Traditional and Artisanal Beverages in Nigeria: Microbial Diversity and Safety Issues
Abstract
1. Introduction
2. Milk and Milk-Like Products
2.1. Plant-Based Milk Products
2.1.1. Soymilk
2.1.2. Tiger Nut Milk
2.2. Animal-Based Milk Products
2.2.1. Nono
2.2.2. Yoghurt
3. Soft Drinks
3.1. Kunu
3.2. Zobo
4. Alcoholic Beverages
4.1. Palm Wine
4.2. Pito
4.3. Burukutu
5. Significance of Organisms Found
5.1. Hygiene Indicators, Pathogens and Toxic Compound Producers
5.2. Molecular Characterization
5.3. Practical Implications of the Genera Found in Beverages
5.3.1. Bacillus
5.3.2. Escherichia
5.3.3. Staphylococcus
5.3.4. Positive Aspects of Beverage Fermentation
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Okaru, A.O.; Rehm, J.; Sommerfeld, K.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. The threat to quality of alcoholic beverages by unrecorded consumption. In Alcoholic Beverages, 7th ed.; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing (Elsevier): Kidlington, UK, 2019; pp. 1–34. [Google Scholar]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, N.; Soliman, A.T.; Elsedjy, H.; di Maio, S.; Kholy, M.; Piscina, B. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: A significant public health hazard. Acta Biomed. 2017, 88, 222–231. [Google Scholar] [PubMed]
- Cífková, R.; Krajčoviechová, A. Alcohol and cardiovascular disease: Position paper of the Czech Society of Cardiology. Cent. Eur. J. Publ. Health 2019, 27, S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Ayoub-Charette, S.; Liu, Q.; Khan, T.A.; Au-Yeung, F.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.; Leiter, L.A.; Kendall, C.; Sievenpiper, J.L. Important food sources of fructose-containing sugars and incident gout: A systematic review and meta-analysis of prospective cohort studies. BMJ Open 2019, 9, e024171. [Google Scholar] [CrossRef] [PubMed]
- Collin, L.J.; Judd, S.; Safford, M.; Vaccarino, V.; Welsh, J.A. Association of sugary beverage consumption with mortality risk in US Adults: A secondary analysis of data from the REGARDS Study. JAMA Netw. Open 2019, 2, e193121. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2016.00377 (accessed on 2 July 2020). [CrossRef]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Cocolin, L.; Gobbetti, M.; Neviani, E.; Daffonchio, D. Ensuring safety in artisanal food microbiology. Nat. Microbiol. 2016, 1, 16171. Available online: https://www.nature.com/articles/nmicrobiol2016171 (accessed on 4 July 2020). [CrossRef]
- Adams, M.; Mitchell, R. Fermentation and pathogen control: A risk assessment approach. Int. J. Food Microbiol. 2002, 79, 75–83. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Russo, P. Microbiological Safety and the Management of Microbial Resources in Artisanal Foods and Beverages: The Need for a Transdisciplinary Assessment to Conciliate Actual Trends and Risks Avoidance. Microorganisms 2020, 8, 306. [Google Scholar] [CrossRef]
- Nwaiwu, O. Use of fragments from D1/D2 Domain of 26S rRNA gene to select Saccharomyces cerevisiae from palm wine. J. Appl. Life Sci. Int. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Florence, A.A.; Courage, K.S.S.; Francis, K.A.; Hellie, G. Shelf life improvement of sorghum beer (Pito) through the addition of Moringa oleifera and pasteurization. Afr. J. Biotechnol. 2016, 15, 2627–2636. [Google Scholar] [CrossRef]
- Okiki, P.A.; Adeniji, C.A.; Oyetunji, O.A.; Yusuf, O.A.; Peters, O.A. Assessment of the physicochemical and bacteriological qualities of Nono—A fermented cow milk. Potravinarstvo 2018, 12, 26–32. [Google Scholar] [CrossRef][Green Version]
- Ani, E.; Amove, J.; Igbabul, B. Physicochemical, Microbiological, sensory properties and storage stability of plant-based yoghurt produced from Bambaranut, soybean and Moringa oleifera seed milks. Am. J. Food Nutr. 2018, 6, 115–125. [Google Scholar]
- Ajiboye, T.O.; Iliasu, G.A.; Ojewuyi, O.B.; Abdulazeez, A.T.; Muhammed, A.O.; Kolawole, F.L. Sorghum-based alcoholic beverage, Burukutu, perturbs the redox status of the liver of male rats. Food Sci. Nutr. 2014, 2, 591–596. [Google Scholar] [CrossRef]
- Ukwuru, M.U.; Ogbodo, A.C. Effect of processing treatment on the quality of tigernut milk. Pakistan J. Nutr. 2011, 10, 95–100. [Google Scholar] [CrossRef]
- Ozoh, C.N.; Umeaku, C.N. Public health implication of ready-to drink soymilk and soymilk yogurt sold in Onitsha Urban Anambra State, Nigeria. J. Multidiscip. Eng. Sci. Technol. 2016, 3, 5386–5393. [Google Scholar]
- Adelekan, A.O.; Alamu, A.E.; Arisa, N.U.; Adebayo, Y.O.; Dosa, A.S. Nutritional, microbiological and sensory characteristics of malted soy-kunu zaki: An improved traditional beverage. Adv. Microbiol. 2013, 3, 389–397. [Google Scholar] [CrossRef]
- Izah, C.S.; Kigigha, L.T.; Aseibai, R.E.; Okowa, P.I.; Orutugu, L.A. Advances in preservatives and condiments used in Zobo (a food-drink) production. Biotechnol. Res. 2016, 2, 104–119. [Google Scholar]
- Omoleke, S.A.; Ajibola, O.; Ajiboye, J.O.; Raji, R.O. Quagmire of epidemic disease outbreaks reporting in Nigeria. BMJ Glob. Health 2018, 3. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 25 January 2020).
- Ohimain, E.I. Methanol contamination in traditionally fermented alcoholic beverages: The microbial dimension. SpringerPlus 2016, 5, 1607. [Google Scholar] [CrossRef] [PubMed]
- Nwaiwu, O.; Itumoh, M. Chemical contaminants associated with palm wine from Nigeria are potential food safety hazards. Beverages 2017, 3, 16. [Google Scholar] [CrossRef]
- Raji, M.I.O.; Jiya, M.H. Evaluation of pathogenic bacteria in packaged milk products sold in Sokoto metropolis, Nigeria. Asian J. Appl. Sci. 2019, 12, 85–90. [Google Scholar] [CrossRef]
- Ukwuru, M.U.; Adama, A. Chemical evaluation and storage stability of a beverage formulated from soybean and papaya pulp flour blends. Plant Foods Hum. Nutr. 2003, 58, 1–11. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, W.; Xu, B. Food Quality improvement of soy milk made from short-time germinated soybeans. Foods 2013, 2, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Adegoke, A.A.; Akinjogunla, O.J. Microbial and physico-chemical quality of powdered soymilk samples in Akwa Ibom, South Southern Nigeria. Afr. J. Biotechnol. 2009, 8, 3066–3071. [Google Scholar]
- Liamngee, K.; Terna, T.P.; Bem, A.A.; Orpin, J.B.; Mzungu, I.; Obaje, M.; Anum, T. Microbial analysis of soyabean milk sold in Makurdi metropolis. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 3, 97–104. [Google Scholar] [CrossRef]
- Adeleke, O.E.; Adeniyi, B.A.; Akinrinmisi, A.A. Microbiological quality of local soymilk: A public health appraisal. Afr. J. Biomed. Res. 2000, 3, 89–92. [Google Scholar]
- Agboke, A.A.; Osonwa, U.E.; Opurum, C.C.; Ibezim, E.C. Evaluation of microbiology quality of some soybean milk products consumed in Nigeria. Pharmacologia 2012, 3, 513–518. [Google Scholar] [CrossRef][Green Version]
- Anagu, L.; Okolocha, E.; Ikegbunam, M.; Ugwu, M.; Oli, A.; Esimone, C. Potential spread of pathogens by consumption of locally produced Zobo and soya milk drinks in Awka metropolis, Nigeria. Br. Microbiol. Res. J. 2015, 5, 424–431. [Google Scholar] [CrossRef]
- Brooks, A.A.; Asamudo, N.U.; Udoukpo, F.C. Microbiological and physico-chemical analysis of soymilk and soy flour sold in Uyo metropolis, Nigeria. Glob. J. Pure Appl. Sci. 2003, 9, 457–463. [Google Scholar]
- Ezekiel, C.N.; Fapohunda, S.O. Mycological and nutrient profile of soymilk. Glob. Adv. Res. J. Food Sci. Technol. 2012, 2, 025–030. [Google Scholar]
- Agwa, O.K.; Ossai-Chidi, L.N. Surveillance of the microbial quality of soybean products sold within markets in Port Harcourt metropolis, Rivers State, Nigeria. Food Public Health 2016, 6, 130–139. Available online: http://article.sapub.org/10.5923.j.fph.20160605.04.html (accessed on 16 July 2020).
- Mbajiuka, C.S.; Obeagu, E.I.; Ifediora, A.C.; Ugwu, G.U. Isolation and identification of microorganisms involved in the spoilage of soymilk. IOSR J. Pharm. Biol. Sci. 2014, 9, 29–36. [Google Scholar]
- Akani, N.P.; Barika, P.N. Fungi associated with soymilk during storage. Niger. J. Mycol. 2019, 11, 93–101. [Google Scholar]
- Carvajal-Campos, A.; Manizan, A.; Tadrist, S.; Akaki, D.; Koffi-Nevry, R.; Moore, G.; Fapohunda, S.; Bailly, S.; Montet, D.; Oswald, I.; et al. Aspergillus korhogoensis, a novel aflatoxin producing species from the Côte d’Ivoire. Toxins 2017, 9, 353. [Google Scholar] [CrossRef]
- Chang, P.K.; Scharfenstein, L.L.; Luo, M.; Mahoney, N.; Molyneux, R.J.; Yu, J.; Brown, R.L.; Campbell, B.C. Loss of MsnA, a putative stress regulatory gene, in Aspergillus parasiticus and Aspergillus flavus increased production of conidia, aflatoxins and Kojic Acid. Toxins 2011, 3, 82–104. [Google Scholar] [CrossRef]
- Ahmed Abdullah Murshed, S.; Bacha, N.; Alharazi, T. Detection of total aflatoxins in groundnut and soybean samples in Yemen using enzyme-linked immunosorbent assay. J. Food Qual. 2019, 2019, 1614502. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of food safety objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Kizzie-Hayford, N.; Jaros, D.; Zahn, S.; Rohm, H. Effects of protein enrichment on the microbiological, physicochemical and sensory properties of fermented tiger nut milk. LWT Food Sci. Technol. 2016, 74, 319–324. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger nut (Cyperus Esculentus) Commercialization: Health aspects, composition, properties, and food applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Akoma, O.; Elekwa, U.O.; Afodurinbi, T.T.; Onyeukwu, G.C. Yoghurt from Coconut and Tigernuts. J. Food Technol. Afr. 2000, 5, 132–134. Available online: https://www.ajol.info/index.php/jfta/article/view/19270 (accessed on 13 July 2020).
- Sebastià, N.; El-Shenawy, M.; Mañes, J.; Soriano, J.M. Assessment of microbial quality of commercial and home-made tiger-nut beverages. Lett. Appl. Microbiol. 2012, 54, 299–305. [Google Scholar] [CrossRef]
- Onovo, J.C.; Ogaraku, A.O. Studies on some microorganisms associated with exposed tiger nut (Cyperus esculentus) milk. J. Biol. Sci. 2007, 7, 1548–1550. [Google Scholar]
- Okorie, S.U.; Adedokun, I.; Duru, N. Effect of blending and storage conditions on the microbial quality and sensory characteristics of soy-tiger nut milk beverage. Food Sci. Qual. Manag. 2014, 31, 96–103. Available online: https://iiste.org/Journals/index.php/FSQM/article/view/15581 (accessed on 16 July 2020).
- Ogbodo, A.C.; Agwaranze, D.I.; Nwaneri, C.B.; Yakubu, M.N.; Hussaini, Z.J. Comparative Study on the Bacteriological Quality of Kunun-Aya sold in Wukari, Nigeria. Int. J. Res. Stud. Microbiol. Biotechnol. 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Badua, M.H.; Bilyaminu, D.; Ogori, A.F.; Charles, B.; Ogori, J. Microbial quality evaluation of tiger nut beverage (Kunun Aya) processed sold in University of Maiduguri. EC Nutr. 2018, 13, 138–142. Available online: https://www.ecronicon.com/ecnu/pdf/ECNU-13-00439.pdf (accessed on 16 July 2020).
- Maduka, N. Molecular characterization of non-lactic bacteria in lactic fermented tigernut-milk drink and effect of ambient and refrigeration temperature storage on sensory properties of the drink spiced with ginger and garlic. Microbiol. Res. J. Int. 2017, 22, 1–11. [Google Scholar] [CrossRef]
- Shu’aibu, I.; Hadiza, J.A.; Yusha’u, M.; Kabiru, M.Y.; Ahmad, M.M.; Lawal, G.; Adamu, M.T.; Khairiyya, M. Assessment of foods and drinks for the presence of extended spectrum beta lactamase (ESBL) producing bacteria in Gombe metropolis, Nigeria. Indian J. Sci. Technol. 2016, 9. [Google Scholar] [CrossRef]
- Umar, Z.D.; Bashir, A.; Raubilu, S.A. Study on bacteriological quality of kunun aya (tigernut juice) sold at Umaru Musa Yar’adua university (UMYU) campus, Katsina. Int. J. Environ. 2014, 3, 87–97. [Google Scholar] [CrossRef]
- Osho, M.B.; Shobande, O.E. Microbiological assessment of tiger nut milk as a potential probiotic product. Niger. J. Biotechnol. 2019, 36, 186–193. [Google Scholar] [CrossRef]
- Wakil, S.M.; Ayenuro, O.T.; Oyinlola, K.A. Microbiological and nutritional assessment of starter-developed fermented tigernut milk. Food Nutr. Sci. 2014, 5, 495–506. [Google Scholar] [CrossRef]
- Udeozor, L.O.; Awonorin, S.O. Comparative microbial analysis and storage of tigernut-soy milk extract. Austin J. Food Nutri. Sci. 2014, 2, 1026. [Google Scholar]
- Shamsuddeen, U.; Aminu, H.A. Occurrence of aflatoxin in Cyperus esculentus (Tiger Nut) sold and consumed raw in Kaduna. Int. J. Sci. Res. Educ. 2016, 4, 5189–5195. Available online: http://ijsae.in/ijsaeems/index.php/ijsae/article/view/1189 (accessed on 16 July 2020). [CrossRef][Green Version]
- Bankole, M.O.; Okagbue, R.N. Properties of “Nono,” a Nigerian fermented milk food. Ecol. Food Nutr. 1992, 27, 145–149. [Google Scholar] [CrossRef]
- Anyanwu, N.C.J.S. Microbiological and comparative analysis of indigenous and semi-industrial fermented milk drinks (Fura Da Nono and Fura Da Yoghurt) sold in Nigeria’s capital. Int. J. Bioassays 2019, 8, 5716–5723. [Google Scholar]
- Olasupo, N.A.; Smith, S.I.; Akinsinde, K.A. Examination of the microbial status of selected indigenous fermented foods in Nigeria. J. Food Saf. 2002, 22, 85–93. [Google Scholar] [CrossRef]
- Yabaya, A.; Manga, S.S.; Lucy, M.; Alhassan, H.M. Bacteriological quality of fermented milk sold locally in Samaru and Sabongari market, Zaria-Nigeria. Cont. J. Microbiol. 2012, 6, 14–18. Available online: https://zenodo.org/record/824031#.XxDWkShKjIU (accessed on 16 July 2020).
- Okonkwo, O.I. Microbial analysis and safety evaluation of Nono: A fermented milk product consumed in most part of Northern Nigeria. Int. J. Dairy Sci. 2011, 6, 181–189. [Google Scholar]
- Reuben, R.C.; Owuna, G. Antimicrobial resistance patterns of Escherichia coli 0157:H7 from Nigerian fermented milk samples in Nasarawa state, Nigeria. Int. J. Pharm. Sci. Invent. 2013, 2, 38–44. [Google Scholar]
- Obi, C.N.; Ikenebomeh, M.J. Studies on the microbiology and nutritional qualities of a Nigerian fermented milk product (Nono). Int. J. Dairy Sci. 2007, 2, 95–99. [Google Scholar]
- Dafur, G.S.; Iheukwumere, C.C.; Azua, E.T.; Dafur, B.S. Evaluation of the Microbial Quality of ‘Nono’ Sold in Mangu Local Government Area of Plateau State, Nigeria. Available online: https://www.journalsajrm.com/index.php/SAJRM/article/view/29245 (accessed on 16 July 2020).
- Maikai, B.V.; Madaki, P.D. Enumeration of coliforms in fermented milk product (Nono) sold in Samaru, Kaduna State, Nigeria. Sokoto J. Vet. Sci. 2019, 16, 50–57. [Google Scholar] [CrossRef]
- Vantsawa, P.A.; Maryah, U.T.; Bulus, T. Isolation and identification of lactic acid bacteria with probiotic potential from fermented cow milk (Nono) in Unguwar Rimi, Kaduna State Nigeria. Am. J. Mol. Biol. 2017, 7, 99–106. [Google Scholar] [CrossRef][Green Version]
- Belewu, O.A.; Aina, O.S. Microbial evaluation of indigenous milk products with special reference to the bacteria flora of some public health importance in Nigeria. Afr. J. Clin. Exp. Microbiol. 2000, 1, 13–19. [Google Scholar]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of Lactic Acid Bacteria on the Yogurt Flavour: A Review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef]
- Omola, E.; Kawo, A.; Shamsudden, U. Physico-Chemical, sensory and microbiological qualities of yoghurt brands sold in kano metropolis, Nigeria. Bayero J. Pure Appl. Sci. 2015, 7, 26–30. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Amadi, E.C.; Tochukwu Nwamaka, N.; Chike, A.E. Bacteria population of some commercially prepared yoghurt sold in Enugu State, Eastern Nigeria. Afr. J. Microbiol. Res. 2010, 4, 984–988. [Google Scholar]
- Makut, M.D.; Ogbonna, A.I.; Dalami, H. An Assessment of the Bacteriological Quality of Different Brands of Yoghurt Sold in Keffi, Nasarawa State, Nigeria. J. Nat. Sci. Res. 2014, 4, 19–22. [Google Scholar]
- Agu, K.C.; Archibong, E.J.; Anekwe, D.C.; Ago, C.A.; Okafor, A.C.; Awah, N.S. Assessment of bacteria present in yoghurt sold on Awka metropolis. Sch. J. Appl. Med. Sci. 2014, 2, 3071–3075. [Google Scholar]
- De, N.; Goodluck, T.M.; Bobai, M. Microbiological quality assessment of bottled yogurt of different brands sold in Central Market, Kaduna Metropolis, Kaduna, Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 20–27. [Google Scholar]
- Dirisu, C.G.; Lily, G.; Igwe, E. Microbiological load of yoghurt sold in Omoku schools, Rivers State, Nigeria. Afr. J. Microbiol. Res. 2015, 9, 1960–1963. [Google Scholar]
- Mepba, H.D.; Achinewhu, S.C.; Aso, S.N.; Wachukwu, C.K. Microbiological quality of selected street foods in Port Harcourt, Nigeria. J. Food Saf. 2007, 27, 208–218. [Google Scholar] [CrossRef]
- Aguoru, C.U.; Dania, G.V. Microbiological examination of commercial probiotic yoghurt produced and sold in Makurdi, Benue state, Nigeria. Int. Sci. Res. J. 2010, 2, 52–55. Available online: http://www.academicpublications.org/isrj/Microbiological_Examination_Aguoru_Dania_52-55.pdf (accessed on 16 July 2020).
- Obire, O.; Berembo, B.T. Microorganisms associated with street vended yoghurt in mile 1 Diobu area of Port Harcourt, Nigeria. J. Sci. Technol. 2014, 5, 179–186. Available online: http://ejournals.teiath.gr/index.php/ejst/article/view/812 (accessed on 16 July 2020).
- Ifeanyi, V.O.; Ihesiaba, E.O.; Muomaife, O.M.; Ikenga, C. Assessment of microbiological quality of yogurt sold by street vendors in Onitsha metropolis, Anambra state, Nigeria. Br. Microbiol. Res. J. 2013, 3, 198–205. [Google Scholar] [CrossRef][Green Version]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLOS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef]
- Bede, E.N.; Okeke, C.E.; Amandikwa, C. Physicochemical properties and sensory evaluation of Kunu-Zaki beverage produced by substitution of sweet potatoes with date fruits. IOSR J. Environ. Sci. Toxicol. Food. Technol. 2015, 9, 81–84. [Google Scholar]
- Gaffa, T.; Jideani, I.A.; Nkama, I. Traditional production, consumption and storage of Kunu-a non alcoholic cereal beverage. Plant Foods Hum. Nutr. 2002, 57, 73–81. [Google Scholar] [CrossRef]
- Mbachu, A.E.; Etok, C.A.; Agu, K.C.; Okafor, O.I.; Awah, N.S.; Chidi-Onuorah, L.C.; Ekwueme, V.C.; Okpala, J.; Ogbue, M.O.; Ikele, M.O. Microbial quality of Kunu drink sold in Calabar, Cross River state, Nigeria. J. Glob. Biosci. 2014, 3, 511–515. Available online: https://pdfs.semanticscholar.org/ce3e/cb2e0b6001fb0f6fe895d005a2b99f22e263.pdf (accessed on 16 July 2020).
- Onyemekara, N.N.; Nwanebu, F.C.; Njoku, M.N.; Chinakwe, E.C.; Nwogwugwu, N.U.; Mike-Anosike, E. Microbiological and Proximate Analysis of a Local Food Beverage-Kunu Zaki, Sold in Owerri Municipal. Available online: https://www.futojnls.org/issues/type/volume-4-issue-2-2018/science (accessed on 16 July 2020).
- Orutugu, L.A.; Izah, S.C.; Aseibai, E.R. Microbiological quality of Kunu drink sold in some major markets of Yenagoa metropolis. Cont. J. Biomed. Sci. 2015, 9, 9–16. Available online: https://www.academia.edu/26125017/microbiological_quality_of_kunu_drink_sold_in_some_major_markets_of_yenagoa_metropolis_nigeria_langley_ayibawanaimi_orutugu_sylvester_chibueze_izah_and_ebinyo_rebecca_aseibai (accessed on 16 July 2020).
- Amusa, N.A.; Odunbaku, O.A. Microbiological and nutritional quality of hawked kunun (a sorghum based non-alcoholic beverage) widely consumed in Nigeria. Pak. J. Nutr. 2009, 8, 20–25. [Google Scholar] [CrossRef]
- Elmahmood, A.M.; Doughari, J.H. microbial quality assessment of kunun-zaki beverage sold in Girei town of Adamawa State, Nigeria. Afr. J. Food Sci. 2007, 1, 11–15. [Google Scholar]
- Blessed, K.Y.; Dadah, A.J.; Uba, A. Isolation of enteric bacteria from hawked “Kunun-Zaki” in Chikun Local Government Area of Kaduna State. Am. J. Lab. Med. 2017, 2, 96. [Google Scholar] [CrossRef]
- Rosemary, C.U.; Seghosime, R.A.; Onah Gloria, T. Assessment of the Microbiological Quality of Kunu Zaki Sold at Gariki, Enugu State, Nigeria. Biol. Environ. Sci. J. Trop. 2016. [Google Scholar] [CrossRef]
- Braide, W.; Ukagwu, N.; Lugbe, P.B.; Akien, A.A.; Adeleye, S. Chemical properties and microbiological profile of kunu zaki, a non-alcoholic beverage. Biomed. J. Sci. Tech. Res. 2018, 4, 3731–3735. [Google Scholar] [CrossRef]
- Umaru, G.A.; Tukur, I.S.; Akensire, U.A.; Adamu, Z.; Bello, O.A.; Shawulu, A.H.B.; Audu, M.; Sunkani, J.B.; Adamu, S.G.; Adamu, N.B. Microflora of Kunun-Zaki and Sobo drinks in relation to public health in Jalingo metropolis, North-Eastern Nigeria. Int. J. Food Res. 2014, 1, 16–21. Available online: http://www.bluepenjournals.org/ijfr/pdf/2014/September/Umaru_et_al.pdf (accessed on 16 July 2016).
- Gaffa, T.; Gaffa, A. Microbial succession during “kunun Zaki” production with sorghum (Sorghum Bicolor) grains. World J. Microb. Biot. 2004, 20, 449–453. [Google Scholar] [CrossRef]
- Adeniji, P.O. Nutritional, sensory and microbiological quality assessment of fortified zobo drink: A home-prepared traditional Nigerian beverage. J. Nutr. Food Sci. 2017, 07, 1–3. [Google Scholar]
- Shruthi, V.H.; Ramachandra, C.T.; Nidoni, U.; Hiregoudar, S.; Naik, N.; Kurubar, A.R. Roselle (Hibiscus sabdariffa L.) as a source of natural colour: A Review. Plant Arch. 2016, 2, 515–522. [Google Scholar]
- Fasoyiro, S.B.; Babalola, S.O.; Owosibo, T. Chemical Composition and Sensory Quality of Fruit-Flavoured Roselle (Hibiscus Sabdariffa) Drinks. World J. Agric. Sci. 2005, 1, 161–164. [Google Scholar]
- Omemu, A.M.; Edema, M.O.; Atayese, A.O.; Obadina, A.O. A Survey of the microflora of Hibiscus sabdariffa (Roselle) and the eesulting “Zobo” Juice. Afr. J. Biotechnol. 2006, 5, 254–259. [Google Scholar]
- Odu, N.N.; Adeniji, A.O. Microbiological analysis of some packaged fruit juices sold in Port Hacourt metropolis. Niger. Nat. Sci. 2013, 11, 30–40. [Google Scholar]
- Oku, Y.I.; Alagoa, K.J.; Daworiye, P.S.; Izon-ebi, B.M. Microbial content of Zobo drink from five different producers within Yenagoa City Bayelsa State, Nigeria. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 4, 74–89. Available online: https://ijasre.net/index.php/ijasre/article/view/868/1480 (accessed on 16 July 2020).
- Ezeigbo, O.; Ekaiko, M.; Agomo, N.; Ojukwu, K.; Nnadozie, A. Antimicrobial effect of lime juice treatment on the shelf-life of zobo drink. Br. Microbiol. Res. J. 2015, 6, 147–153. [Google Scholar] [CrossRef]
- Onuoha, S.C.; Fatokun, K. Effect of Citrus Aurantifolia Juice on the Shelf-Life of Zobo drink produced locally in Afikpo Ebonyi State, Nigeria. Am. J. BioSci. 2014, 2, 45. Available online: http://www.sciencepublishinggroup.com/journal/paperinfo?journalid=219&doi=10.11648/j.ajbio.20140202.14 (accessed on 15 July 2020).
- Ezearigo, O.; Adeniji, P.; Ayoade, F. Screening of natural spices for improving the microbiological, nutritional and organoleptic qualities of the Zobo drink. J. Appl. Biosci. 2014, 76. [Google Scholar] [CrossRef]
- Umar, M.; Mohammed, I.B.; Abdulkarim, I.M.; Yusuf, G.; Yaya, A.A.; Leo, G. Comparative studies on the prevalence of Salmonella species in two home-made fermented beverages (Zobo and Kunun-Zaki) sold at Samaru, Zaria, Kaduna, Nigeria. Int. J. Sci. Res. Publ. 2016, 6, 428–435. Available online: http://www.ijsrp.org/research-paper-0316.php?rp=P515200 (accessed on 16 July 2020).
- Ibitoye, F.; Oyetayo, A.; Aribisala, O.; Giwa, O. Investigation of bacteria associated with the spoilage of zobo drink fortified with scent leaf and ginger. Cell. Autom. Res. Ind. 2017, 9, 1–7. Available online: https://www.journalacri.com/index.php/ACRI/article/view/18448/34113 (accessed on 15 July 2020). [CrossRef]
- Bukar, A.; Uba, A.; Oyeyi, T.I. Occurrence of Some Enteropathogenic Bacteria in Some Minimally and Fully Processed Ready-to-Eat Foods in Kano Metropolis, Nigeria. Afr. J. Food Sci. 2010, 4, 32–36. [Google Scholar]
- Ayandele, A.A. Microbiological analyses of hawked kunun and zobo drinks within LAUTECH campus, Ogbomoso, Oyo State, Nigeria. IOSR J. Environ. Sci. 2015, 9, 52–56. [Google Scholar]
- Nwaiwu, O.; Itumoh, M. Molecular phylogeny of yeasts from palm wine and enological potentials of the drink. Annu. Res. Rev. Biol. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Fossi, B.T.; Natalia, B.E.; Nchanji, G.T.; Ngah, B.G.; Anyangwe, I.A.; Samuel, W.S. Probiotic properties of lactic acid bacteria isolated from fermented sap of palm tree (Elaeis guineensis). J. Microbiol. Antimicrob. 2015, 7, 42–52. [Google Scholar]
- Nwaiwu, O. Suitability of Palm Wine as a Multifunctional Beverage. Available online: https://encyclopedia.pub/315 (accessed on 25 February 2020).
- Okafor, N. Palm-wine yeasts from parts of Nigeria. J. Sci. Food Agric. 1972, 23, 1399–1407. [Google Scholar] [CrossRef]
- Faparusi, S.I.; Bassir, O. Microflora of fermenting palm-wine. J. Food Sci. Technol. 1971, 8, 206–212. [Google Scholar]
- Nwachukwu, I.; Ekaiko, M.U.; Stephen, C. Microbiological quality of palm wine (Elaeis Guineensis and Raphia Hookeri) sold within Aba metropolis, Abia state, south eastern Nigeria. Genet. Eng. Biotechnol. J. 2016, 3, 38–44. Available online: https://www.idpublications.org/wp-content/uploads/2016/03/Full-Paper-microbiological-quality-of-palm-wine-elaeis-guineensis-and-raphia-hookeri-sold-within-aba-metropolis.pdf (accessed on 14 July 2020).
- Onwumah, M.; Okoronkwo, P.; Effiong, E. Molecular characterization of yeast isolated from palm wine in Alakahia, Rivers State, Nigeria. World Sci. News 2019, 130, 297–304. [Google Scholar]
- Eze, C.O.; Berebon, D.P.; Gugu, T.H.; Nworu, C.S.; Esimone, C.O. Effects of Lactobacillus Spp. isolated from the sap of palm tree Elaeis guineensis (palm wine) on cellular and innate immunity. Afr. J. Microbiol. Res. 2019, 13, 33–39. [Google Scholar] [CrossRef]
- Olowonibi, O.O. Isolation and characterization of palm wine strains of Saccharomyces cerevisiae potentially useful as bakery yeasts. Eur. J. Exp. Biol. 2017, 7. [Google Scholar] [CrossRef]
- Nwakanma, C.; Unachukwu, N.M.; Onah, P.; Engwa, A.G. Isolation and sensory evaluation of Saccharomyces cerevisiae from palm wine (Elaeis guinneensis) gotten from different sites in Enugu. Eur. J. Pharm. Sci. 2015, 2, 19–26. Available online: https://www.ejbps.com/ejbps/abstract_id/677 (accessed on 15 July 2020).
- Femi-Ola, T.O.; Falegan, C.R.; Adebule, O.M. Isolation and distribution of Bacillus strains in some Nigerian food and drinks. Int. J. Agric. Innov. Res. 2014, 2, 717–719. Available online: https://ijair.org/index.php/issues?view=publication&task=show&id=181 (accessed on 14 July 2020).
- Chilaka, C.A.; Obidiegwu, J.E.; Akpor, O.B. evaluation of the efficiency of yeast isolates from palm wine in diverse fruit wine production. Afr. J. Food Sci. 2010, 4, 764–774. [Google Scholar]
- Gberikon, G.M.; Ichor, T.; Omeche, E.T. Effect of bitter leaf extract (Vernonia amygdalina) on culturable microorganisms isolated from palm wine in Makurdi metropolis. Res. J. Microbiol. 2016, 11, 112–118. [Google Scholar] [CrossRef]
- Nkemnaso, C.; Ifeanyi, N. Microbiological and nutritional status of palm wine from Umudike and its Environs. Int. J. Bioinform. Biomed. Eng. 2019, 4, 62–69. Available online: http://www.aiscience.org/journal/paperInfo/ijbbe?paperId=4265 (accessed on 14 July 2020).
- Danmadami, R.N.; Yabaya, A.; Yahaya, O.; Abraham, O.J.; Orukotan, A.A. The efficiency of Saccharomyces cerevisiae strain isolated from palm wine in the production of Burukutu. Int. J. Res. 2018. [Google Scholar] [CrossRef]
- Ebbah, L.; Laryea, D.; Barimah, J.; Djameh, C. Effect of Steeping temperature on the quality of malt and Pito (an indigenous Ghanaian drink). J. Inst. Brew. 2015, 121, 518–523. [Google Scholar]
- Ekundayo, J.A. The production of Pito, a Nigerian fermented beverage. Int. J. Food Sci. Technol. 1969, 4, 217–225. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Kayode, R.M.O.; Akinduyo, B. Proximate and microbial analyses of Burukutu and Pito produced in Ilorin, Nigeria. Afr. J. Biotechnol. 2007, 6, 587–590. [Google Scholar]
- Gazuwa, S.; Jaryum, K.; Mafulul, S. Organic contaminants and microbial load of native beers locally prepared within Jos metropolis. J. Appl. Life Sci. Int. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Fadahunsi, I. Biomass yield and antimicrobial substances production in limiting nutrient cultures of lactobacillus Species. Trakia J. Sci. 2015, 13, 51–58. [Google Scholar] [CrossRef]
- Eneji, I.S.; Asan, A.A.; Itodo, A.U. Physicochemical and microbial analysis of locally fermented drinks (burukutu and pito) from cereals in north central Nigeria. FUW Trends Sci. Technol. J. 2020, 5, 433–438. Available online: https://pdfs.semanticscholar.org/409f/d485dbb18fcf946f6b5acc1a4868d3d25c4e.pdf (accessed on 12 July 2020).
- Adesina, I.A.; Ojokoh, A.O.; Arotupin, D.J. Inhibitory properties of lactic acid bacteria again moulds associated with spoilage of bakery products. Adv. Microbiol. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Sanni, A.I.; Lonner, C. Identification of yeasts isolated from Nigerian traditional alcoholic beverages. Food Microbiol. 1993, 10, 517–523. [Google Scholar] [CrossRef]
- Jimoh, S.O.; Ado, S.A.; Ameh, J.B.; Whong, C.M.Z. Characteristics and diversity of yeast in locally fermented beverages sold in Nigeria. World J. Eng. Pure Appl. Sci. 2012, 2, 41. Available online: https://www.academia.edu/9236428/Characteristics_and_Diversity_of_Yeast_in_Locally_Fermented_Beverages_Sold_in_Nigeria (accessed on 12 July 2020).
- Adelekan, A.O.; Arisa, N.U.; Alamu, A.; Adebayo, Y.O.; Omolara, O. The effect of some fruits addition on the nutritional, microbiological and sensory qualities of sorghum (Sorghum bicolour) based Pito. Int. J. Food Sci. 2013, 2, 61–69. [Google Scholar]
- Orji, M.U.; Mbata, T.I.; Aniche, G.N.; Ahonkhai, I. The Use of starter cultures to produce ‘Pito’, a Nigerian fermented alcoholic beverage. World J. Microbiol. Biot. 2003, 19, 733–736. [Google Scholar] [CrossRef]
- Faparusi, S.I.; Olofinboba, J.A.; Ekundayo, J.A. The microbiology of Burukutu beer. Z. Allg. Mikrobiol. 1973, 13, 563–568. [Google Scholar] [CrossRef]
- Chilaka, C.A.; de Boevre, M.; Atanda, O.O.; de Saeger, S. Quantification of Fusarium mycotoxins in Nigerian traditional beers and spices using a multi-mycotoxin LC-MS/MS method. Food Control 2018, 87, 203–210. [Google Scholar] [CrossRef]
- Owuama, C.I. Production of Burukutu with Saccharomyces cerevisiae variants. Appl. Microbiol. Biotechnol. 1991, 35, 21–22. [Google Scholar] [CrossRef]
- Anaukwu, C.G.; Nwangwu, F.C.; Okafor, O.I.; Ezemba, C.C.; Orji, C.C.; Agu, K.C.; Archibong, E.J. Microbiological analysis of Burukutu beverage produced in Southern part of Nigeria. Eur. J. Exp. Biol. 2015, 5, 18–22. [Google Scholar]
- Falegan, C.R.; Akoja, S.O. Microbiological and Physicochemical Studies of Two Nigerian Fermented Alcoholic Drinks (Palmwine and Burukutu) in Ekiti State, Nigeria. Available online: http://www.eajournals.org/wp-content/uploads/Microbiological-and-Physicochemical-Studies-of-Two-Nigerian-Fermented-Alcoholic-Drinks-Palmwine-and-Burukutu-In-Ekiti-State-Nigeria.pdf (accessed on 13 July 2020).
- Lynn, M.; Alibe, W.; Brisca, J.; De, N. Isolation of some pathogens in Burukutu, a local drink, sold in Sengere Village, Girie Local Government, Adamawa State. Greener J. Microbiol. Antimicrob. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Alo, M.N.; Eze, U.A.; Eda, N.E. Microbiological Qualities of Burukutu Produced from a Mixture of Sorghum and Millet. Available online: https://www.academia.edu/5908608/Microbiological_qualities_of_burukutu_produced_from_a_mixture_of_sorghum_and_millet (accessed on 13 July 2020).
- Stanley, M.C.; Ifeanyi, E.; Chinedum, O.K.; Christopher, O.N. Effect of ginger and garlic on the microbial load and shelf-life of Burukutu. Int. J. Microbiol. Res. 2014, 5, 117–123. [Google Scholar]
- Yabaya, A. Microorganisms associated with starter cultures of traditional Burukutu liquor in Madakiya, Kaduna state, Nigeria. Sci. World J. 2008, 3, 9–11. [Google Scholar] [CrossRef][Green Version]
- Eze, V.; Eleke, O. Microbiological and Nutritional Qualities of Burukutu Sold in Mammy Market Abakpa, Enugu State, Nigeria. Available online: https://pdfs.semanticscholar.org/8fed/bc93603285299d1014e3b7a0751b58008805.pdf?_ga=2.120659776.955855744.1594937732-672785399.1575931509 (accessed on 14 July 2020).
- Olaniyi, O.O.; Akinyele, J.B. Isolation of toxigenic Aspergillus flavus and evaluation of aflatoxins in “Burukutu”, sorghum fermented beverage sold in Akure, Nigeria. J. Food Safe. Hyg. 2019, 5, 30–38. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Oni, R. Use of Microbiological indicators for assessing hygiene controls for the manufacture of powdered infant formula. J. Food Prot. 2012, 75, 989–997. [Google Scholar] [CrossRef]
- Camargo, A.C.; Cossi, M.; Silva, W.; Bersot, L.; Landgraf, M.; Baranyi, J.; Franco, B.; Luís Augusto, N. Microbiological Testing for the Proper Assessment of the Hygiene Status of Beef Carcasses. Microorganisms 2019, 7, 86. [Google Scholar] [CrossRef]
- Usman, R.Z.; Mustapha, B.M.; Mohammed, F.I. Isolation and Identification of Methicillin Resistant Staphylococcus aureus (MRSA) from Traditionally Fermented Milk “Nono” and Yoghurt in Zaria Metropolis, Nigeria. Available online: http://ijclris.com/papers/2016/feb2016/ijclris1.pdf (accessed on 14 July 2020).
- Martin, N.H.; Trmcic, A.; Hsieh, T.H.; Boor, K.J.; Wiedmann, M. The evolving role of coliforms as indicators of unhygienic processing conditions in dairy foods. Front. Microbiol. 2016, 7, 1549. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 493–537. [Google Scholar] [CrossRef]
- Ezeronye, O.U.; Legras, J.L. Genetic analysis of Saccharomyces cerevisiae strains isolated from palm wine in eastern Nigeria. Comparison with other African strains. J. Appl. Microbiol. 2009, 106, 1569–1578. [Google Scholar]
- Nwaiwu, O.; Ibekwe, V.I.; Amadi, E.S.; Udebuani, A.C.; Nwanebu, F.C.; Oguoma, O.I.; Nnokwe, J.C. Evaluation of fermentation products of palm wine yeasts and role of Sacoglottis gabonensis supplement on products abundance. Beverages 2016, 2, 9. [Google Scholar] [CrossRef]
- Okolie, P.I.; Opara, C.N.; Emerenini, E.C.; Uzochukwu, S.V.A. Evaluation of bacterial diversity in palm wine by 16S RDNA analysis of community DNA. Niger. Food J. 2013, 31, 83–90. [Google Scholar] [CrossRef][Green Version]
- Banwo, K.; Sanni, A.; Tan, H.; Tian, Y. Phenotypic and genotypic characterization of lactic acid bacteria isolated from some Nigerian traditional fermented foods. Food Biotechnol. 2012, 26, 124–142. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.I.; Ezeokoli, O.T.; Sulyok, M.; van Wyk, D.A.B.; Oyedele, O.A.; Akinyemi, O.M.; Chibuzor-Onyema, I.E.; Adeleke, R.A.; Nwangburuka, C.C.; et al. High-throughput sequence analyses of bacterial communities and multi-mycotoxin profiling during processing of different formulations of Kunu, a traditional fermented beverage. Front. Microbiol. 2019, 10, 3282. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Kellingray, L.; Akinyemi, N.; Adefiranye, O.O.; Olaonipekun, A.B.; Bayili, G.R.; Ibezim, J.; du Plessis, A.S.; Houngbédji, M.; Kamya, D.; et al. Comparison of the microbial composition of african fermented foods using amplicon sequencing. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.K.; Heffron, J.D.; Bozue, J.A.; Welkos, S.L. Bacillus anthracis and other bacillus species. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; Volume 3, pp. 1789–1844. [Google Scholar]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhiming, Y.; Wenyin, L.; Jianxin, Z.; Hao, Z.; Qixiao, Z.; Wei, C. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins -The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Rouzeau-Szynalski, K.; Stollewerk, K.; Messelhäusser, U.; Ehling-Schulz, M. Why be serious about emetic Bacillus cereus: Cereulide production and industrial challenges. Food Microbiol. 2020, 85, 103279. [Google Scholar] [CrossRef]
- Chai, S.J.; Gu, W.; O’Connor, K.A.; Richardson, L.C.; Tauxe, R.V. Incubation periods of enteric illnesses in foodborne outbreaks, United States, 1998-2013. Epidemiol. Infect. 2019, 147. [Google Scholar] [CrossRef]
- Xing, Y.; Willie, W.F. Bacillus spore awakening: Recent discoveries and technological developments. Curr. Opin. Biotechnol. 2020, 64, 110–115. [Google Scholar] [CrossRef]
- Lentz, S.A.M.; Rivas, P.M.; Cardoso, M.R.I.; Morales, D.L.; Centenaro, F.C.; Martins, A.F. Bacillus cereus as the main casual agent of foodborne outbreaks in southern Brazil: Data from 11 Years. Cad. Saude Publica 2018, 34, e00057417. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Fortunato, F.; Tafuri, S.; Cozza, V.; Chironna, M.; Germinario, C.; Pedalino, B.; Prato, R. Lessons learnt from a birthday party: A Bacillus cereus outbreak, Bari, Italy, January 2012. Ann. Ist. Super. Sanita 2013, 49, 391–394. [Google Scholar] [PubMed]
- Chen, D.; Li, Y.; Lv, J.; Liu, X.; Gao, P.; Zhen, G.; Zhang, W.; Wu, D.; Jing, H.; Li, Y.; et al. A foodborne outbreak of gastroenteritis caused by norovirus and Bacillus cereus at a University in the Shunyi district of Beijing, China 2018: A retrospective cohort study. BMC Infect. Dis. 2019, 19, 910. [Google Scholar] [CrossRef] [PubMed]
- Delbrassinne, L.; Botteldoorn, N.; Andjelkovic, M.; Dierick, K.; Denayer, S. An emetic Bacillus cereus outbreak in a kindergarten: Detection and quantification of critical levels of cereulide toxin. Foodborne Pathog. Dis. 2015, 12, 84–87. [Google Scholar] [CrossRef]
- Thirkell, C.E.; Sloan-Gardner, T.S.; Kacmarek, M.C.; Polkinghorne, B. An Outbreak of Bacillus Cereus toxin-mediated emetic and diarrhoeal syndromes at a restaurant in Canberra, Australia 2018. Commun. Dis. Intell. 2019, 43. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, C.; Li, L.; Song, X.; Xu, K.; Wang, J.; Liu, Y.; Fu, K.; Bao, H.; Song, D.; et al. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157:H7 detection. Anal. Biochem. 2018, 542, 58–62. [Google Scholar] [CrossRef]
- Chong, Y.; Shinji Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 Outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Mirhoseini, A.; Amani, J.; Nazarian, S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microb. Pathog. 2018, 117, 162–169. [Google Scholar] [CrossRef]
- Abat, C.; Rolain, J.M.; Colson, P. Investigations by the Institut Hospitalo-Universitaire Méditerranée Infection of Food and Food-Borne Infections in the Mediterranean Basin and in Sub-Saharan Africa. New Microbes New Infect. 2018, 26, S37–S42. [Google Scholar] [CrossRef]
- Aijuka, M.; Buys, E.M. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: Implications for food safety and public health. Food Microbiol. 2019, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Di Ciccio, P.; Ianieri, A.; Nychas, G.E. Methicillin-resistant food-related Staphylococcus aureus: A review of current knowledge and biofilm formation for future studies and applications. Res. Microbiol. 2017, 168, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oniciuc, E.A.; Nicolau, I.A.; Hernández, M.; Rodríguez-Lázaro, D. Presence of methicillin-resistant Staphylococcus aureus in the food chain. Trends Food Sci. Technol. 2017, 61, 49–59. [Google Scholar] [CrossRef]
- Rubab, M.; Shahbaz, H.M.; Olaimat, A.N.; Oh, D.H. Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens. Bioelectron. 2018, 105, 49–57. [Google Scholar] [CrossRef]
- Shankar, N.; Soe, P.; Tam, C.C. Prevalence and risk of acquisition of methicillin-resistant Staphylococcus aureus among households: A Systematic review. Int. J. Infect. Dis. 2020, 92, 105–113. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Garcia, C.; Fessard, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F. Nutritional and microbiological quality of tiger nut tubers (Cyperus esculentus), derived plant-based and lactic fermented beverages. Fermentation 2019, 5, 3. [Google Scholar] [CrossRef]
- Salvetti, E.; Orrù, L.; Capozzi, V.; Lamontanara, A.; Keller, D.; Cash, H.; Cattivelli, L.; Torriani, S.; Spano, G. Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl. Microbiol. Biotechnol. 2016, 100, 4595–4605. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Production of fungal biomass for feed, fatty acids, and glycerol by Aspergillus oryzae from fat-rich dairy substrates. Fermentation 2017, 3, 48. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of co-inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the industrial production of Negroamaro wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Russo, P.; Fragasso, M.; Berbegal, C.; Grieco, F.; Spano, G.; Capozzi, V. Microorganisms able to produce biogenic amines and factors affecting their activity. In Biogenic Amines in Food: Analysis, Occurrence and Toxicity, 1st ed.; Saad, B., Tofalo, R., Eds.; Royal Society of Chemistry Publishing: Cambridge, UK, 2020; pp. 18–40. [Google Scholar]

| S/n | Bacteria | Yoghurt [70,71,72,73,74,75,76,77,78,79] | Nono [58,59,60,61,62,63,64,65,66,67] | Soymilk [28,29,30,31,32,33,34,35,36,37] | Tiger Nut Milk [46,47,48,49,50,51,52,53,54,55] | Zobo [96,97,98,99,100,101,102,103,104,105] | Kunu [83,84,85,86,87,88,89,90,91,92] | Pito [123,124,125,126,127,128,129,130,131,132] | Burukutu [132,133,134,135,136,137,138,139,140,141,142,143] | Palm Wine [123,124,125,126,127,128,129,130,131,132] |
|---|---|---|---|---|---|---|---|---|---|---|
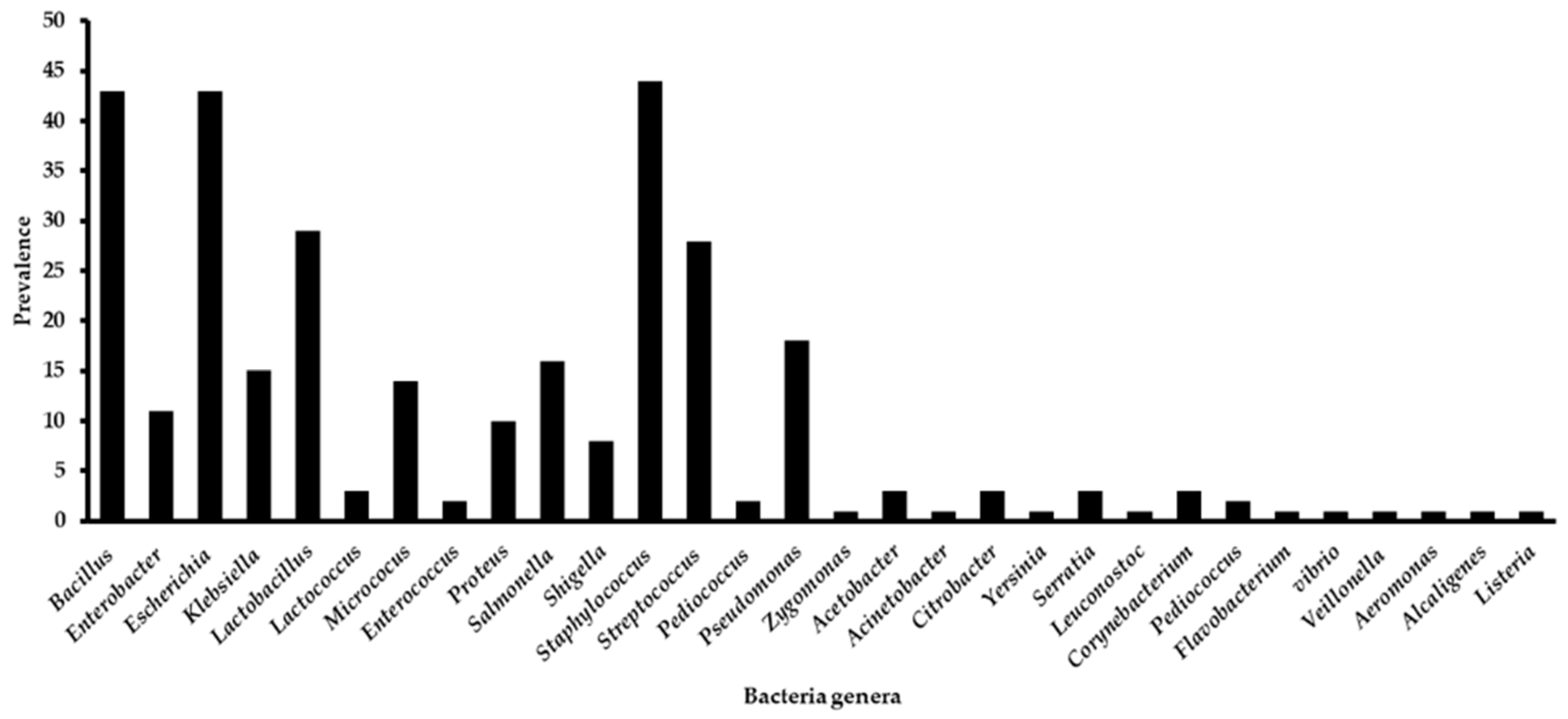
| 1 | Bacillus | 5 | 2 | 4 | 6 | 8 | 7 | 2 | 5 | 4 |
| 2 | Enterobacter | 3 | 2 | 1 | 3 | 1 | 1 | |||
| 3 | Escherichia | 3 | 7 | 5 | 4 | 7 | 9 | 2 | 5 | 1 |
| 4 | Klebsiella | 2 | 4 | 2 | 2 | 2 | 1 | 2 | ||
| 5 | Lactobacillus | 4 | 2 | 1 | 3 | 3 | 5 | 3 | 5 | 3 |
| 6 | Lactococus | 1 | 1 | 1 | ||||||
| 7 | Micrococus | 1 | 1 | 2 | 5 | 2 | 1 | 1 | 1 | |
| 8 | Enterococcus | 1 | 1 | |||||||
| 9 | Proteus | 2 | 1 | 2 | 3 | 1 | 1 | |||
| 10 | Salmonella | 3 | 2 | 4 | 3 | 2 | 1 | 1 | ||
| 11 | Shigella | 2 | 2 | 1 | 2 | 1 | ||||
| 12 | Staphylococcus | 6 | 4 | 6 | 4 | 7 | 6 | 2 | 6 | 3 |
| 13 | Streptococcus | 4 | 1 | 3 | 2 | 3 | 5 | 3 | 4 | 3 |
| 14 | Pediococcus | 1 | 1 | |||||||
| 15 | Pseudomonas | 2 | 2 | 3 | 2 | 5 | 3 | 1 | ||
| 16 | Zygomonas | 1 | ||||||||
| 17 | Acetobacter | 1 | 1 | 1 | ||||||
| 18 | Acinetobacter | 1 | ||||||||
| 19 | Citrobacter | 1 | 1 | 1 | ||||||
| 20 | Yersinia | 1 | ||||||||
| 21 | Serratia | 1 | 1 | 1 | ||||||
| 22 | Leuconostoc | 1 | ||||||||
| 23 | Corynebacterium | 1 | 1 | 1 | ||||||
| 24 | Pediococcus | 1 | 1 | |||||||
| 25 | Flavobacterium | 1 | ||||||||
| 26 | Vibrio | 1 | ||||||||
| 27 | Veillonella | 1 | ||||||||
| 28 | Aeromonas | 1 | ||||||||
| 29 | Alcaligenes | 1 | ||||||||
| 30 | Listeria | 1 | ||||||||
| Fungi | ||||||||||
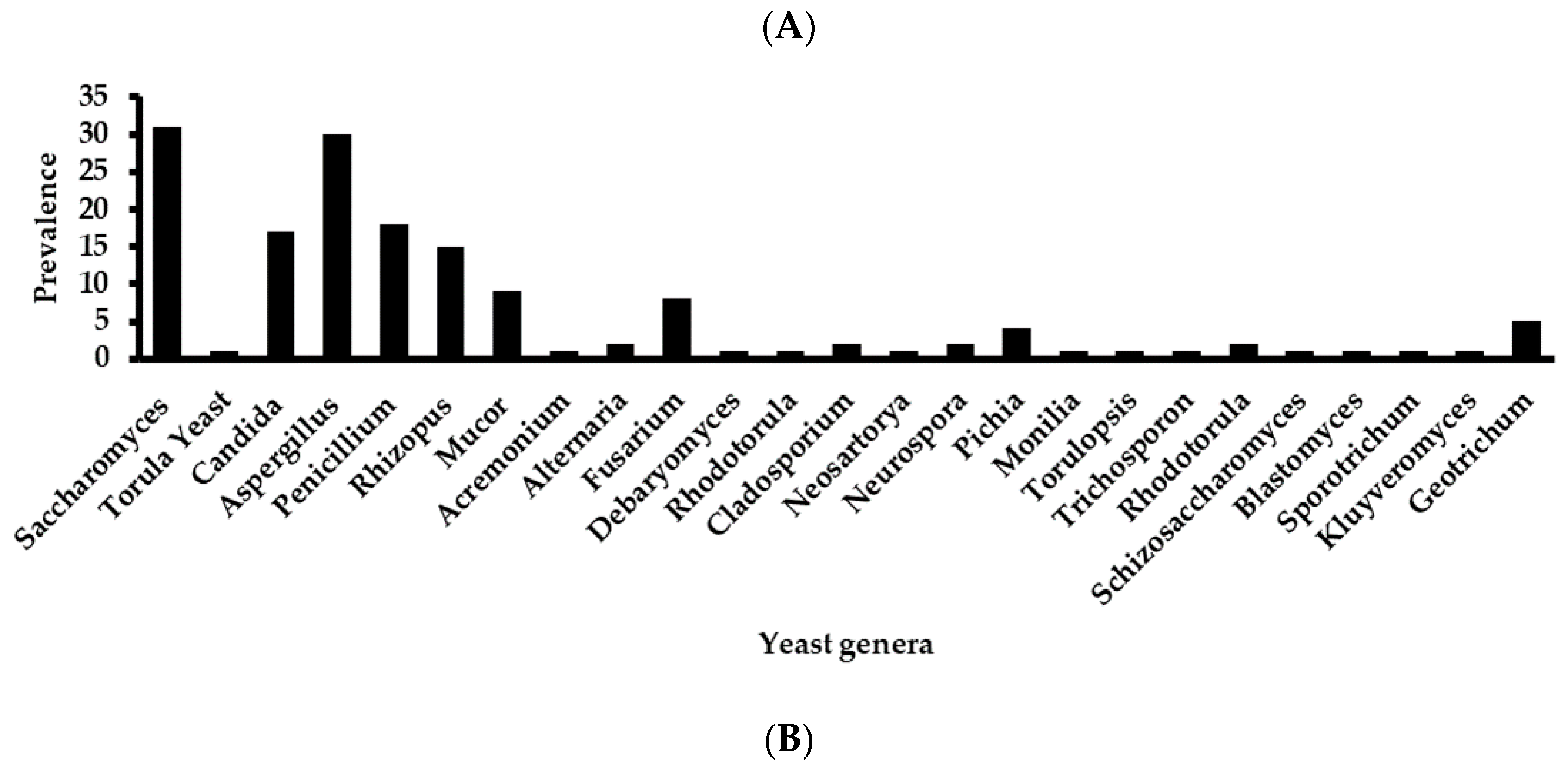
| 1 | Saccharomyces | 2 | 1 | 3 | 3 | 1 | 5 | 4 | 7 | 5 |
| 2 | * Torula yeast | 1 | ||||||||
| 3 | Candida | 1 | 2 | 1 | 3 | 2 | 2 | 2 | 1 | 3 |
| 4 | Aspergillus | 3 | 1 | 7 | 4 | 3 | 4 | 2 | 5 | 1 |
| 5 | Penicillium | 1 | 1 | 3 | 2 | 4 | 4 | 1 | 1 | 1 |
| 6 | Rhizopus | 2 | 4 | 1 | 2 | 2 | 2 | 2 | ||
| 7 | Mucor | 2 | 1 | 1 | 1 | 1 | 2 | 1 | ||
| 8 | Acremonium | 1 | ||||||||
| 9 | Alternaria | 1 | 1 | |||||||
| 10 | Fusarium | 1 | 1 | 1 | 3 | 1 | 1 | |||
| 11 | Debaryomyces | 1 | ||||||||
| 12 | Rhodotorula | 1 | ||||||||
| 13 | Cladosporium | 1 | 1 | |||||||
| 14 | Neosartorya | 1 | ||||||||
| 15 | Neurospora | 1 | 1 | |||||||
| 16 | Pichia | 1 | 1 | 2 | ||||||
| 17 | Monilia | 1 | ||||||||
| 18 | Torulopsis | 1 | ||||||||
| 19 | Trichosporon | 1 | ||||||||
| 20 | Rhodotorula | 1 | 1 | |||||||
| 21 | Schizo-saccharomyces | 1 | ||||||||
| 22 | Blastomyces | 1 | ||||||||
| 23 | Sporotrichum | 1 | ||||||||
| 24 | Kluyveromyces | 1 | ||||||||
| 25 | Geotrichum | 1 | 2 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwaiwu, O.; Aduba, C.C.; Igbokwe, V.C.; Sam, C.E.; Ukwuru, M.U. Traditional and Artisanal Beverages in Nigeria: Microbial Diversity and Safety Issues. Beverages 2020, 6, 53. https://doi.org/10.3390/beverages6030053
Nwaiwu O, Aduba CC, Igbokwe VC, Sam CE, Ukwuru MU. Traditional and Artisanal Beverages in Nigeria: Microbial Diversity and Safety Issues. Beverages. 2020; 6(3):53. https://doi.org/10.3390/beverages6030053
Chicago/Turabian StyleNwaiwu, Ogueri, Chiugo Claret Aduba, Victor Chukwunenye Igbokwe, Chizoba Evelyn Sam, and Michael Ukwuru Ukwuru. 2020. "Traditional and Artisanal Beverages in Nigeria: Microbial Diversity and Safety Issues" Beverages 6, no. 3: 53. https://doi.org/10.3390/beverages6030053
APA StyleNwaiwu, O., Aduba, C. C., Igbokwe, V. C., Sam, C. E., & Ukwuru, M. U. (2020). Traditional and Artisanal Beverages in Nigeria: Microbial Diversity and Safety Issues. Beverages, 6(3), 53. https://doi.org/10.3390/beverages6030053








