Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapevine Cultivars and Yeast Strains
2.2. Yeast Growth and Inocula Preparation
2.3. Vinifications
- S. cerevisiae ScXG3
- L. thermotolerans Lt93
- L. thermotolerans Lt93 + S. cerevisiae ScXG3 by sequential fermentation (Lt93 + ScXG3)
- No yeast addition (spontaneous fermentation with indigenous yeast from the must) (Spo)
- S. cerevisiae Sc71B
- L. thermotolerans Lt93
- L. thermotolerans Lt93 + S. cerevisiae Sc71B by sequential inoculation (Lt93 + Sc71B)
- No yeast addition (spontaneous fermentation with indigenous yeast from the must) (Spo)
2.4. Microbiological Control
2.5. Chemical Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results
3.1. Fermentation Kinetics and Dinamic of Yeast Population
3.1.1. Treixadura
3.1.2. Mencía
3.2. Chemical Characteristics of Wines
3.2.1. Treixadura
3.2.2. Mencía
3.3. Sensory Evaluation of Treixadura Wines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleet, G.H.; Heard, G.M. Yeasts-Growth during fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Reading, UK, 1993; pp. 28–47. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Role and Use of Non-Saccharomyces Yeasts in Wine Production. S. Afr. J. Enol. Vitic. 2006, 27, 15–39. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 87. [Google Scholar] [CrossRef]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous yeast interactions in dual-starter fermentations may improve the varietal expression of Moschofilero wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 14312. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.; Mulet, A. Growth of Yeast Species During the Fermentation of Musts Inoculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1990, 41, 156–159. [Google Scholar]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and composition of airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non- Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Bokulich, N.; Collins, T.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Fermentation Behavior Suggest Microbial Contribution to Regional. MBio 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Hranilovic, A.; Bely, M.; Masneuf-Pomarede, I.; Jiranek, V.; Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 2017, 12, e0184652. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Yeast diversity on grapes from Galicia, NW Spain: Biogeographical patterns and the influence of the farming system. Oeno ONE 2019, 53, 573–587. [Google Scholar] [CrossRef]
- Blanco, P.; Castrillo, D.; Graña, M.J.; Lorenzo, M.J.; Soto Vázquez, E. Evaluación de levaduras vínicas no convencionales para afrontar las consecuencias del cambio climático en bodega. Enoviticultura 2019, 58, 16–25. [Google Scholar]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological potential of non-Saccharomyces yeasts to mitigate effects of climate change in winemaking: Impact on aroma and sensory profiles of Treixadura wines. FEMS Yeast Res. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Treixadura (Vitis vinifera L.) musts and wines, and on the aromatic composition and sensory profiles of its wines. Food Chem. 2018, 240, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E.; Mirás-Avalos, J. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages 2018, 4, 76. [Google Scholar] [CrossRef]
- Blanco, P.; Mirás-Avalos, J.M.; Pereira, E.; Orriols, I. Fermentative aroma compounds and sensory profiles of Godello and Albariño wines as influenced by Saccharomyces cerevisiae yeast strains. J. Sci. Food Agric. 2013, 93, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Mirás-Avalos, J.M.; Suárez, V.; Orriols, I. Inoculation of Treixadura musts with autochthonous Saccharomyces cerevisiae strains: Fermentative performance and influence on the wine characteristics. Food Sci. Technol. Int. 2013, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL Medium to Profile Native Flora. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Huerta, T.; Ramon, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Office International de la Vigne et du Vin Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2015. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Etievant, P.X. Wine. In Volatile Compounds of Food and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Escudero, A.; Graña, M.; Cacho, J. Volatile composition and sensory properties of North West Spain white wines. Food Res. Int. 2013, 54, 562–568. [Google Scholar] [CrossRef]
- Nissen, P.; Nielsen, D.; Arneborg, N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 2003, 20, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Branco, P.; Almeida, M.G.; Caldeira, J.; Albergaria, H.; Arneborg, N. Cell-to-cell contact and antimicrobial peptides play a combined role in the death of Lachanchea thermotolerans during mixed-culture alcoholic fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef]
- Blanco, P.; Mirás-Avalos, J.M.; Pereira, E.; Fornos, D.; Orriols, I. Modulation of chemical and sensory characteristics of red wine from Mencía by using indigenous Saccharomyces cerevisiae yeast strains. J. Int. Sci. Vigne Vin 2014, 48, 63–74. [Google Scholar] [CrossRef]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Callejon, R.M.; Clavijo, A.; Ortigueira, P.; Troncoso, A.M.; Paneque, P.; Morales, M.L. Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal. Chim. Acta 2010, 660, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the volatile fraction of young wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta 2006, 563, 145–153. [Google Scholar] [CrossRef]
- Shinohara, T. Gas Chromatographic Analysis of Volatile Fatty Acids in Wines. Agric. Biol. Chem. 1985, 49, 2211–2212. [Google Scholar]
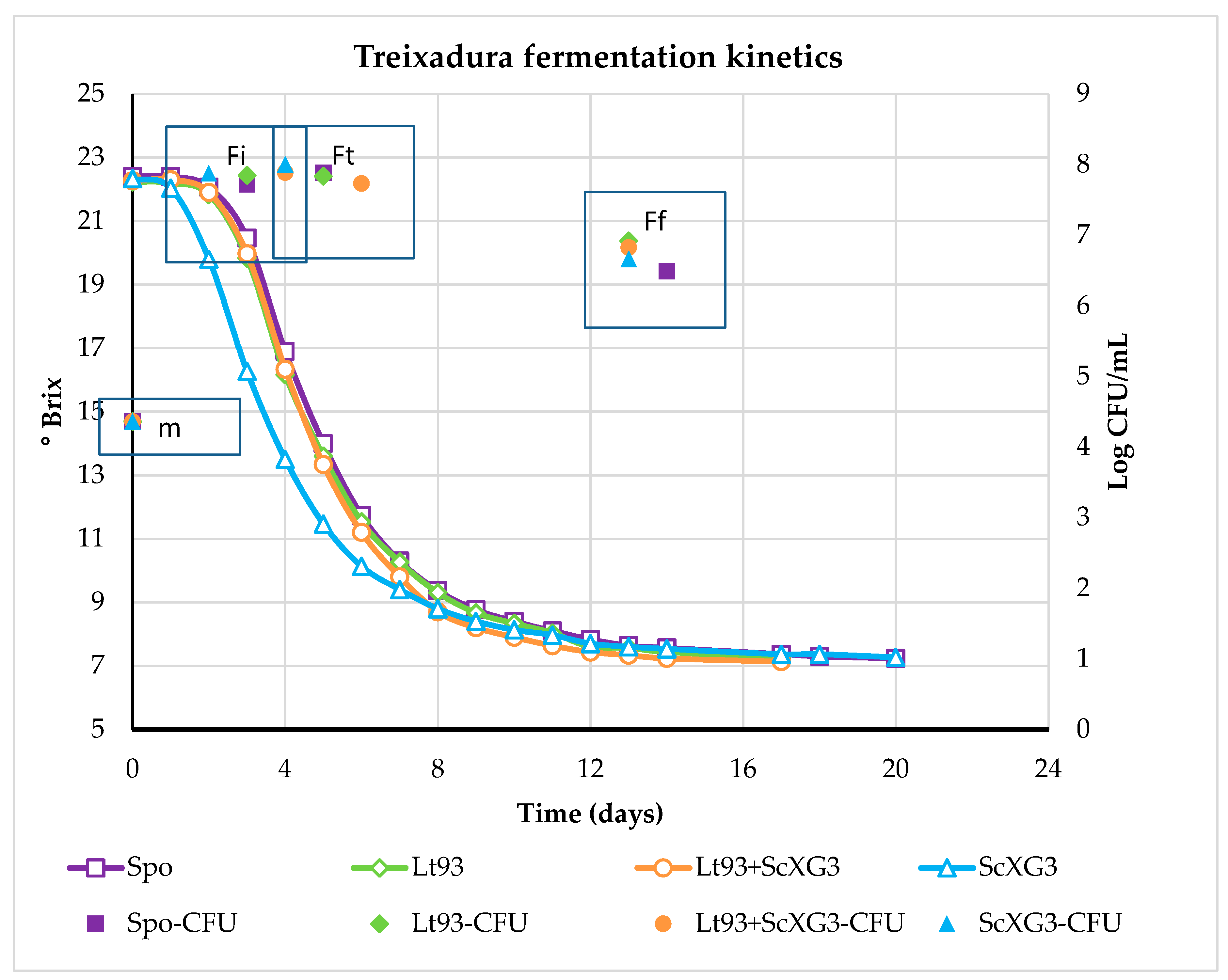
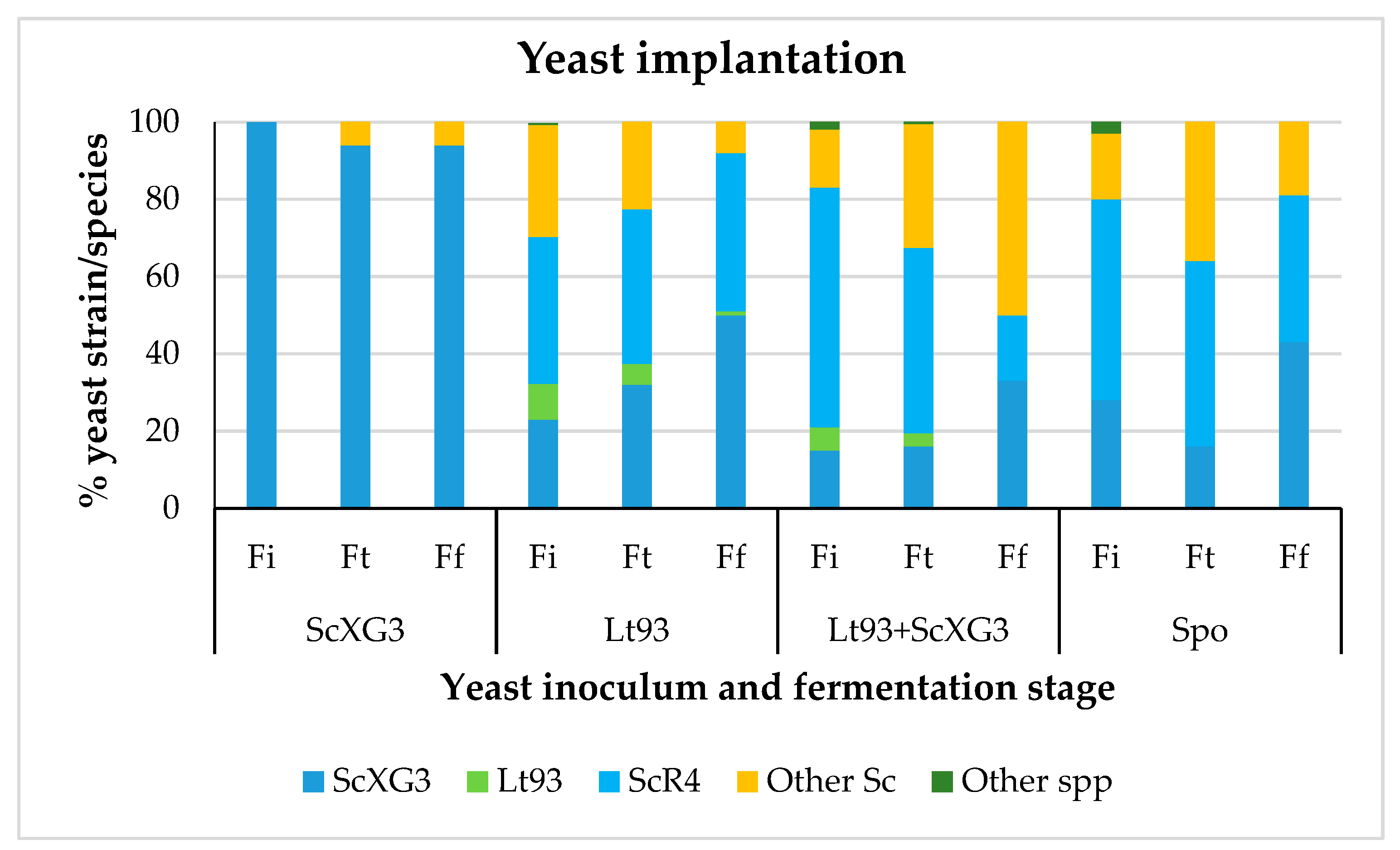
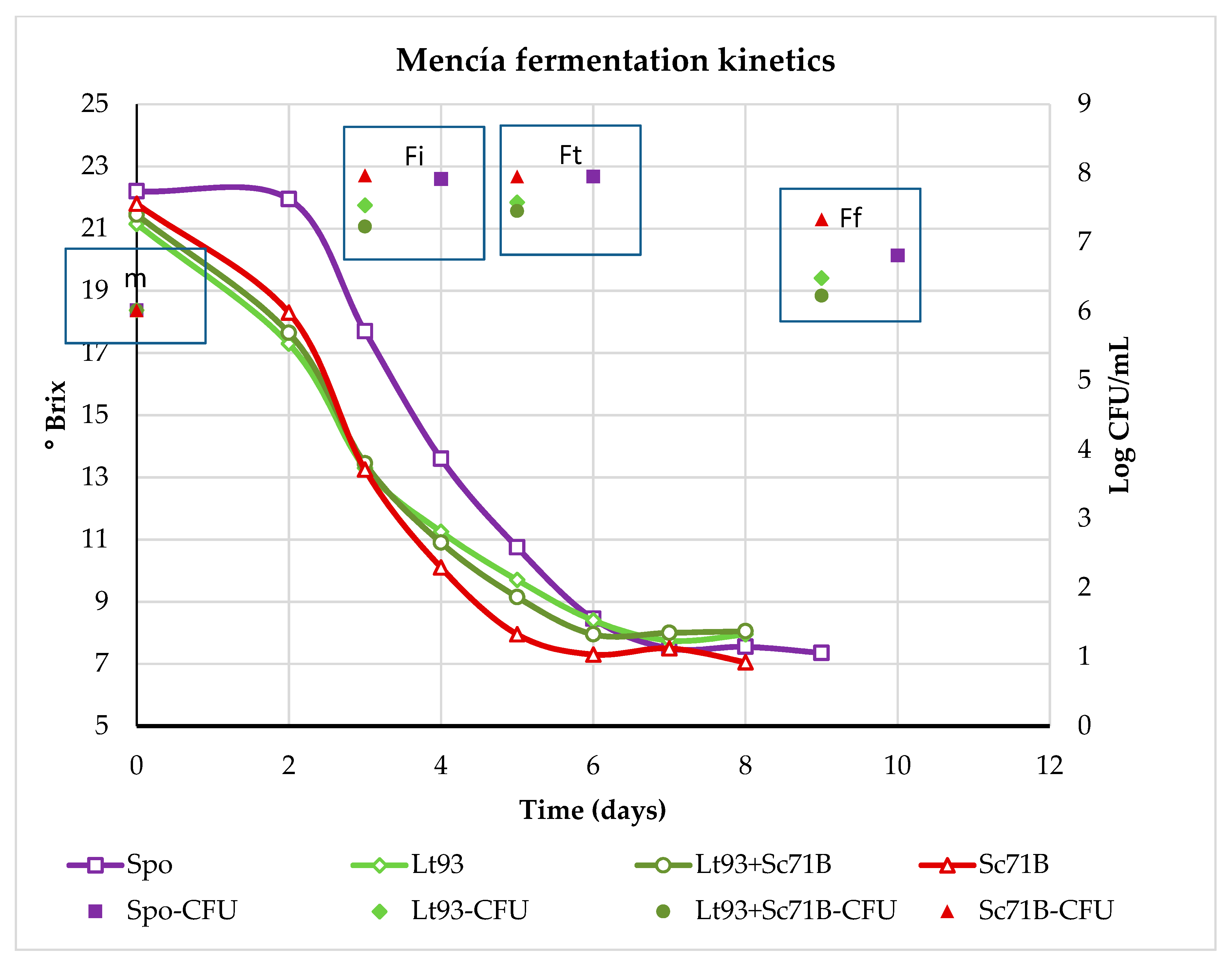
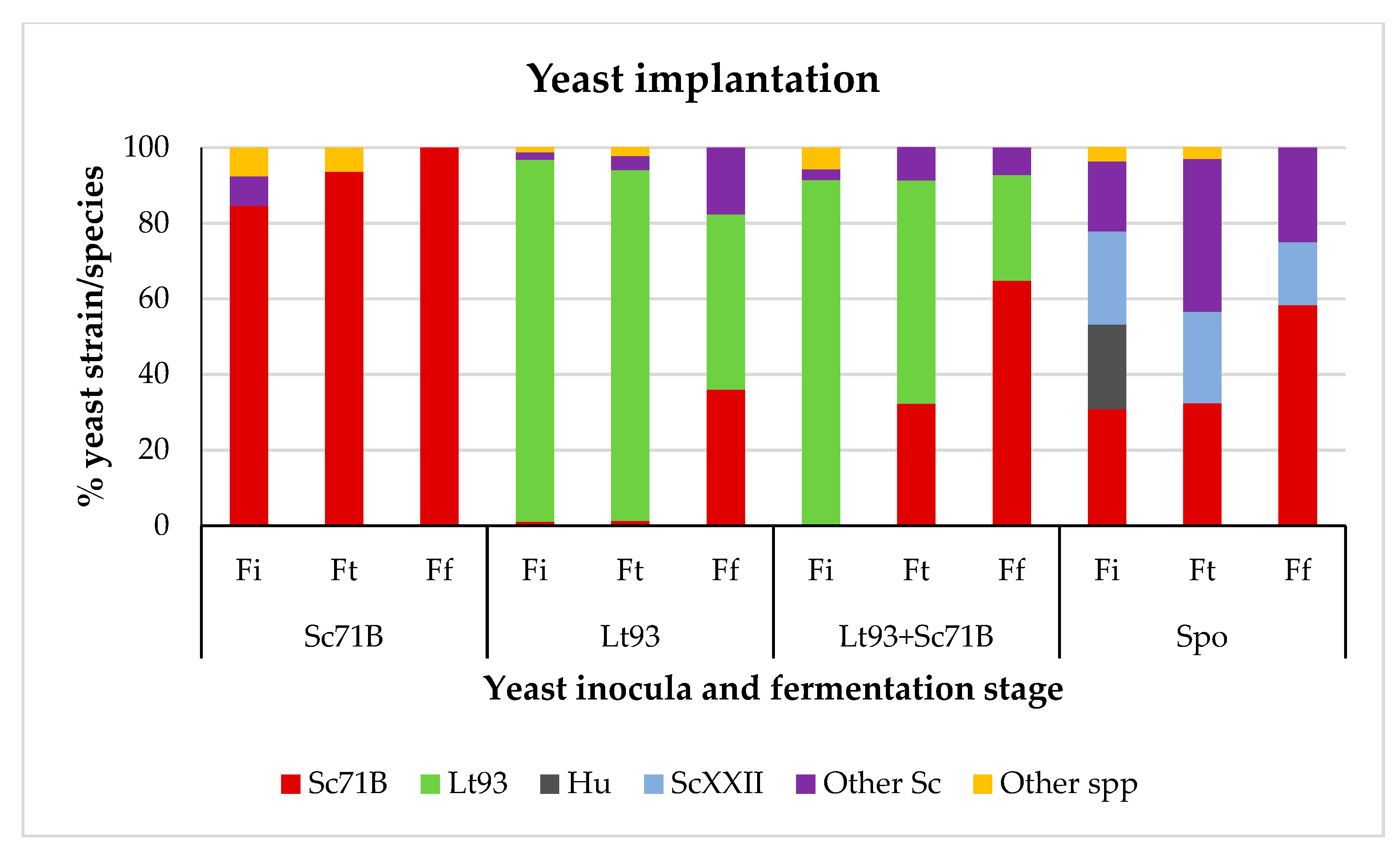

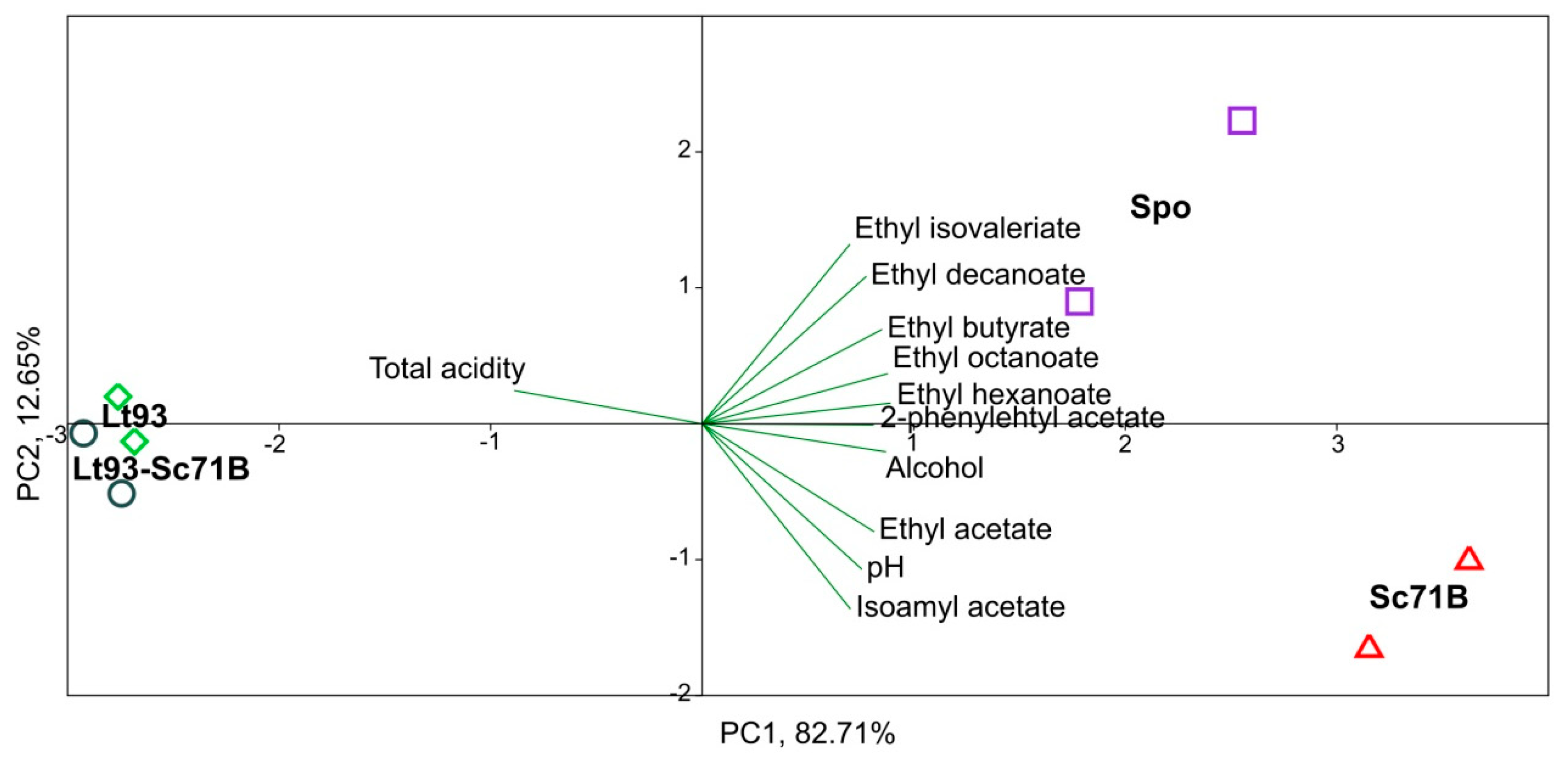
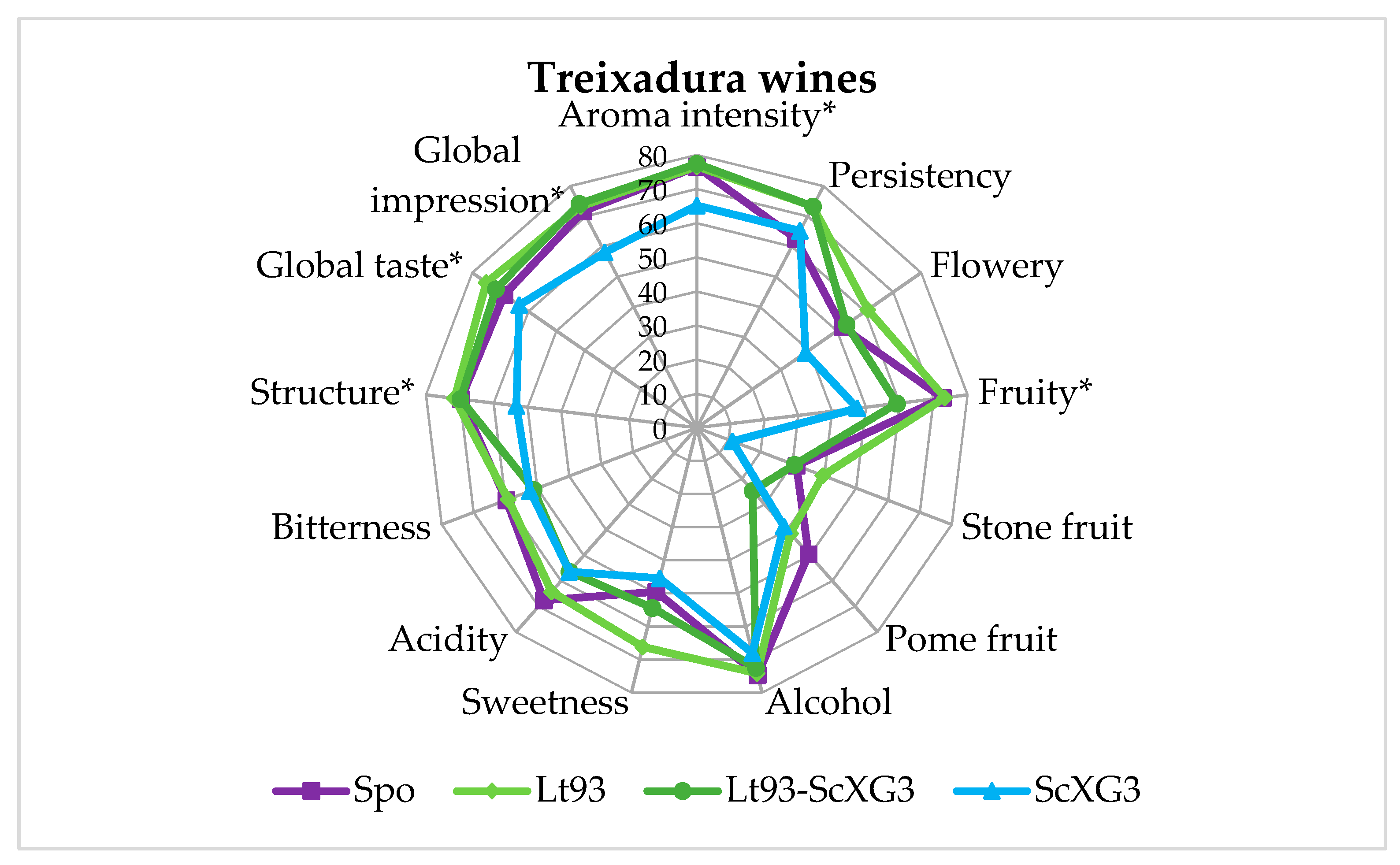
| Parameter | Treixadura | Mencía |
|---|---|---|
| ° Brix | 22.4 | 21.5 |
| Sugars (g/L) | 219.5 | 209.0 |
| Total acidity (g tartaric acid/L) | 5.7 | 4.6 |
| pH | 3.51 | 3.57 |
| Malic acid (g/L) | 2.3 | 1.4 |
| Tartaric acid (g/L) | 4.7 | 4.8 |
| Chemical Parameter | ScXG3 | Lt93 | Lt93+ScXG3 | Spo |
|---|---|---|---|---|
| Alcohol content (%vol) | 13.5 ± 0.2 | 13.5 ± 0.1 | 13.6 ± 0.1 | 13.6 ± 0.0 |
| Glucose + fructose (g/L) | 4.2 ± 2.3 | 3.5 ± 0.7 | 1.6 ± 1.1 | 2.9 ± 0.3 |
| Total acidity (g tartaric acid /L) * | 5.6 ± 0.1 a | 5.8 ± 0.1 b | 5.7 ± 0.1 ab | 5.6 ± 0.0 a |
| Volatile acidity (g acetic acid/L) | 0.45 ± 0.04 | 0.36 ± 0.05 | 0.39 ± 0.01 | 0.38 ± 0.02 |
| Lactic acid (g/L) * | 0.1 ± 0.0 a | 0.2 ± 0.0 b | 0.2 ± 0.1 ab | 0.1 ± 0.0 a |
| Malic acid (g/L) | 1.8 ± 0.0 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.0 |
| Tartaric acid (g/L) | 3.4 ± 0.0 | 3.7 ± 0.0 | 3.4 ± 0.2 | 3.4 ± 0.1 |
| pH (-) * | 3.45 ± 0.02 a | 3.39 ± 0.02 b | 3.42 ± 0.03 ab | 3.46 ± 0.02 a |
| Glycerol (g/L) * | 3.9 ± 0.1 a | 3.3 ± 0.1 b | 3.5 ± 0.1 b | 3.6 ± 0.1 ab |
| Total sulfur dioxide (mg/L) * | 36.7 ± 3.0 a | 54.3 ± 5.5 b | 49.7 ± 1.1 b | 37.3 ± 2.1 a |
| Compounds | Spo | Lt93 | Lt93+ScXG3 | ScXG3 | Statistical Significance |
|---|---|---|---|---|---|
| Alcohols (mg/L) | |||||
| Methanol | 38.33 ± 1.53 | 37.33 ± 1.53 | 41.00 ± 1.73 | 38.67 ± 1.15 | ns |
| 1-propanol | 20.39 ± 0.32 | 20.08 ± 0.98 | 23.90 ± 3.23 | 23.21 ± 1.66 | ns |
| 2-methyl-1-propanol | 37.25 ± 2.51 b | 26.05 ± 3.77 a | 36.35 ± 2.42 b | 53.46 ± 2.08 c | *** |
| 2-methyl-1-butanol | 16.07 ± 0.14 a | 15.99 ± 1.23 a | 17.50 ± 1.23 a | 23.33 ± 2.37 b | *** |
| 3-methyl-1-butanol | 145.94 ± 2.68 ab | 139.31 ± 8.52 a | 155.57 ± 11.06 bc | 168.98 ± 6.75 c | ** |
| ∑Higher alcohols | 219.64 ± 5.31b | 195.80 ± 6.29 a | 233.32 ± 17.68 b | 268.97 ± 11.28 c | *** |
| Other major compounds (mg/L) | |||||
| Acetaldehyde | 82.00 ± 15.87 | 47.67 ± 13.50 | 65.00 ± 31.48 | 46.67 ± 34.93 | ns |
| Ethyl acetate | 37.33 ± 2.52 | 46.00 ± 3.00 | 38.67 ± 12.74 | 40.33 ± 4.93 | ns |
| Higher alcohol acetates(µg/L) | |||||
| 2-phenylethyl acetate | 282 ± 29 b | 197 ± 34 a | 215 ± 25 a | 242 ± 20 ab | * |
| Hexyl acetate | 128 ± 35 | 115 ± 64 | 70 ± 26 | 92 ± 55 | ns |
| Isoamyl acetate | 1257 ± 155 | 1548 ± 352 | 1474 ± 264 | 1142 ± 133 | ns |
| ∑Higher alcohol acetates | 1667 ± 193 | 1859 ± 442 | 1759 ± 299 | 1476 ± 114 | ns |
| Ethyl esters (µg/L) | |||||
| Ethyl hexanoate | 258 ± 38 b | 381 ± 47 c | 119 ± 104 a | 242 ± 3 b | ** |
| Ethyl octanoate | 360 ± 145 | 528 ± 77 | 479 ± 109 | 343 ± 39 | ns |
| Ethyl decanoate | 65 ± 49 | 107 ± 44 | 91 ± 26 | 57 ± 24 | ns |
| ∑Ethyl esters | 683 ± 197 a | 1017 ± 96 b | 689 ± 85 a | 641 ± 64 a | * |
| Fatty acids (µg/L) | |||||
| Butyric acid | 1163 ± 68 b | 1473 ± 155 c | 1436 ± 37 c | 964 ± 51 a | *** |
| Isobutyric acid | 1347 ± 123 b | 1127 ± 103 a | 1287 ± 53 ab | 2703 ± 70 c | *** |
| Isovaleric acid | 395 ± 38 a | 341 ± 26 a | 387 ± 40 a | 676 ± 25 b | *** |
| ∑Short-chain acids (C4–C5) | 2905 ± 159 a | 2942 ± 205 a | 3106 ± 71 a | 4343 ± 136 b | *** |
| Hexanoic acid | 2560 ± 273 b | 3269 ± 328 c | 2874 ± 102 bc | 2050 ± 89 a | *** |
| Octanoic acid | 4678 ± 978 ab | 5590 ± 595 b | 4928 ± 502 b | 3539 ± 213a | * |
| Decanoic acid | 912 ± 279 ab | 1287 ± 55 b | 931 ± 268 ab | 607 ± 241 a | * |
| Dodecanoic acid | 178 ± 67 | 121 ± 67 | 118 ± 28 | 86 ± 11 | ns |
| ∑Medium-long chain acids (C6–C12) | 8328 ± 1593 b | 10,267 ± 883 b | 8851 ± 898 b | 6282 ± 543 a | * |
| ∑Total fatty acids | 11,233 ± 1511 | 13,209 ± 1021 | 11,957 ± 853 | 10,626 ± 617 | ns |
| Chemical Parameter | Sc71B | Lt93 | Lt93+Sc71B | Spo |
|---|---|---|---|---|
| Alcohol content (%vol.) * | 12.5 ± 0.1 a | 11.7 ± 0.0 b | 11.9 ± 0.1 b | 12.3 ± 0.1 a |
| Glucose + fructose (g/L) * | 0.2 ± 0.1 a | 3.6 ± 0.3 b | 1.85 ± 0.2 c | 0.2 ± 0.1 a |
| Total acidity (g tartaric acid /L) * | 5.3 ± 0.1 a | 10.1 ± 0.0 c | 10.3 ± 0.0 c | 6.8 ± 0.0 b |
| Volatile acidity (g acetic acid/L) * | 0.24 ± 0.06 ab | 0.36 ± 0.01 bc | 0.42 ± 0.01 c | 0.20 ± 0.00 a |
| Lactic acid (g/L) * | 0.2 ± 0.1 a | 7.1 ± 0.1 b | 7.2 ± 0.8 b | 0.4 ± 0.1 a |
| Malic acid (g/L) * | 1.4 ± 0.1 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 2.5 ± 0.0 c |
| Tartaric acid (g/L) * | 2.6 ± 0.0 a | 3.2 ± 0.0 c | 3.1 ± 0.0 b | 2.6 ± 0.0 a |
| pH (-) * | 3.74 ± 0.00 a | 3.54 ± 0.02 b | 3.57 ± 0.00 b | 3.61 ± 0.01 b |
| Glycerol (g/L) * | 10 ± 0.0 a | 8.6 ± 0.0 c | 9.1 ± 0.0 b | 8.2 ± 0.0 d |
| Total sulfur dioxide (mg/L) * | 27.5 ± 0.7 a | 40.0 ± 0.7 b | 43.0 ± 0.7 b | 38.5 ± 0.7 b |
| Compounds | Spo | Lt93 | Lt93+Sc71B | Sc71B | Statistical Significance |
|---|---|---|---|---|---|
| Alcohols | |||||
| Benzyl alcohol | 14 ± 2 | 8 ± 0 | 6 ± 1 | 14 ± 4 | ns |
| 2-methyl-1-propanol | 1622 ± 211 a | 180 ± 69 c | 1264 ± 112 ab | 846 ± 59 b | *** |
| 3-methyl-1-butanol | 6350 ± 163 b | 5311 ± 198 c | 5081 ± 185 c | 7155 ± 61 a | *** |
| 2-phenylethyl alcohol | 5604 ± 360 ab | 3949 ± 638 bc | 3266 ± 349 c | 6491 ± 159 a | ** |
| ∑Higher alcohols | 13,577 ± 733 a | 9440 ± 370 b | 9612 ± 53 b | 14,492 ± 161 a | *** |
| Ethyl esters | |||||
| Ethyl butyrate | 150 ± 20 a | 56 ± 3 b | 55 ± 5 b | 133 ± 16 a | ** |
| Ethyl isovalerate | 20 ± 1 a | 8 ± 2 c | 9 ± 1 c | 13 ± 0 b | *** |
| Ethyl hexanoate | 1148 ± 153 a | 499 ± 47 b | 476 ± 37 b | 1258 ± 20 a | *** |
| Ethyl octanoate | 1405 ± 124 a | 274 ± 12 b | 229 ± 31 b | 1360 ± 69 a | *** |
| Ethyl decanoate | 344 ± 105 a | 86 ± 6 ab | 41 ± 16 b | 248 ± 75 ab | * |
| Ethyl dodecanoate | 22 ± 4 | 12 ± 9 | 10 ± 3 | 27 ± 7 | ns |
| ∑Ethyl esters | 3090 ± 407 a | 935 ± 66 b | 819 ± 52 b | 3040 ± 49 a | *** |
| Diethyl succinate | 943 ± 46 a | 84 ± 26 b | 51 ± 11 b | 1208 ± 183 a | *** |
| Acetates | |||||
| Ethyl acetate | 7450 ± 1972 ab | 3702 ± 493 b | 4087 ± 219 b | 10761 ± 648 a | ** |
| Isoamyl acetate | 1917 ± 318 b | 1659 ± 107 b | 1648 ± 205 b | 2845 ± 128 a | * |
| Hexyl acetate | 188 ± 56 | 143 ± 2 | 152 ± 13 | 216 ± 47 | ns |
| 2-phenylehtyl acetate | 250 ± 46 ab | 185 ± 17 ab | 123 ± 26 b | 304 ± 41 a | * |
| ∑Higher alcohol acetates | 2355 ± 327 b | 1987 ± 91 b | 1923 ± 192 b | 3365 ± 134 a | ** |
| Fatty acids (C6–C10) | |||||
| Hexanoic acid | 132 ± 25 a | 55 ± 7 b | 41 ± 9 b | 142 ± 13 a | ** |
| Octanoic acid | 244 ± 42 a | 32 ± 7 b | 19 ± 6 b | 242 ± 20 a | *** |
| Decanoic acid | 49 ± 2 a | 17 ± 0 b | 10 ± 1 b | 44 ± 11 a | ** |
| ∑Fatty acids | 425 ± 69 a | 105 ± 14 b | 70 ± 16 b | 428 ± 44 a | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, P.; Rabuñal, E.; Neira, N.; Castrillo, D. Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics. Beverages 2020, 6, 36. https://doi.org/10.3390/beverages6020036
Blanco P, Rabuñal E, Neira N, Castrillo D. Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics. Beverages. 2020; 6(2):36. https://doi.org/10.3390/beverages6020036
Chicago/Turabian StyleBlanco, Pilar, Eva Rabuñal, Noemi Neira, and David Castrillo. 2020. "Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics" Beverages 6, no. 2: 36. https://doi.org/10.3390/beverages6020036
APA StyleBlanco, P., Rabuñal, E., Neira, N., & Castrillo, D. (2020). Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics. Beverages, 6(2), 36. https://doi.org/10.3390/beverages6020036




