Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Fermentation
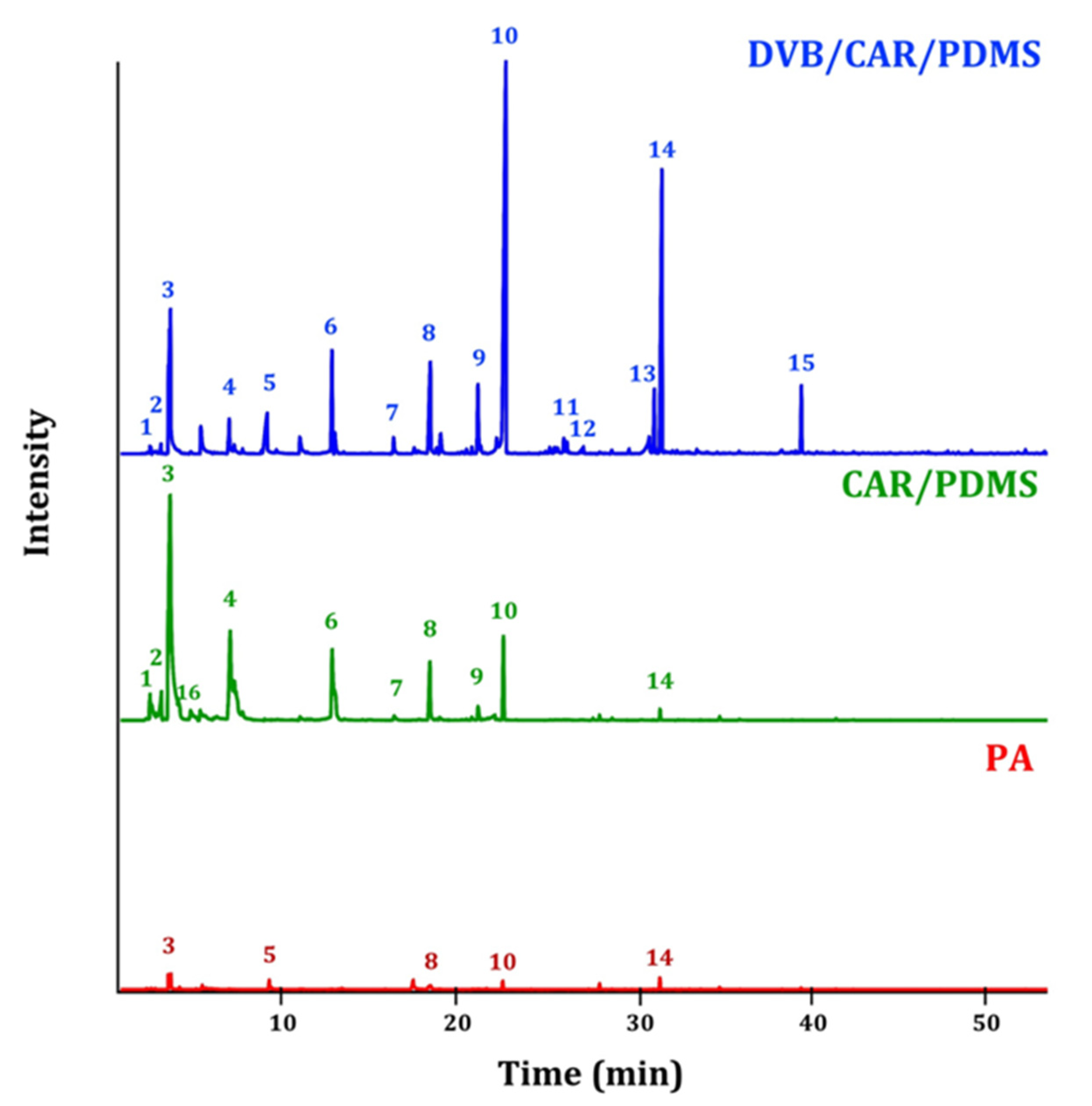
2.2. Solid Phase Microextraction (SPME) Optimization
2.3. Gas Chromatography–Mass Spectrometry Analysis
3. Results and Discussion
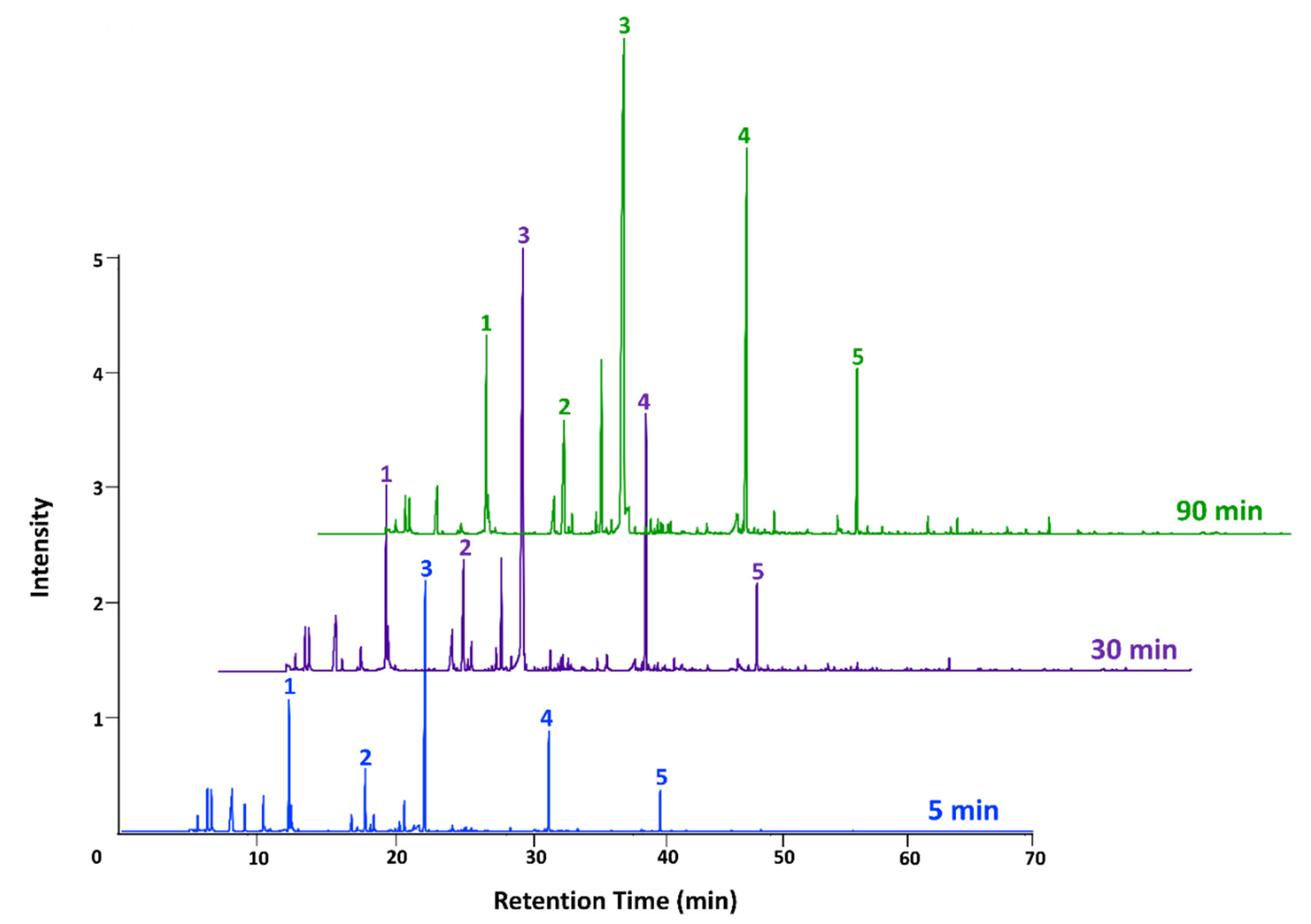
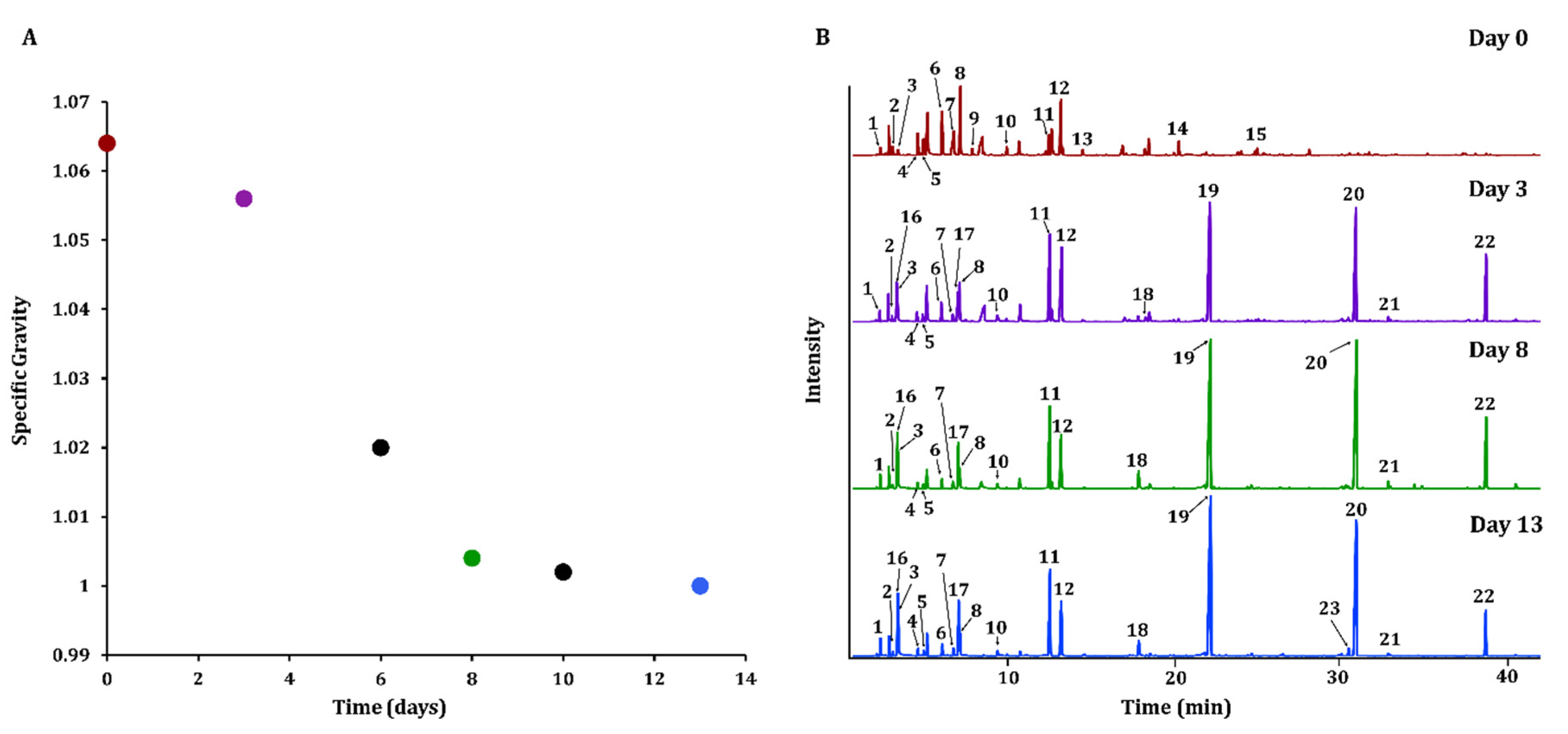
3.1. Cider VOC Development throughout the Fermentation Process
3.2. Cider VOC Profiles Resulting from Different Yeast Strains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diamond, D.H. Origins of Pioneer Apple Orchards in the American West: Random Seeding versus Artisan Horticulture. Agric. Hist. 2010, 84, 423–450. [Google Scholar] [CrossRef]
- Singh, S. Cider Market by Product (Apple Flavored, Fruit Flavored, and Perry), Distribution Channel (On-trade and Off-trade), and Packaging (Draught, Cans, Glass Bottles, Plastic Bottles, and Others)—Global Opportunity Analysis and Industry Forecast, 2017–2023. Available online: https://www.alliedmarketresearch.com/cider-market (accessed on 20 January 2020).
- Lofholm, N. An apple revival near Four Corners is restoring hundreds of historic fruits and the local ag economy. Available online: https://coloradosun.com/2019/11/28/colorado-heritage-apples-orchard-restoration-hard-cider/ (accessed on 28 November 2019).
- Qin, Z.H.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Symoneaux, R.; Guichard, H.; Le Quere, J.M.; Baron, A.; Chollet, S. Could cider aroma modify cider mouthfeel properties? Food Qual. Prefer. 2015, 45, 11–17. [Google Scholar] [CrossRef]
- Rosend, J.; Kuldjarv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar]
- Rebiere, L.; Clark, A.C.; Schmidtke, L.M.; Prenzler, P.D.; Scollary, G.R. A robust method for quantification of volatile compounds within and between vintages using headspace-solid-phase micro-extraction coupled with GC-MS—Application on Semillon wines. Anal. Chim. Acta 2010, 660, 149–157. [Google Scholar] [CrossRef]
- da Silva, G.C.; da Silva, A.A.S.; da Silva, L.S.N.; Godoy, R.; Nogueira, L.C.; Quiterio, S.L.; Raices, R.S.L. Method development by GC-ECD and HS-SPME-GC-MS for beer volatile analysis. Food Chem. 2015, 167, 71–77. [Google Scholar] [CrossRef]
- Peng, B.Z.; Li, F.L.; Cui, L.; Guo, Y.D. Effects of Fermentation Temperature on Key Aroma Compounds and Sensory Properties of Apple Wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, G.; Roman, T.; Carlin, S.; Malacarne, M.; Nardin, T.; Bertoldi, D.; Larcher, R. Characterisation of single-variety still ciders produced with dessert apples in the Italian Alps. J. Inst. Brew. 2018, 124, 457–466. [Google Scholar] [CrossRef]
- Pello-Palma, J.; Mangas-Alonso, J.J.; de la Fuente, E.D.; Gonzalez-Alvarez, J.; Diez, J.; Alvarez, M.D.G.; Abrodo, P.A. Characterization of Volatile Compounds in New Cider Apple Genotypes Using Multivariate Analysis. Food Anal. Methods 2016, 9, 3492–3500. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Villiere, A.; Arvisenet, G.; Bauduin, R.; Le Quere, J.M.; Serot, T. Influence of cider-making process parameters on the odourant volatile composition of hard ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Goodrich, K.M.; Neilson, A.P.; Hurley, E.K.; Peck, G.M.; Stewart, A.C. Characterization of the Polyphenol Composition of 20 Cultivars of Cider, Processing, and Dessert Apples (Malus x domestica Borkh.) Grown in Virginia. J. Agric. Food Chem. 2014, 62, 10181–10191. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.L.; Peck, G.M.; Ma, S.H.; Neilson, A.P.; Stewart, A.C. Management of Apple Maturity and Postharvest Storage Conditions to Increase Polyphenols in Cider. Hortscience 2019, 54, 143. [Google Scholar] [CrossRef]
- Way, M.L.; Jones, J.E.; Swarts, N.D.; Dambergs, R.G. Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods. Beverages 2019, 5, 53. [Google Scholar] [CrossRef]
- Staples, M.; Poechlinger, G.; Gossinger, M. Impact of different yeasts on organoleptic parameters of apple wine. Mitt. Klosterneubg. 2016, 66, 245–254. [Google Scholar]
- dos Santos, C.M.E.; Alberti, A.; Pietrowski, G.D.M.; Zielinski, A.A.F.; Wosiacki, G.; Nogueira, A.; Jorge, R.M.M. Supplementation of amino acids in apple must for the standardization of volatile compounds in ciders. J. Inst. Brew. 2016, 122, 334–341. [Google Scholar] [CrossRef]
- dos Santos, C.M.E.; Pietrowski, G.D.M.; Braga, C.M.; Rossi, M.J.; Ninow, J.; dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Zea, L.; Moyano, L.; Medina, M. Aroma active compounds during the drying of grapes cv. Pedro Ximenez destined to the production of sweet Sherry wine. Eur. Food Res. Technol. 2010, 230, 429–435. [Google Scholar] [CrossRef]
- Alberti, A.; Vieira, R.G.; Drilleau, J.F.; Wosiacki, G.; Nogueira, A. Apple Wine Processing with Different Nitrogen Contents. Braz. Arch. Biol. Technol. 2011, 54, 551–558. [Google Scholar] [CrossRef]
- Braga, C.M.; Zielinski, A.A.F.; da Silva, K.M.; de Souza, F.K.F.; Pietrowski, G.D.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef]
- Jolicoeur, C. The New Cider Maker’s Handbook: A Comprehensive Guide for Craft Producers; Watson, B., Ed.; Chelsea Green Publishing: Hartford, VT, USA, 2013. [Google Scholar]
- Vas, G.; Vekey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.M.; Rabin, S.I.; Prada, P.A.; Furton, K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005, 31, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Schmutzer, G.R.; Magdas, A.D.; David, L.I.; Moldovan, Z. Determination of the Volatile Components of Apple Juice Using Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry. Anal. Lett. 2014, 47, 1683–1696. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. Lwt-Food Sci. Technol. 2016, 73, 567–575. [Google Scholar] [CrossRef]
- Jordan, M.J.; Margaria, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef]
- Sansone-Land, A.; Takeoka, G.R.; Shoemaker, C.F. Volatile constituents of commercial imported and domestic black-ripe table olives (Olea europaea). Food Chem. 2014, 149, 285–295. [Google Scholar] [CrossRef]
- Pino, J.A.; Tolle, S.; Gok, R.; Winterhalter, P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012, 132, 1436–1441. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortin, N.; Escudero, A.; Lopez, R.; Cacho, J. Chemical characterization of the aroma of Grenache rose wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melus, M.A.; Ortin, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, J.; Garcia-Martinez, T.; Millan, M.C.; Mauricio, J.C.; Moreno, J. Proteins involved in wine aroma compounds metabolism by a Saccharomyces cerevisiae flor-velum yeast strain grown in two conditions. Food Microbiol. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Luo, J.; Niu, Y.W.; Wang, P.P.; Wang, R.L.; Sun, X.X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019, 116, 211–222. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Vedova, A.D.; Stella, L.; Flamini, R. Changes in Chemical Composition of a Red Wine Aged in Acacia, Cherry, Chestnut, Mulberry, and Oak Wood Barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef]
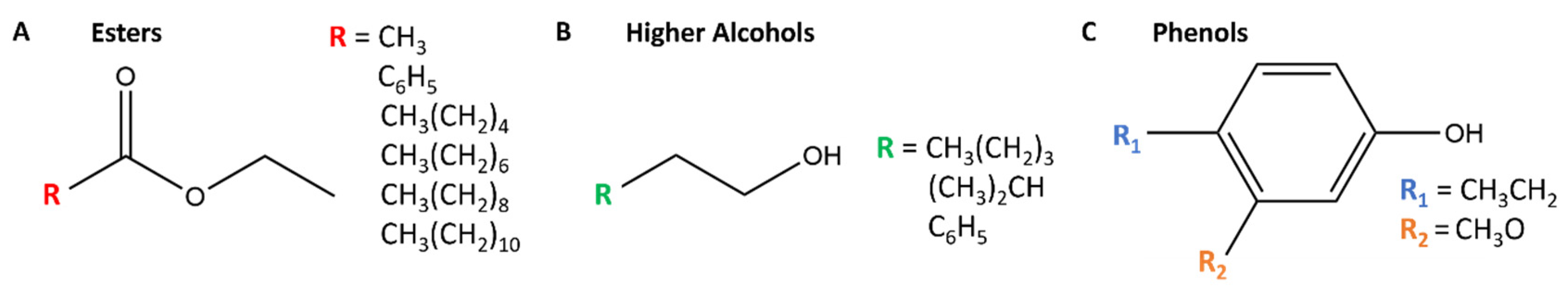
| Edinburgh Yeast | Relative Peak Area | Retention Time (min) | NIST (%) a | Odor Descriptor | Perception Threshold (μg L−1) |
|---|---|---|---|---|---|
| Ethyl octanoate | 1.00 | 22.1 | 92.3 | Fruity, candy, pineapple b,c | 580 b |
| Ethyl decanoate | 0.85 | 31.0 | 89.1 | Fruity, grape b | 200 b |
| 3-methyl-1-butanol | 0.18 | 3.1 | 69.0 | Alcohol, nail polish, whiskey b | 30,000 b |
| Ethyl hexanoate | 0.16 | 12.3 | 85.5 | fruity, strawberry, green apple b | 14 b |
| Ethyl benzoate | 0.14 | 20.6 | 77.3 | Camomile, flower, celery, fruit d | 60 e |
| Ethyl dodecanoate | 0.11 | 38.9 | 82.3 | Candy, floral, waxy, soap b | 1500 b |
| 4-ethyl-2-methoxyphenol | 0.11 | 25.6 | 81.1 | Phenolic, smoked f | 6.9 f |
| 2-phenylethyl alcohol | 0.11 | 17.8 | 84.3 | Rose, honey g | 390 g |
| 1-hexanol | 0.09 | 6.5 | 69.0 | Herbaceous, fatty, floral b | 110 b |
| 4-ethylphenol | 0.08 | 20.7 | 85.3 | Phenolic, leather g,h | 21 g |
| Decanoic acid | 0.07 | 30.3 | 97.3 | Rancid fat, animal b | 1000 b |
| Belgian II Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| Ethyl octanoate | 1.00 | 22.1 | 89.1 | Fruity, candy, pineapple b,c | 580 b |
| Ethyl decanoate | 0.86 | 31.0 | 88.9 | Fruity, grape b | 200 b |
| 2-phenylethyl alcohol | 0.23 | 17.8 | 83.5 | Rose, honey g | 390 g |
| Ethyl hexanoate | 0.19 | 12.3 | 88.6 | Fruity, strawberry, green apple b | 14 b |
| 3-methyl-1-butanol | 0.14 | 3.1 | 41.7 | Alcohol, nail polish, whiskey b | 30,000 b |
| Ethyl benzoate | 0.12 | 20.6 | 67.0 | Camomile, flower, celery, fruit d | 60 e |
| 4-ethyl-2-methoxyphenol | 0.09 | 25.6 | 80.4 | Phenolic, smoked f | 6.9 f |
| 1-hexanol | 0.07 | 6.5 | 66.1 | Herbaceous, fatty, floral b | 110 b |
| Decanoic acid | 0.07 | 30.2 | 98.0 | Rancid fat, animal b | 1000 b |
| Ethyl dodecanoate | 0.06 | 38.9 | 85.9 | Candy, floral, waxy, soap b | 1500 b |
| French Saison Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| Ethyl octanoate | 1.00 | 22.1 | 93.3 | Fruity, candy, pineapple b,c | 580 b |
| Ethyl decanoate | 0.50 | 31.0 | 88.6 | Fruity, grape b | 200 b |
| 3-methyl-1-butanol | 0.15 | 3.1 | 42.5 | Alcohol, nail polish, whiskey b | 30,000 b |
| Ethyl hexanoate | 0.15 | 12.3 | 91.9 | Fruity, strawberry, green apple b | 14 b |
| 2-phenylethyl alcohol | 0.12 | 17.8 | 86.3 | Rose, honey g | 390 g |
| Ethyl dodecanoate | 0.09 | 38.9 | 83.3 | Candy, floral, waxy, soap b | 1500 b |
| Ethyl benzoate | 0.09 | 20.5 | 71.4 | Camomile, flower, celery, fruit d | 60 e |
| Ethyl-9-decenoate | 0.07 | 30.5 | 99.0 | Roses b | 100 b |
| Abbey Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| Ethyl octanoate | 1.00 | 22.1 | 92.4 | Fruity, candy, pineapple b,c | 580 b |
| Ethyl decanoate | 0.65 | 31.0 | 90.3 | Fruity, grape b | 200 b |
| 3-methyl-1-butanol | 0.21 | 3.1 | 44.8 | Alcohol, nail polish, whiskey b | 30,000 b |
| Ethyl hexanoate | 0.18 | 12.3 | 92.8 | Fruity, strawberry, green apple b | 14 b |
| Ethyl benzoate | 0.18 | 20.6 | 95.0 | Camomile, flower, celery, fruit d | 60 e |
| 2-phenylethyl alcohol | 0.16 | 17.8 | 87.5 | Rose, honey g | 390 g |
| 4-ethyl-2-methoxyphenol | 0.10 | 25.6 | 81.4 | Phenolic, smoked f | 6.9 f |
| Decanoic acid | 0.07 | 30.3 | 98.0 | Rancid fat, animal g | 1000 g |
| 1-hexanol | 0.06 | 6.5 | 66.7 | Herbaceous, fatty, floral b | 110 b |
| Edinburgh Yeast | Relative Peak Area | Retention Time (min) | NIST (%) a | Odor Descriptor | Perception Threshold (μg L−1) |
|---|---|---|---|---|---|
| Ethyl nonanoate | 0.012 | 26.5 | 90.7 | Fatty, oily, fruity, nutty b | − |
| Ethyl acetate | 0.010 | 1.9 | 86.0 | Solvent, fruity, balsamic b | 12,000 b |
| 3-methylbutyl octanoate | 0.009 | 33.0 | 70.7 | Fruity, flore b | 125 d |
| Phenethyl acetate | 0.008 | 24.6 | 85.3 | Rose, honey e | 3,317,000 f |
| Ethyl hexadecanoate | 0.007 | 52.7 | 98.7 | Waxy, greasy g | 1500 g |
| 3-methylbutyl n-decanoate | 0.006 | 40.7 | 96.3 | Fruity h | − |
| Belgian II Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| Ethyl acetate | 0.031 | 2.0 | 95.7 | Solvent, fruity, balsamic b | 12,000 b |
| Dodecanoic acid | 0.010 | 37.8 | 97.0 | Fatty c | − |
| Ethyl tetradecanoate | 0.007 | 46.1 | 96.7 | Lily g | − |
| Ethyl hexadecanoate | 0.007 | 52.7 | 99.0 | Waxy, greasy g | 1500 g |
| French Saison Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| 1-hexanol | 0.042 | 6.5 | 67.6 | Herbaceous, fatty, floral b | 110 b |
| 1-octanol | 0.019 | 15.8 | 49.5 | Citrus, green i | 6.9 i |
| Decanoic acid | 0.027 | 30.2 | 98.0 | Rancid fat, animal g | 1000 g |
| Methyl salicylate | 0.018 | 21.7 | 96.0 | Peppermint g | 0.1 g |
| 4-ethyl-2-methoxyphenol | 0.010 | 25.6 | 92.0 | Phenolic, smoked j | 3 j |
| 4-ethylphenol | 0.008 | 20.8 | 94.7 | Phenolic i | 21 h |
| Ethyl nonanoate | 0.006 | 26.5 | 87.7 | Fatty, oily, fruity, nutty b | − |
| Ethyl acetate | 0.006 | 2.0 | 92.4 | solvent, fruity, balsamic g | 12,000 g |
| 2-phenylethyl acetate | 0.005 | 24.6 | 80.0 | Rose, honey j | 108 j |
| Abbey Yeast | RPA | RT (min) | NIST (%) | Odor Descriptor | PT (μg L−1) |
| Ethyl acetate | 0.034 | 2.0 | 92.5 | Solvent, fruity, balsamic b | 12,000 b |
| Ethyl dodecanoate | 0.031 | 38.8 | 66.7 | Candy, floral, waxy, soap b | 1500 b |
| 3-methylbutyl acetate | 0.030 | 6.8 | 87.9 | Banana i | 30 i |
| Ethyl-9-decenoate | 0.020 | 30.5 | 98.7 | Roses g | 100 g |
| Hexyl acetate | 0.008 | 13.0 | 87.3 | Banana, apple, pear j | 0.2 k |
| 1-octanol | 0.007 | 15.8 | 90.0 | Citrus, green i | 6.9 i |
| 2-phenylethyl acetate | 0.007 | 24.7 | 74.7 | Roses, honey j | 108 j |
| Ethyl 2-methylbutanoate | 0.006 | 5.8 | 95.7 | Strawberry, candy, fruit g | 18 g |
| Diethyl butanedioate | 0.004 | 21.3 | 78.7 | Wine, caramel, fruity g | 200,000 g |
| Analyte (Odor) | Edinburgh Yeast | Belgian II Yeast | French Saison Yeast | Abbey Yeast | Perception Threshold (mg·L−1) |
|---|---|---|---|---|---|
| Ethyl acetate (solvent, fruity, balsamic) b | 1.89 ± 0.42 a | 33.00 ± 7.26 | 2.52 ± 0.55 | 41.28 ± 9.08 | 12 b |
| Ethyl octanoate (fruity, candy, pineapple) b | 0.13 ± 0.03 | 0.16 ± 0.04 | 0.29 ± 0.08 | 0.20 ± 0.05 | 0.014 b |
| 2-Phenylethyl alcohol (Rose, honey) c | 6.85 ± 1.03 | 11.95 ± 1.79 | 9.59 ± 1.44 | 8.98 ± 1.35 | 0.390 c |
| 3-methyl-1-butanol (Alcohol, nail polish, whiskey) b | 13.40 ± 3.58 | 32.36 ± 8.64 | 44.47 ± 11.87 | 38.51 ± 10.28 | 30 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingman, M.T.; Stellick, C.E.; Pelkey, J.P.; Scott, J.M.; Cole, C.A. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages 2020, 6, 40. https://doi.org/10.3390/beverages6020040
Bingman MT, Stellick CE, Pelkey JP, Scott JM, Cole CA. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages. 2020; 6(2):40. https://doi.org/10.3390/beverages6020040
Chicago/Turabian StyleBingman, Matthew T., Claire E. Stellick, Jordanne P. Pelkey, Jared M. Scott, and Callie A. Cole. 2020. "Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis" Beverages 6, no. 2: 40. https://doi.org/10.3390/beverages6020040
APA StyleBingman, M. T., Stellick, C. E., Pelkey, J. P., Scott, J. M., & Cole, C. A. (2020). Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages, 6(2), 40. https://doi.org/10.3390/beverages6020040