, the objectives solicited by an eco-friendly extraction process should encompass the use of alternative non-toxic and reusable solvents, high extraction yields, production of a safe and high-quality extract/product, and minimum energy consumption [8]. It is of vital importance to choose a suitable solvent, as its physical-chemical properties will largely determine the extraction performance, as well as the means of appropriate downstream processing (e.g., evaporation, adsorption) [8].
Cyclodextrins (CDs) are a group of cyclic oligosaccharides consisting of subunits of α(1→4)-linked D-glucopyranose. Alpha-, β- and γ-CDs are the most common CDs, consisting of six, seven and eight glucose units, respectively, and they are obtained by enzymic starch degradation. Their shape has a truncated cone form, with hydroxyl functions directed towards the cavity’s outer surface (Figure 1).
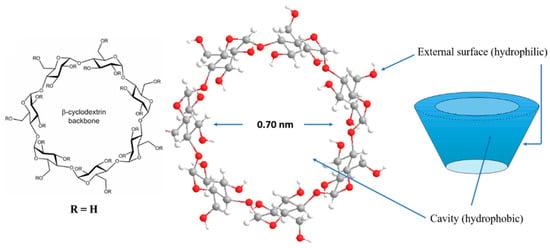
Figure 1.
Structure of β-cyclodextrin.
This three-dimensional structure of the CD molecules is characterized by an external hydrophilic surface and an internal hydrophobic cavity, providing both water solubility and the ability to encapsulate appropriately sized hydrophobic molecules inside the cavity, producing inclusion complexes [9]. The use of CDs for the extraction of polyphenolic compounds is a state-of-the-art trend, offering unparalleled “green extraction” possibilities. This is because, while common organic solvents used for the recovery of polyphenols (e.g., ethanol, ethyl acetate, etc.) show excellent potential for the dissolution and extraction of polyphenols, their use is of significant environmental concern [7]. Aqueous systems containing CDs may be regarded as green extraction media, with a potential of replacing organic solvents in such processes.
The use of various cyclodextrins has been stressed as a way towards fostering aqueous polyphenols extraction and achieving increased yields. This has been demonstrated for various polyphenol classes of red grape pomace (RGP) [10], polyphenols of vine shoots [11], catechin and epicatechin of RGP [12], flavone glycosides and rosmarinic acid from Salvia fruticosa [13], polyphenols of potato peels [14], and flavanone glycosides from orange peels [15].
In such a frame, the study presented herein had as objective the thorough investigation of polyphenol extraction from RGP, using β-CD. The scope was to investigate (i) the effect of the ultrasonication pretreatment on the extraction performance and (ii) to examine whether β-CD could function as an extraction booster with selectivity towards specific polyphenolic constituents. The methodology developed was integrated by a pretreatment step using ultrasonication, which has been previously proven to significantly enhance the extraction performance [16]. The relevant mechanisms implicate extraction enhancement by cavitation phenomena. The result is the implosion of cavitation bubbles on the surface of the solid matrix, producing micro-jetting and other associated effects (surface peeling, erosion, and particle breakdown), but also micro mixing [8]. Critical parameters such as the ultrasonication time, β-CD concentration and liquid-to-solid ratio were jointly appraised by response surface methodology, while the performance of the technique was assessed in comparison to commonly used water/ethanol mixtures and detailed investigation of the polyphenolic composition and antioxidant activity of the extracts produced. To the best of the authors’ knowledge, this is a novel methodology involving a green pretreatment technique (ultrasonication) and a food-grade extraction booster (β-CD) to produce RGP extracts enriched in functional bio-phenols.
2. Materials and Methods
2.1. Chemicals and Reagents
Folin–Ciocalteu reagent, L-ascorbic acid (99.5%), β-cyclodextrin (β-CD), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), catechin, quercetin, rutin (quercetin 3-O-rutinoside) (>94%), and 2,2-diphenylpicrylhydrazyl (DPPH) were from Sigma-Aldrich (Darmstadt, Germany). Iron chloride hexahydrate and citric acid anhydrous were from Merck (Darmstadt, Germany). Sodium carbonate anhydrous (99%) was from Penta (Prague, Czech Republic). Gallic acid hydrate was from Panreac (Barcelona, Spain). Pelargonin (pelargonidin 3,5-di-O-glucoside) chloride was from Extrasynthese (Genay, France). Solvents used for chromatographic purposes were HPLC grade.
2.2. Red Grape Pomace (RGP)
RGP originated from industrial processing of Vitis vinifera cv. Muscat of Hamburg grapes, and it was kindly donated by a local winery (Karditsa, Greece). The material was collected following a 7-day pomace contact, and it was transferred to the laboratory within 2 h after collection, where it was stored at −40 °C until used. A suitable mass of RGP was thawed just prior to experimentations and placed on a tray to form approximately 0.5 cm thick layers. RGP was dried in a laboratory oven (Binder BD56, Bohemia, NY, USA), at 80 °C, for 450 min. Following drying, RGP was pulverized in a ball mill to yield a material with average particle diameter of 0.384 mm. This feed was placed in air-tight plastic vessels and stored at 4 °C, protected from light.
2.3. Extraction of RGP
All extractions were performed for 180 min, at ambient temperature (27 ± 1 °C) and 900 rpm, using a magnetic stirrer (Witeg, Wertheim, Germany). The extraction medium was deionized water, containing 1 g L−1 citric acid, adjusted at pH 2. This regulation was deemed necessary, to maintain acidic pH and avoid polyphenol losses through oxidation. Then suitable β-CD amount was dissolved in 20 mL of the extraction medium and mixed with predetermined amount of dried RGP. Samples were ultrasonicated for various periods prior to every extraction. The concentration of CD (CCD), the liquid-to-solid ratio (RL/S) and the time of ultrasonication pretreatment (tUS) were determined by the experimental design.
Ultrasonications were carried out with an Elma S 100 (H) heated ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany), at nominal power of 550 W and frequency of 37 Hz. The actual ultrasonication power (P) dissipated into the system, and the acoustic energy densities (AED) were determined as follows:
where m is the mass of the coupling liquid (water) into the ultrasonication bath (in g), Cp the specific heat capacity of water (4.2 J g−1 K−1) and the temperature rise per s, which was determined by fitting temperature change (dT), measured by a thermocouple, versus time [17]. P and AED thus determined were 103.4 W and 15.12 W L−1, respectively.
2.4. Experimental Design—Response Surface Methodology
The appraisal of three critical factors, namely, the liquid-to-solid ratio (RL/S), the CD concentration and the ultrasonication time (tUS), was accomplished by implementing response surface methodology. The setup of the experiments was based on a Box–Behnken design with three central points and a total of 15 design points. The choice regarding the ranges for every independent (process) variable was based on previous investigations [13,16]. Codification of actual variable levels (Table 1) was carried out as described elsewhere [13]. The overall significance of the model (R2, p) and the significance of the individual polynomial coefficients for each model were based on ANOVA and lack-of-fit tests, with a significance level of at least 95%.

Table 1.
Independent variables used for the response surface methodology and their codification.
2.5. Kinetics and Temperature Effect
The first-order model, as previously implemented for RGP extraction, was used [18]:
YTP(t) = YTP(s)(1 − e−kt)
YTP(t) and YTP(s) correspond to the TP yield at any time t and at saturation (equilibrium), respectively. YTP(s) is the maximum YTP attained under the given set of experimental conditions, and k is the first-order extraction rate constant.
Temperature effects were assessed using the Arrhenius equation:
k0 represents a temperature-independent factor (min−1), R the universal gas constant (8.314 J K−1 mol−1), T the absolute temperature (K) and Ea the activation energy (J mol−1). The linear form of Equation (4) is
Ea could be determined graphically, by plotting lnk against 1/T (slope =).
2.6. Total Polyphenol (TP) Determination
For TP determination, all samples were diluted 1:50 with 0.5% (v/v) aqueous formic acid prior to analysis, and a previously described micro-scale methodology was employed [19]. Volume of 0.1 mL diluted sample and 0.1 mL Folin–Ciocalteu reagent were thoroughly mixed in a 1.5-mL Eppendorf tube and allowed to react for 2 min. Then 0.8 mL Na2CO3 solution (5% w/v) was added, the sample was vortexed and placed in a water bath regulated at 40 °C, for 20 min. Following incubation, the sample was cooled down with tap water, and the absorbance at 740 nm was read, using appropriate blank. Total polyphenol concentration (CTP) was calculated from a calibration curve, using gallic acid as standard (10–80 mg L−1). Results were expressed as mg gallic acid equivalents (GAE) L−1.
2.7. Antioxidant Activity
The antioxidant activity of the extracts produced was evaluated by two complementary tests, the antiradical activity (AAR) and the ferric-reducing power (PR). AAR was determined employing a stoichiometric methodology, with the DPPH as the radical probe. Results were provided as μmol DPPH g−1 dm. The ferric-reducing power was determined with a modified FRAP assay, and results were expressed as μmol ascorbic acid equivalents (AAE) per g dry mass. The protocols followed in both cases have been reported in full detail elsewhere [20].
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
The equipment employed was a A Shimadzu CBM-20A liquid chromatograph (Shimadzu Europa GmbH, Duisburg, Germany), with a SIL-20AC auto sampler and a CTO-20AC column oven. The detector was a Shimadzu SPD-M20A interfaced by a Shimadzu LC solution software. Detail concerning the column used, as well as the elution program, have been analytically reported elsewhere [16]. Quantification was performed at 280, 320, 360 and 520 nm for gallic acid and flavanols, hydroxycinnamates, flavonols and anthocyanins, respectively, based on calibration curves (1–50 μg mL−1), constructed with gallic acid (R2 = 0.9990), catechin (R2 = 0.9999), chlorogenic acid (R2 = 0.9999), quercetin (R2 = 0.9999), rutin (R2 = 0.9990) and pelargonin (R2 = 0.9999). Chromatograms of standards can be found in the Supplementary File (Figures S1–S4).
2.9. Liquid Chromatography—Mass Spectrometry
A Finnigan (San Jose, CA, USA) MAT Spectra System P4000 pump, a UV6000LP diode array detector and a Finnigan AQA mass spectrometer were used. Chromatographic analyses were accomplished on a Fortis RP-18 column, 150 mm × 2.1 mm, 3 μm, with a 10-μL injection loop, at 40 °C. Mass spectra were acquired with electrospray ionization (ESI) in both positive and negative ion mode. Details regarding mass spectrometry settings and elution have been reported elsewhere [21].
2.10. Statistical Analyses
For the response surface methodology, the experimental setup and all associated statistical analyses (ANOVA, lack-of-fit) were performed with JMP™ Pro 13 (SAS, Cary, NC, USA). Linear and non-linear regressions, as well as kinetics model fitting, were conducted with SigmaPlot™ 12.5 (Systat Software Inc., San Jose, CA, USA). Extractions were carried out at least twice and all determinations in triplicate. Values reported are averages ± standard deviation (SD).
3. Results and Discussion
3.1. Process (Extraction) Optimization
The process deployed was set up to evaluate and optimize the effect of three key extraction parameters, RL/S, CCD and tUS, and to detect significant synergistic functions between them. The evaluation of the fitted model and response surface suitability were based on lack-of-fit and ANOVA tests (Figure 2), taking into consideration the closeness of the predicted and measured values (Table 2). The mathematical model derived (second-degree polynomial equation), composed only of the significant terms, was as follows:
YTP = 23.20 + 2.02X1 + 3.32X3 + 2.61X1X2 + 2.20X1X3 − 2.80X12 + 4.81X32
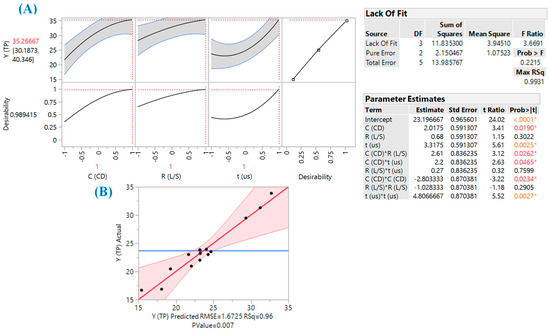
Figure 2.
Data on the desirability function (A) and correlation between actual and predicted values (B). Inset tables show statistical information regarding the model derived from the implementation of response surface methodology. Asterisk denotes statistically significant values.

Table 2.
The experimental design used to implement response surface methodology, displaying all points considered and the measured and predicted values of the response.
The square correlation coefficient (R2) of the model was 0.96, suggesting a low total variability around the mean given by the model. Since the p value (0.0070) for lack-of-fit (assuming a confidence interval of 95%) was highly significant, it can be supported that equation exhibited excellent adjustment to the experimental data. The three-dimensional plots generated based on the model (Figure 3) depict at-a-glance the effect of the experimental variables on the response (YTP). The desirability function permitted the determination of the optimal values for all three independent (process) variables, which were CCD = 1.50% (w/v), RL/S = 90 mL g−1, and tUS = 30 min. Under these conditions, the predicted maximum response (YTP) was estimated to be 35.27 ± 5.08 mg GAE g−1 dm.
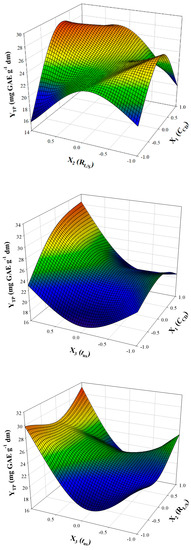
Figure 3.
3D graphs illustrating the effect of simultaneous changes in process (independent) variables on the response.
CCD had a direct and positive effect on YTP, as shown by the model (Equation (6)), but its quadratic effect was negative. However, the range of CCD could not be much further extended upwards, because of constrains arising by the solubility of β-CD in water, which at 25 °C, is 18.5 mg mL−1 [22]. The finding that shifting CCD within the range tested could significantly impact YTP indicated that increases in CCD up to 1.50% may provide statistically higher YTP. In previous studies on β-CD-aided extraction of polyphenols from RGP, it was shown that raising CCD from 0 to 8 mM (approximately up to 1.00%) did have a significant effect on YTP, but an increase from 8 to 13 mM (approximately up to 1.60%) did not [12]. Recent investigations carried out with 2-hydroxypropyl β-cyclodextrin (HP-β-CD) extraction of polyphenols from potato peels demonstrated that switching HP-β-CD concentration from 0.25 to 0.5 mM did provoke a significant increase in YTP, but further increase up to 8 mM gave no statistically significant effect [15].
At this point, the role of RL/S should be appraised in conjunction with changes in CCD. Considering the model, cross terms between CCD (X1) and RL/S (X2) were positive and significant. This outcome suggested emphatically that concomitant rising in both these variables contributed to attaining statistically higher YTP. Therefore, the efficiency of β-CD in assisting polyphenol recovery from RGP may largely depend on the RL/S used. This is in close agreement with recent data on cyclodextrin-aided polyphenol extraction from Salvia fruticosa, where the optimum RL/S determined was on average 96 mL g−1 [13]. This value is practically the same with the optimum one determined in this study (90 mL g−1) and strongly indicates that cyclodextrin-aided polyphenol extraction might require high RL/S.
This might occur because higher RL/S results in more dilute extracts. Therefore, for a given CCD, the formation of inclusion complexes between polyphenols and β-CD would be facilitated, leading to enhanced polyphenol solubility and thus more extended polyphenol extraction. By contrast, if a low RL/S is used, then after the elapse of a certain time, all β-CD molecules would form inclusion complexes, due to high polyphenol concentration, and further polyphenol solubilization would not be favored. Nevertheless, this hypothesis might depend on the polyphenolic load of the plant material and the rate by which the liquid phase is enriched in solute (polyphenols). For example, β-CD-aided polyphenol extraction from coffee pulp, a material that contains significantly less polyphenols than RGP, was found to require RL/S of 30 mL g−1 [23]. It should also be stressed that, irrespective of the presence of β-CD, the magnitude of RL/S is correlated with the concentration gradient between the liquid phase (extraction medium) and the solid particle, which is directly implicated in mass transfer phenomena. If RL/S is below a certain level, then fast diffusion of the solute during extraction may not be favored, due to non-negligible resistance to mass transfer [24]. On this theoretical background, it could be argued that for relatively high RL/S, increases in CCD may provide significantly improved YTP.
tUS was also a process variable that exerted significant effect on YTP, and this finding highlighted the importance of ultrasonication pretreatment in the extraction efficiency. Ultrasonication pretreatment followed by stirred-tank extraction was shown to be far more effective for polyphenol recovery from RGP, compared to ultrasonication as a standalone extraction method [25]. This has also been demonstrated for subcritical water extraction of RGP polyphenols, where ultrasonic irradiation prior to extraction boosted polyphenol yield [26]. Similar results were reported for polyphenol extraction from elderberry flowers (Sambucus nigra), using a deep eutectic solvent [16]. On the contrary, ultrasonication pretreatment had a negative effect on the extraction of orange peel polyphenols using HP-β-CD [15].
Another critical issue pertaining to process efficiency was the cross term between CCD (X1) and tUS (X2), which was shown to be statistically significant (Figure 2, inset “Parameter Estimates” table). This outcome implied that ultrasonication and β-CD addition might act jointly towards enhancing extraction efficiency. Such a combined effect has been previously reported for total polyphenol extraction from RGP [12,27] but also for ultrasound-assisted extraction of stilbenes from Polygonum cuspidatum in the presence of β-CD [28], olive pomace polyphenols in the presence of HP-β-CD and methyl β-cyclodextrin (m-β-CD) [29] and red beet polyphenols in the presence of β-CD [30]. It is well known that ultrasonication provokes cavitation phenomena, which can contribute to increasing mass transfer, through microturbulence (microstreamings) as a result of symmetric cavitation. Moreover, cavitational collapse on solid surface or asymmetric cavitation may cause microjets of extractant toward the solid surface, and this can lead to cell disruption, liberating the solute [31]. However, it has also been stressed that the sonochemical effect may be deleterious for polyphenols [32], with negative consequences on the antioxidant activity [33]. On this ground, it could be supported that the role of β-CD might be crucial for both solubilizing and protecting the solute (polyphenols). Nevertheless, the protection that β-CD could offer to polyphenols against ultrasound-induced degradation is a subject that merits investigation.
3.2. Extraction Kinetics and the Effect of Temperature
After establishing the optimal extraction conditions, kinetics was traced to draw information regarding the effect of temperature (Figure 4). The kinetic parameters determined using the first-order model are analytically given in Table 3.
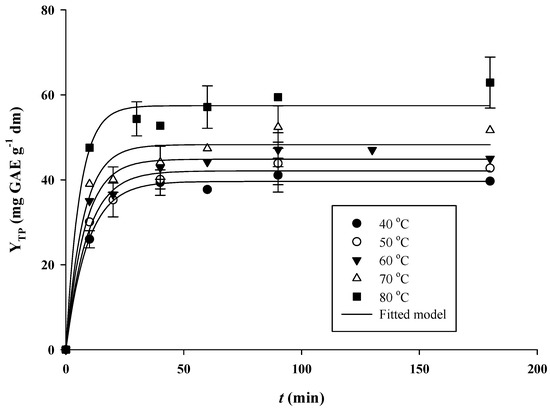
Figure 4.
Kinetics of total polyphenol extraction under optimized conditions (CCD = 1.5%, tus = 30 min, RL/S = 90 mL g−1). Extractions were performed under stirring at 900 rpm, for 180 min.

Table 3.
Kinetic data for polyphenol extraction from RGP, carried out under optimized conditions.
An increase in temperature from 40 to 80 °C provoked a positive effect on total polyphenol extraction, and boosted YTP(s) from 39.64 to 57.47 mg GAE g−1 dm. The effect of temperature on polyphenol extraction from RGP is generally positive [18,34], since increasing temperature favors polyphenol diffusivity and enhances polyphenol solubility [35]. On the other hand, excessive temperature increase may trigger thermal degradation of thermolabile molecules, such as anthocyanins [36]. The highest YTP(s) level achieved (57.47 mg GAE g−1 dm) was similar to 55 mg GAE g−1 dm found for aqueous extraction [37] and comparable to 66.70 mg GAE g−1 dm determined for extraction with aqueous glycerol [18]. However, it is to be stressed that polyphenol yields from RGP extraction reported in the literature may vary from 7.7 mg GAE g−1 dm [38] to as high as 77.80 mg GAE g−1 dm [39].
The extraction rate constant, k, behaved likewise, and it increased from 0.108 to 0.172 min−1. This fact clearly indicated that the progression of the extraction was fast, considering the k values reported in the literature for RGP polyphenol extraction are up to 0.130 min−1 [40]. Nevertheless, for subcritical water extraction in semi-continuous mode, a value of 0.187 min−1 has been attained, at 120 °C [41]. The effect of temperature on k followed the Arrhenius model (Figure 5), and the activation energy (Ea) thus determined was 10.95 kJ mol−1. This barrier is close to 13.94 kJ mol−1 determined for aqueous glycerol extraction of RGP polyphenols [18] but more than twice the level found for ultrasound-assisted extraction [38]. Nevertheless, it is significantly lower than 23 kJ mol−1, reported by other authors for RGP polyphenol extraction [40].
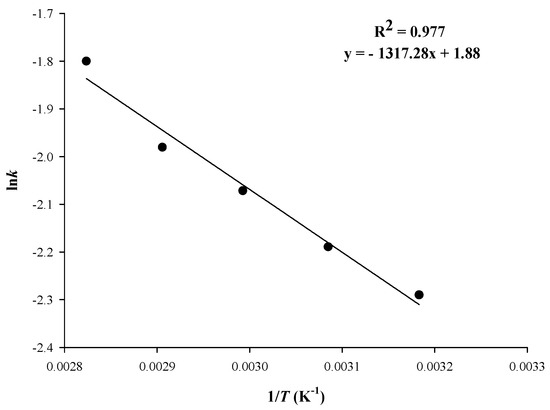
Figure 5.
Arrhenius plot showing the effect of T on the first-order extraction rate constant, k. Extractions were performed under optimized conditions (CCD = 1.5%, tus = 30 min, RL/S = 90 mL g−1), under stirring at 900 rpm, for 180 min.
3.3. Comparative Evaluation of Extracts
After having established the optimized conditions for β-CD-aided aqueous polyphenol extraction from RGP, an additional evaluation was accomplished to verify whether β-CD can significantly boost extraction yield and provide extracts with improved antioxidant properties. Extractions with 60% (v/v) aqueous ethanol were also performed to have a measure of comparison. In Figure 6, the results concerning YTP can be seen. When only water was used as the extraction solvent, the YTP attained was 49.31 mg GAE g−1 dm. On the other hand, extraction with aqueous β-CD afforded YTP of 59.64 mg GAE g−1 dm, boosting the extraction efficiency by 17.6%. Extraction with aqueous ethanol was proven far more effective, giving YTP of 97.93 mg GAE g−1 dm.
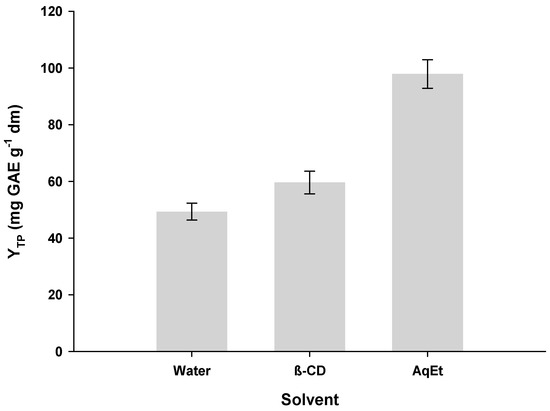
Figure 6.
Bar plot displaying YTP of the extraction media tested. Extractions with β-CD were performed under optimized conditions (CCD = 1.5%, tus = 30 min, RL/S = 90 mL g−1), under stirring at 900 rpm, for 180 min. The same conditions (except for the addition of β-CD) were applied to the extractions with water and 60% aqueous ethanol (AqEt).
In the same line, the extract produced with water had a PR value of 247.98 μmol AAE g−1 dm, whereas that obtained with aqueous β-CD had a PR of 314.72 μmol AAE g−1 (21.2% increase) and the one obtained with aqueous ethanol 522.98 μmol AAE g−1 (Figure 7B). However, with regard to AAR, the results displayed a different pattern. As shown in Figure 7A, extraction with aqueous β-CD did afford extract with AAR enhanced by 51.1% compared to the extract generated with water but also enhanced by 12.2% compared to the extract produced with aqueous ethanol. This finding pointed emphatically to the role of β-CD in the expression of radical-scavenging effects. Similar phenomena have been previously reported for coffee extract [42], although some contradictory outcome has also been demonstrated for the role of β-CD in the manifestation of antiradical effects [43].
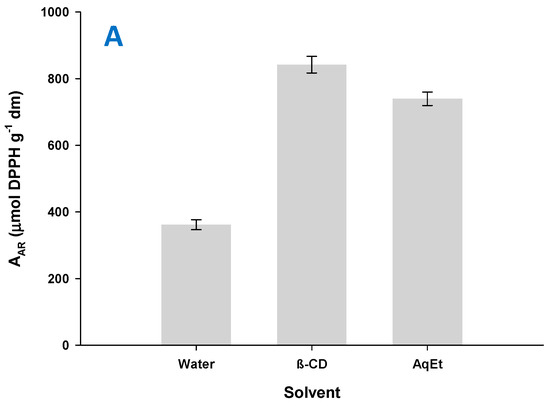
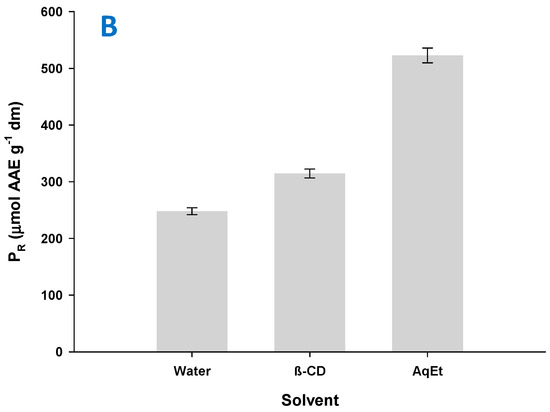
Figure 7.
Bar plot showing AAR (A) and PR (B) of the extracts produced with the extraction media tested. Extractions with β-CD were performed under optimized conditions (CCD = 1.5%, tus = 30 min, RL/S = 90 mL g−1), under stirring at 900 rpm, for 180 min. The same conditions (except for the addition of β-CD) were applied to the extractions with water and 60% aqueous ethanol (AqEt).
Based on evidence emerged from studies on pure compounds, certain polyphenols such as quercetin [44], chlorogenic acid [45] and rosmarinic acid [46] may exhibit enhanced antioxidant performance when tested encapsulated in CDs compared to their free (non-encapsulated) state. A theory proposed to interpret such a behavior was that polyphenol radicals, generated as a result of reaction with other radicals, may be more effectively stabilized when engulfed in a CD hydrophobic cavity, through resonance, by intramolecular hydrogen bonding. This may contribute to lowering the redox potential between the aroxyl radical and the reduced polyphenol, endowing the polyphenol higher radical-scavenging activity [47].
3.4. Polyphenolic Profile
Typical chromatograms of the extract obtained under optimized conditions are presented in Figure 8. Gallic acid, catechin, rutin and quercetin were tentatively identified by comparing their retention times with the corresponding of original standards. Caftaric acid (caffeoyltartaric acid) and malvidin 3-O-glucoside p-coumarate were identified based on liquid chromatography–mass spectrometry data reported elsewhere [48,49]. The quantitative analysis given in Table 4 revealed that the aqueous extraction was more effective than the β-CD-aided extraction only for gallic acid (153.43 and 103.37 μg g−1 dm, respectively), providing a by 33% higher yield. For caftaric acid, rutin and malvidin 3-O-glucoside p-coumarate no statistical difference was observed between the yields attained with aqueous and β-CD-aided extractions.
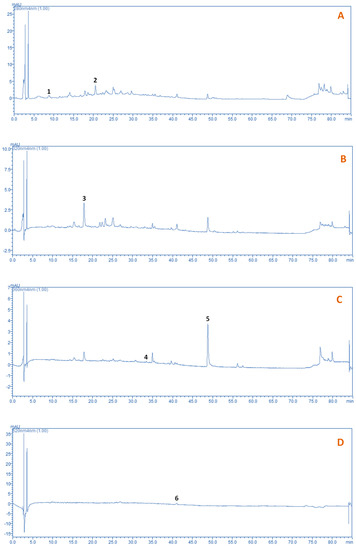
Figure 8.
HPLC traces recorded at 280 (A), 320 (B), 365 (C) and 520 nm (D), of the extract prepared using β-CD. Extractions with β-CD were performed under optimized conditions (CCD = 1.5%, tus = 30 min, RL/S = 90 mL g−1), under stirring at 900 rpm, for 180 min. Peak assignment: 1, gallic acid; 2, catechin; 3, caftaric acid; 4, rutin; 5, quercetin; 6, malvidin 3-O-glucoside p-coumarate.

Table 4.
Quantitative data on the recovery of major polyphenols of RGP, recovered with the different extraction media tested. Values reported represent means ± standard deviation (n = 3).
By contrast, the effect of β-CD was striking for catechin (506.54 μg g−1 dm) and quercetin (151.17 μg g−1 dm) recovery, as it enhanced their yields by 211 and 66%, respectively. It should also be stressed that, although the extraction with aqueous ethanol had the highest performance for most of the polyphenols considered, the β-CD-aided extraction afforded by 91% higher catechin yield compared to the aqueous ethanol. This finding suggested a selective effect of β-CD towards catechin. This fact may also explain the higher AAR found for the extract produced with β-CD compared to the one generated with aqueous ethanol (Figure 7A). Catechin is a powerful antioxidant flavonoid, and its efficiency in the DPPH assay has been well documented [50].
The selectivity displayed by β-CD towards catechin has been previously reported by other studies on RGP extraction. More particularly, extraction with aqueous β-CD, although considerably less effective than the extraction with aqueous ethanol, was proposed as an alternative green means of selectively recovering catechin and epicatechin [12]. Other examinations also highlighted the effect of β-CD concentration, since flavanol extraction yield was shown to increase proportionally, up to a point. The most effective extraction was achieved with 0.5% β-CD [10].
4. Conclusions
The use of various types of cyclodextrins as green enhancers of aqueous polyphenol extraction has been gaining a high appreciation. The investigation presented herein is proposing for the first time a green high-performance process of red grape pomace polyphenol recovery, using ultrasonication pretreatment and β-cyclodextrin-aided extraction. The most important findings may be summarized as follows:
- Optimization through response surface methodology demonstrated that incorporation of β-cyclodextrin in an aqueous medium at a level of 1.5% and ultrasonication pretreatment for 30 min may significantly increase the extraction yield in total polyphenols.
- The maximum yield, after carrying out a temperature assay, was 57.47 mg GAE g−1 dm, at 80 °C. Taking into consideration the bibliographic data from previous studies, but also the relatively low activation energy determined, the process developed is effective, green, with low energy demands.
- The extracts obtained were characterized by high (506.54 μg g−1 dm) and quercetin (151.17 μg g−1 dm) content and relatively high antiradical activity. This outcome may be important for the production of extracts fortified in selected polyphenolic phytochemicals and enable task-specific extractions.
Since β-cyclodextrin is an approved food additive, it could be part of the final product formulation. Thus, polyphenol-enriched extracts could be directly used in foods/pharmaceuticals/cosmetics, the appropriate composition and concentration provided. Such an option would be particularly appealing, since current trends suggest ingredient production based on functionality rather than purity. Therefore, the extracts produced with the methodology proposed could be for specific applications rather than for general use.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/beverages7030059/s1, Figure S1: Gallic acid standard (10 mg L−1); Figure S2: Catechin standard (10 mg L−1); Figure S3: Rutin standard (10 mg L−1); Figure S4: Quercetin standard (10 mg L−1).
Author Contributions
Conceptualization, D.P.M. and S.L.; methodology, D.P.M. and S.L.; validation, A.A., A.L.; formal analysis, A.A., E.B., A.L.; investigation, A.A., E.B., A.L., A.C.; resources, D.P.M., S.L.; data curation, A.A., A.L., A.C.; writing—original draft preparation, D.P.M. and S.L.; writing—review and editing, D.P.M. and S.L.; supervision, D.P.M., A.C. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AAR | antiradical activity (μmol DPPH g−1) |
| AED | acoustic energy density (W L−1) |
| CCD | β-cyclodextrin concentration (% w/v) |
| Cp | specific heat capacity of water (4.2 J g−1 K−1) |
| m | mass (g) |
| P | ultrasonication power (W) |
| PR | ferric-reducing power (μmol AAE g−1) |
| RL/S | liquid-to-solid ratio (mL g−1) |
| tus | ultrasonication time (min) |
| T | temperature (°C) |
| YTP | yield in total polyphenols (mg GAE g−1) |
| YTP(s) | yield in total polyphenols at saturation (equilibrium) (mg GAE g−1) |
References
- Strategic Work of FAO for Sustainable Food and Agriculture. Available online: http://www.fao.org/3/a-i6488e.pdf (accessed on 25 July 2021).
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A. Antioxidant molecules from plant waste: Extraction techniques and biological properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- Burlini, I.; Sacchetti, G. Secondary bioactive metabolites from plant-derived food byproducts through ecopharmacognostic approaches: A bound phenolic case study. Plants 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotech. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of grape pomace as a potential source for value addition. Environ. Pol. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Yammine, S.; Brianceau, S.; Manteau, S.; Turk, M.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M. Extraction and purification of high added value compounds from by-products of the winemaking chain using alternative/nonconventional processes/technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Ratnasooriya, C.C.; Rupasinghe, H.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-assisted extraction of polyphenols from vine shoot cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- López-Miranda, S.; Serrano-Martínez, A.; Hernández-Sánchez, P.; Guardiola, L.; Pérez-Sánchez, H.; Fortea, I.; Gabaldón, J.A.; Núñez-Delicado, E. Use of cyclodextrins to recover catechin and epicatechin from red grape pomace. Food Chem. 2016, 203, 379–385. [Google Scholar] [CrossRef]
- Grigorakis, S.; Benchennouf, A.; Halahlah, A.; Makris, D.P. High-performance green extraction of polyphenolic antioxidants from Salvia fruticosa using cyclodextrins: Optimization, kinetics, and composition. Appl. Sci. 2020, 10, 3447. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a low-temperature and high-performance green extraction process for the recovery of polyphenolic phytochemicals from waste potato peels using hydroxypropyl β-cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-cyclodextrin as a green co-solvent in the aqueous extraction of polyphenols from waste orange peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Sakamoto, T.; Leveque, J.-M.; Sohmiya, H.; Fujita, M.; Ikeda, S.; Ando, T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996, 3, 157–161. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem. Eng. Com. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Res. 2013, 126. [Google Scholar] [CrossRef] [Green Version]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukri, A.; Tsitlakidou, P.; Goula, A.; Assimopoulou, A.N.; Kontogiannopoulos, K.N.; Mourtzinos, I. Green extracts from coffee pulp and their application in the development of innovative brews. Appl. Sci. 2020, 10, 6982. [Google Scholar] [CrossRef]
- Rakotondramasy-Rabesiaka, L.; Havet, J.-L.; Porte, C.; Fauduet, H. Estimation of effective diffusion and transfer rate during the protopine extraction process from Fumaria officinalis L. Separ. Purif. Technol. 2010, 76, 126–131. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Proc. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Elmi Kashtiban, A.; Esmaiili, M. Extraction of phenolic compounds from Siah-Sardasht grape skin using subcritical water and ultrasound pretreatment. J. Food Proc. Preserv. 2019, 43, 14071. [Google Scholar] [CrossRef]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Chemat, F.; Cravotto, G. Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont. Compt. Rendus Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Mantegna, S.; Binello, A.; Boffa, L.; Giorgis, M.; Cena, C.; Cravotto, G. A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum. Food Chem. 2012, 130, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Separ. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Atanassova, D.; Kefalas, P.; Petrakis, C.; Mantzavinos, D.; Kalogerakis, N.; Psillakis, E. Sonochemical reduction of the antioxidant activity of olive mill wastewater. Environ. Int. 2005, 31, 281–287. [Google Scholar] [CrossRef]
- Brahim, M.; Gambier, F.; Brosse, N. Optimization of polyphenols extraction from grape residues in water medium. Ind. Crops Prod. 2014, 52, 18–22. [Google Scholar] [CrossRef]
- Galanakis, C.; Goulas, V.; Tsakona, S.; Manganaris, G.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- González-Centeno, M.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Brandelli, A.; Marczak, L.D.F.; Tessaro, I.C. Kinetic modeling of total polyphenol extraction from grape marc and characterization of the extracts. Separ. Purif. Technol. 2012, 100, 82–87. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Proc. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Lalas, S.; Makris, D.P. Effect of methyl β-cyclodextrin on radical scavenging kinetics of olive leaf extracts and interactions with ascorbic acid. ChemEngineering 2017, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Celik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: Influence of glycosylation on inclusion complexation. J. Inclus. Phenom. Macr. Chem. 2015, 83, 309–319. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocol. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Medronho, B.; Valente, A.J.; Costa, P.; Romano, A. Inclusion complexes of rosmarinic acid and cyclodextrins: Stoichiometry, association constants, and antioxidant potential. Colloid Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Tufan, A.N.; Güçlü, K.; Apak, R. Spectroscopic study and antioxidant properties of the inclusion complexes of rosmarinic acid with natural and derivative cyclodextrins. Spectrochim. Acta A Mol. Biomol. Spectr. 2011, 78, 1615–1624. [Google Scholar] [CrossRef]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Inter. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Kefalas, P.; Makris, D. Liquid chromatography-mass spectrometry techniques in flavonoid analysis: Recent advances. In Natural Antioxidant Phenols: Sources, Structure-Activity Relationship, Current Trends in Analysis and Characterisation; Boskou, D., Gerothanassis, I.P., Kefalas, P., Eds.; Research Signpost: Kerala, India, 2006; pp. 69–123. [Google Scholar]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.L.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).