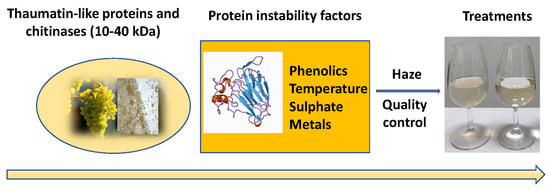
White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview
Abstract
1. Introduction
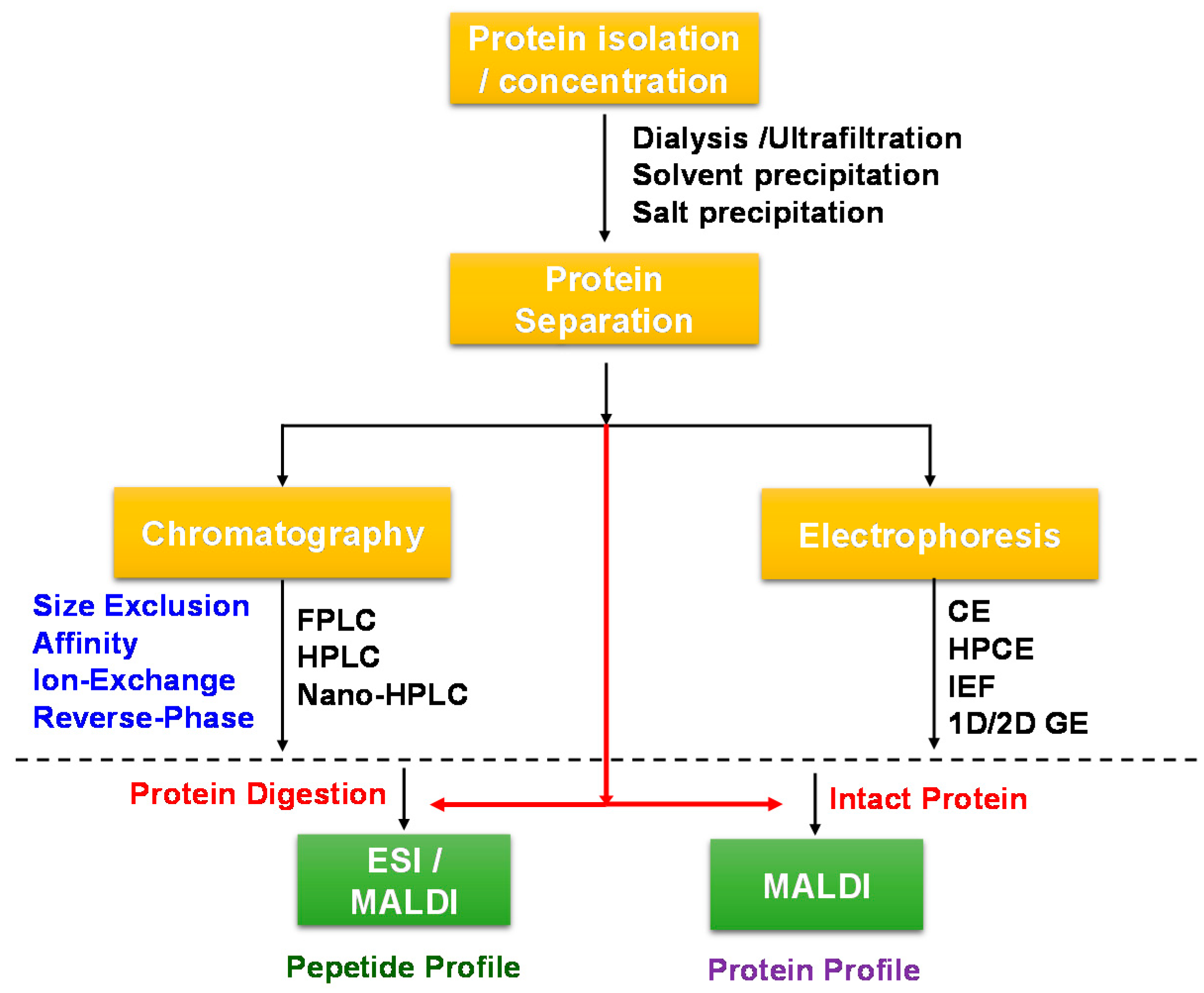
2. Profiling and Characterisation of Wine Proteins
3. Proteins Responsible for Wine Haze
4. Factors That Affect Wine Protein Stabilisation
5. Protein Stability Tests for Wine Quality Control—Advantages and Disadvantages
5.1. Heat Test
5.2. Trichloroacetic Acid Test
5.3. Tannin Test
5.4. Bentotest
5.5. Ethanol Test
5.6. Spectroscopic Methods
6. Wine Protein Stabilisation—Strategies and Treatments
6.1. Winemaking Practices to Prevent or Reduce Wine Protein Instability
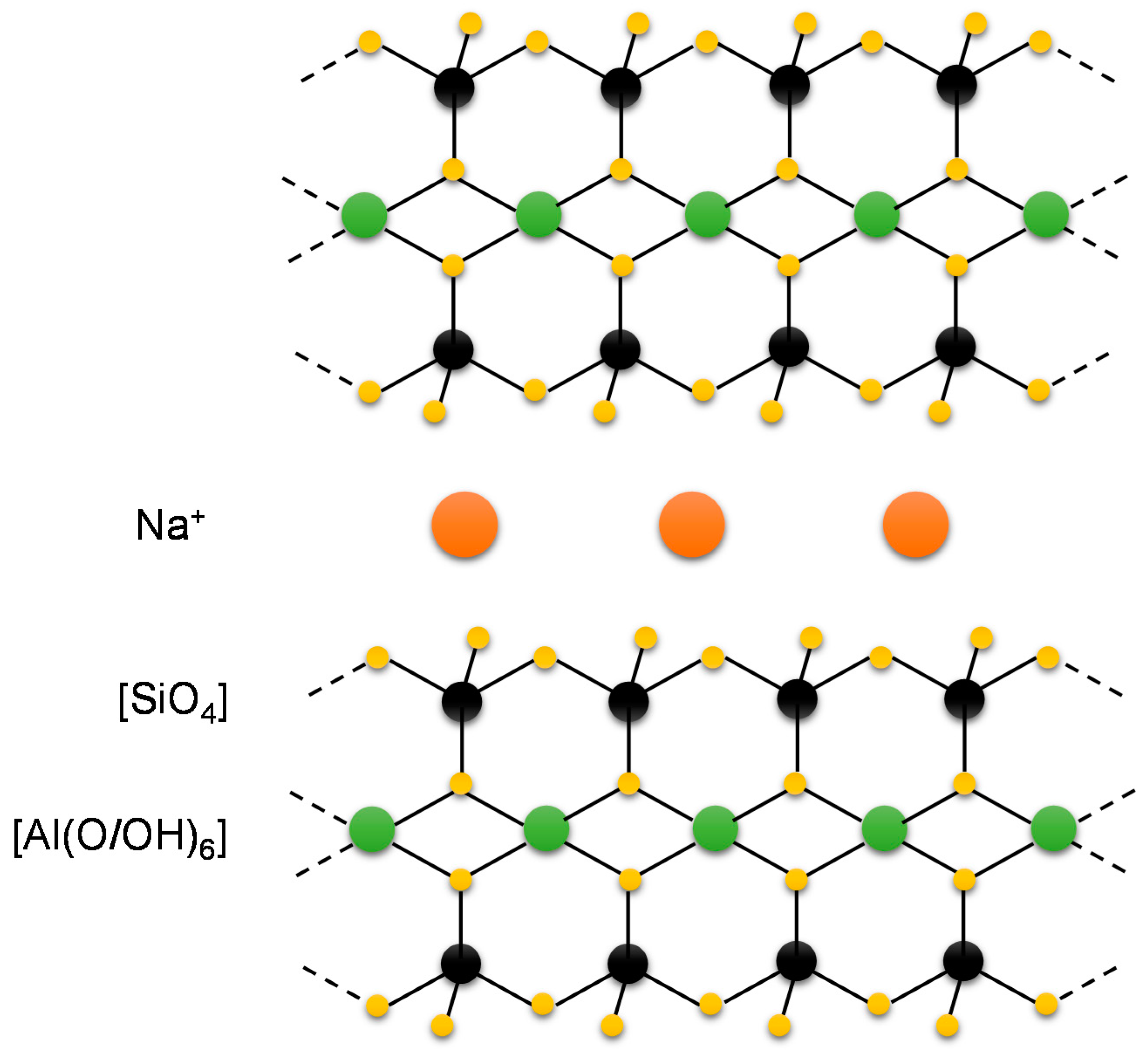
6.2. Bentonite Fining
6.3. Other Adsorbents
6.4. Mannoproteins
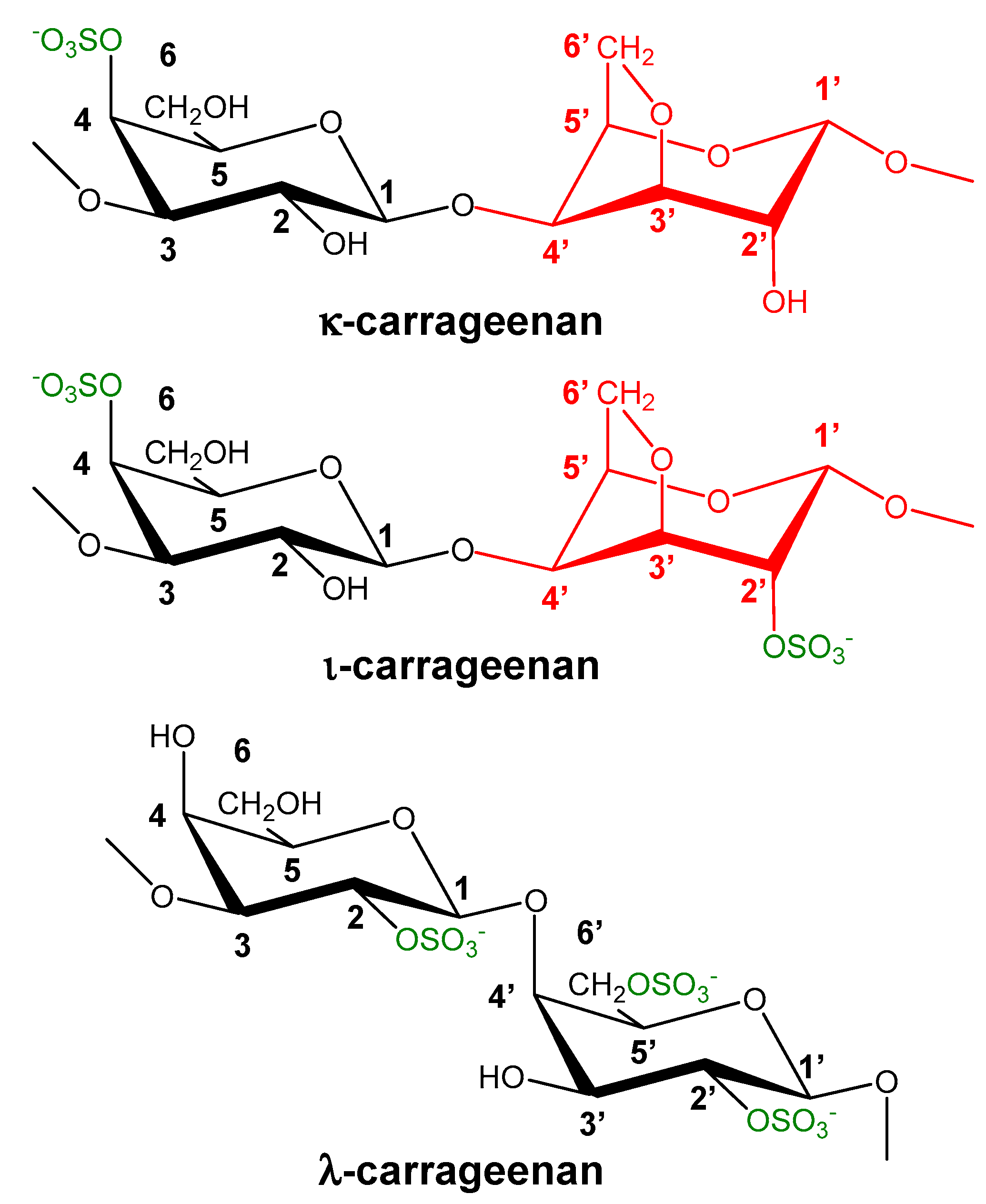
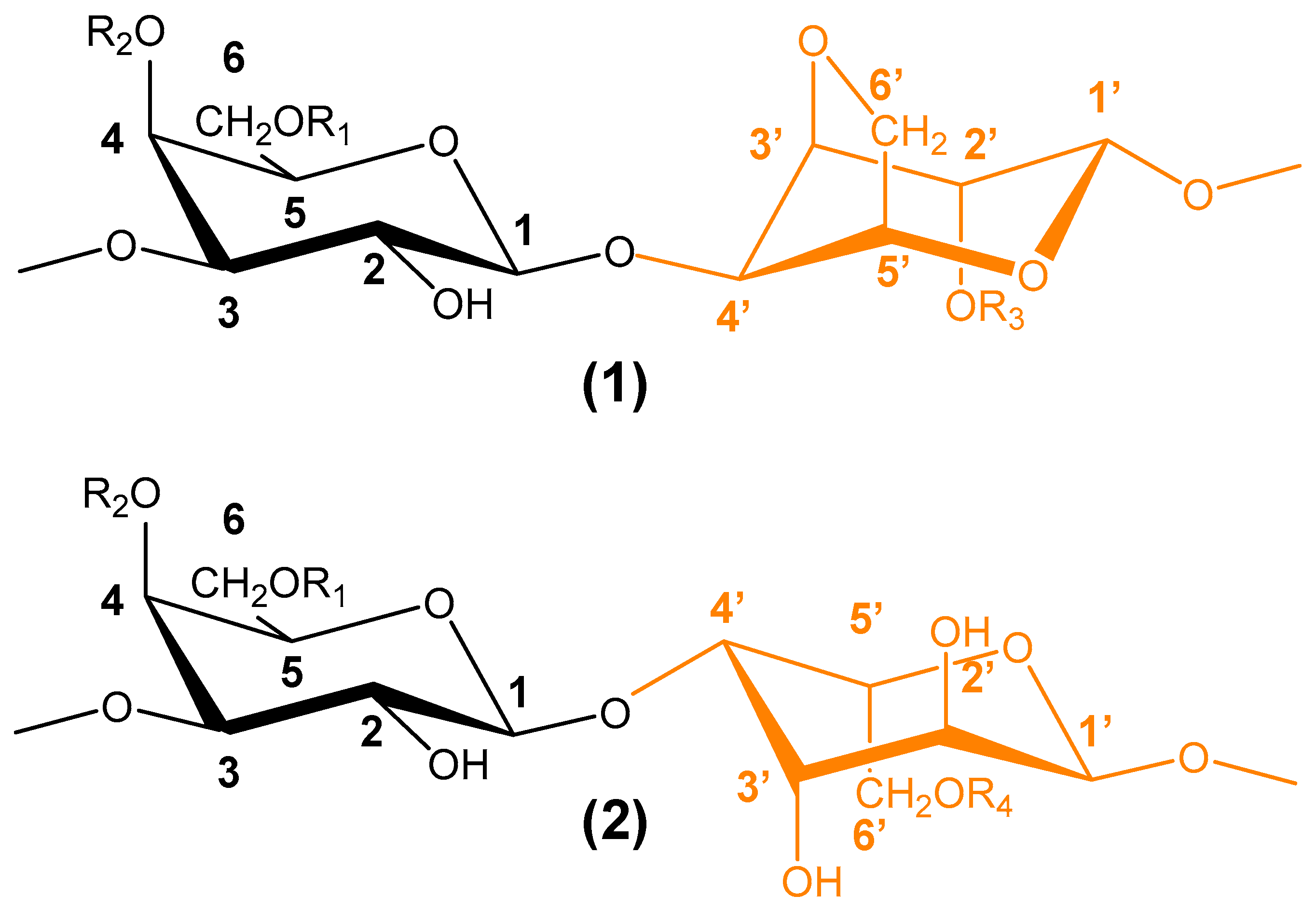
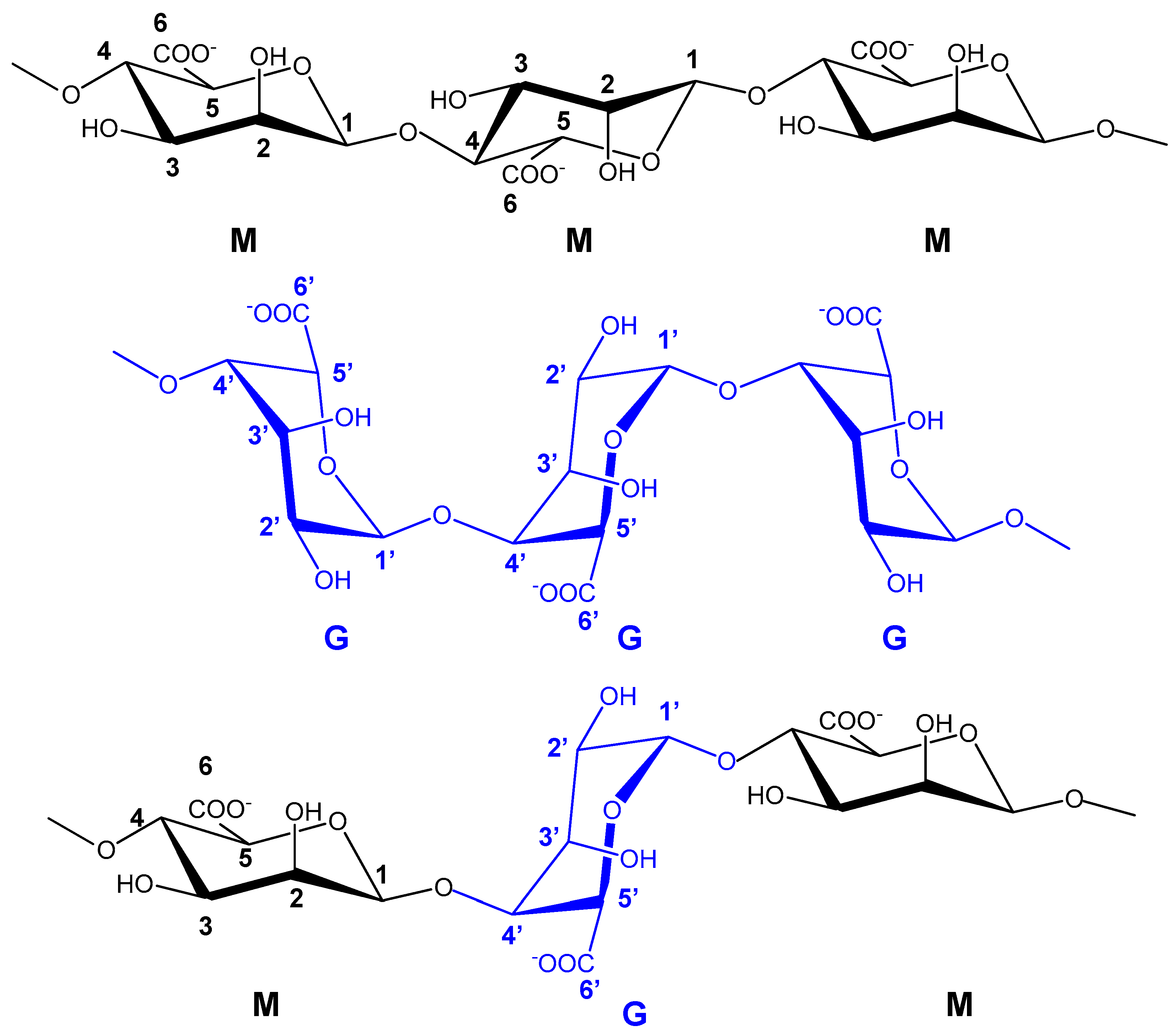
6.5. Polysaccharides from Seaweeds
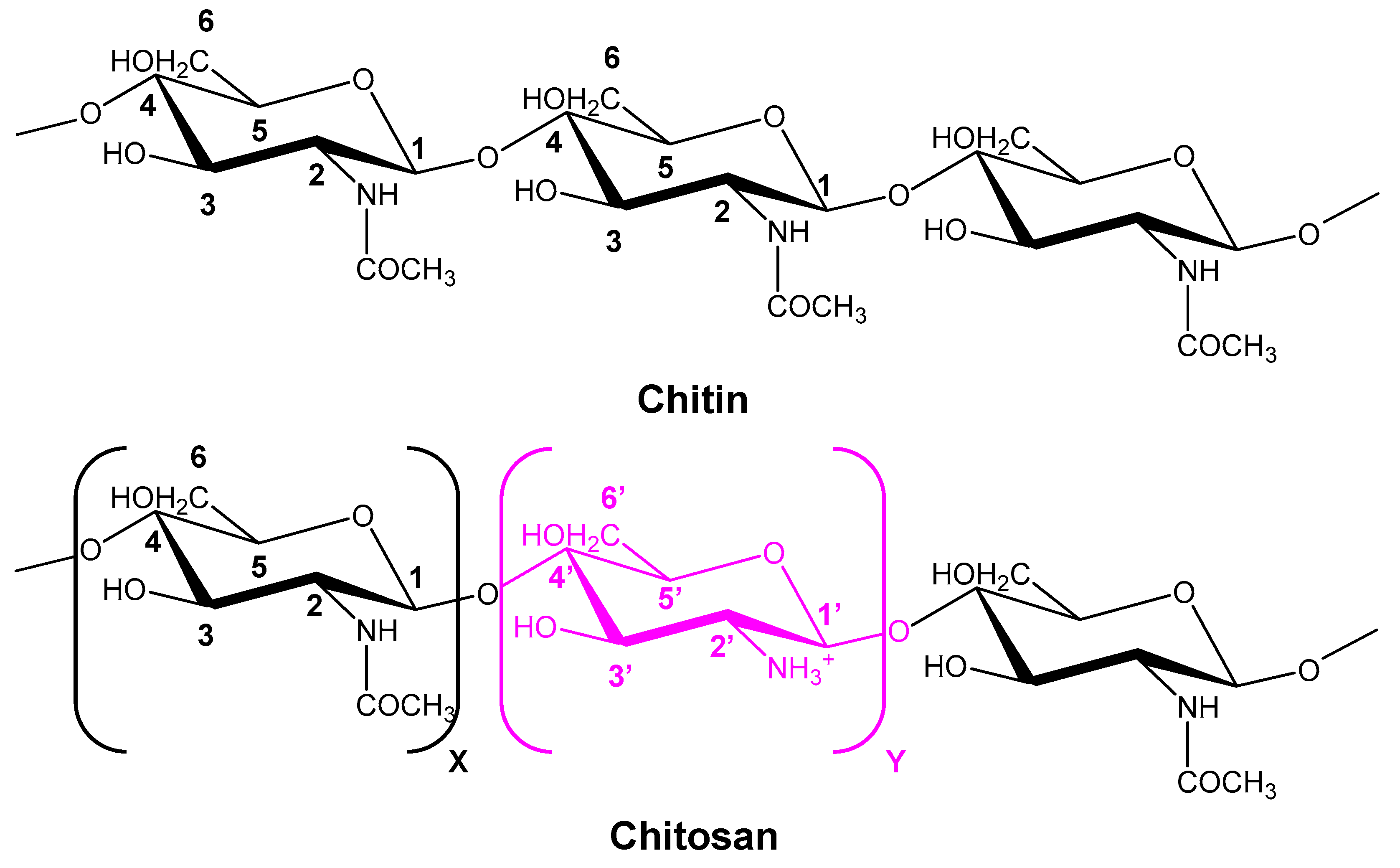
6.6. Chitin/Chitosan
6.7. Ultrafiltration
6.8. Proteases
6.9. Acrylic Acid-Coated Magnetic Nanoparticles
7. Conclusions
Funding
Conflicts of Interest
References
- Sauvage, F.-X.; Bach, B.; Moutonet, M.; Vernhet, A. Proteins in white wines: Thermo-sensivity and differential adsorption by bentonite. Food Chem. 2010, 118, 26–34. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2002, 12, 230–239. [Google Scholar] [CrossRef]
- Dambrouck, T.; Marchal, R.; Marchal-Delahaut, L.; Parmentier, M.; Maujean, A.; Jeandet, P. Immunodetection of proteins from grapes and yeast in a white wine. J. Agric. Food Chem. 2003, 51, 2727–2732. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Høj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Marangon, M.; Stockdale, V.J.; Munro, P.; Trethewey, T.; Schulkin, A.; Holt, H.E.; Smith, P.A. Addition of carrageenan at different stages of winemaking for white wine protein stabilization. J. Agric. Food Chem. 2013, 61, 6516–6524. [Google Scholar] [CrossRef]
- Waters, E.J.; Wallace, W.; Williams, P.J. Identification of heat-unstable wine proteins and their resistance to peptidases. J. Agric. Food Chem. 1992, 40, 1514–1519. [Google Scholar] [CrossRef]
- Høj, P.B.; Tattersall, D.B.; Adams, K.; Pocock, K.F.; Hayasaka, Y.; van Heeswijck, R.; Waters, E. The ‘haze proteins’ of wine—A summary of properties, factors affecting their accumulation in grapes, and the amount of bentonite required for their removal from wine. In Proceedings of the ASEV 50th Anniversary Meeting, Seattle, WA, USA, 19–23 June 2000; American Society of Enology and Viticulture: Davis, CA, USA, 2000; pp. 149–154. [Google Scholar]
- Boulton, R. The nature of wine proteins. In Proceedings of the Sixth Annual Wine Industry Technology Seminar of the Wine Institute, San Francisco, CA, USA, 1980; pp. 46–58. [Google Scholar]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The complexity of protein haze formation in wines. Food Chem. 2009, 112, 169–177. [Google Scholar] [CrossRef]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Protein haze formation in wines revisited. The stabilizing effect of organic acids. Food Chem. 2010, 122, 1067–1075. [Google Scholar] [CrossRef]
- Dufrechou, M.; Poncet-Legrand, C.; Sauvage, F.X.; Vernhet, A. Stability of white wine proteins: Combined effect of pH, ionic strength, and temperature on their aggregation. J. Agric. Food Chem. 2012, 60, 1308–1319. [Google Scholar] [CrossRef]
- Pocock, K.F.; Alexander, G.M.; Hayasaka, Y.; Jones, P.R.; Waters, E.J. Sulfate—A candidate for the missing essential factor that is required for the formation of protein haze in white wine. J. Agric. Food Chem. 2007, 55, 1799–1807. [Google Scholar] [CrossRef]
- Marangon, M.; Sauvage, F.X.; Waters, E.J.; Vernhet, A. Effects of ionic strength and sulfate upon thermal aggregation of grape chitinases and thaumatin-like proteins in a model system. J. Agric. Food Chem. 2011, 59, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Pocock, K.F.; Waters, E.J.; Francis, I.L.; Williams, P.J. Taste properties of grape (Vitis vinifera) pathogenesis-related proteins isolated from wine. J. Agric. Food Chem. 1997, 45, 4639–4643. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Gordon, T.A.; Villalobos-Carvajal, R.; Moreno-Osorio, L.; Salazar, F.N.; Perez-Won, M.; Acuña, S. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine. Food Chem. 2014, 155, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.P.; Davies, C. Molecular biology of grape berry ripening. Aust. J. Grape Wine Res. 2000, 6, 175–188. [Google Scholar] [CrossRef]
- Waters, E.J.; Wallace, W.; Tate, M.E.; Williams, P.J. Isolation and partial characterization of a natural haze protective factor from white wine. J. Agric. Food Chem. 1993, 41, 724–730. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-da-Silva, J.M.; Laureano, O. Interactions between protein fining agents and proanthocyanidins in white wine. Food Chem. 2008, 106, 536–544. [Google Scholar] [CrossRef]
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the structural features of commercial mannoproteins in white wine protein stabilization and chemical and sensory properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; Faveri, D.M. Comparing the impact of bentonite addiction for both must clarification and wine fining on the chemical profile of wine from Chambave Muscat grapes. Int. J. Food Sci. Technol. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Waters, E.J.; Shirley, N.J.; Williams, P.J. Nuisance proteins of wine are grape pathogenesis related proteins. J. Agric. Food Chem. 1996, 44, 3–5. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar]
- Hsu, J.-C.; Heatherbell, D.A.; Flores, J.H.; Watson, B.T. Heat-unstable proteins in grape juice and wine. II. Characterization and removal by ultrafiltration. Am. J. Enol. Vitic. 1987, 38, 17–22. [Google Scholar]
- Flores, J.H.; Heatherbell, D.A.; McDaniel, M.R. Ultrafiltration of wine: Effect of ultrafiltration on white Riesling and Gewürztraminer wine composition and stability. Am. J. Enol. Vitic. 1990, 41, 207–214. [Google Scholar]
- Feuillat, M.; Ferrari, G. Hydrolyse enzymatique des proteins du raisin en vinification. Comptes Rendus des Séances de l’Academie d’Agriculture de France 1982, 68, 1070–1075. [Google Scholar]
- Dizy, M.; Bisson, L.F. White wine protein analysis by capillary zone electrophoresis. Am. J. Enol. Vitic. 1999, 50, 120–127. [Google Scholar]
- Francis, I.L.; Sefton, M.A.; Williams, P.J. The sensory effects of pre- or post-fermentation thermal processing on Chardonnay and Semillon wines. Am. J. Enol. Vitic. 1994, 45, 243–251. [Google Scholar]
- Pocock, K.F.; Høj, P.B.; Adams, K.S.; Kwiatkowski, M.J.; Waters, E.J. Combined heat and proteolytic enzyme treatment of white wines reduces haze forming protein content without detrimental effect. Aust. J. Grape Wine Res. 2003, 9, 56–63. [Google Scholar] [CrossRef]
- Sarmento, M.R.; Oliveira, J.C.; Boulton, R.B. Selection of low swelling materials for protein adsorption from white wines. Int. J. Food Sci. Technol. 2000, 35, 41–47. [Google Scholar] [CrossRef]
- Pashova, V.; Guell, C.; López, F. White wine continuous protein stabilization by Packed Column. J. Agric. Food Chem. 2004, 52, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.N.; Achaerandio, I.; Labbé, M.A.; Güell, C.; López, F. Comparative study of protein stabilisation in white wine using zirconia and bentonite: Physiochemical and wine sensory analysis. J. Agric. Food Chem. 2006, 54, 9955–9958. [Google Scholar] [CrossRef]
- Marangon, M.; Lucchetta, M.; Waters, E.J. Protein stabilisation of white wines using zirconium dioxide enclosed in a metallic cage. Aust. J. Grape Wine Res. 2011, 17, 28–35. [Google Scholar] [CrossRef]
- Mercurio, M.; Mercurio, V.; Gennaro, B.; Gennaro, M.; Grifra, C.; Langella, A.; Morra, V. Natural zeolites and white wines from Campania region (Southern Italy): A new contribution for solving somo oenological problems. Period Miner. 2010, 79, 95–112. [Google Scholar]
- Mierczynska-Vasilev, A.; Wahono, S.K.; Smith, P.-A.; Bindon, K.; Vasilev, K. Using Zeolites To Protein Stabilize White Wines. ACS Sustain. Chem. Eng. 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Vincenzi, S.; Mosconi, S.; Zoccatelli, G.; Pellegrina, C.D.; Veneri, G.; Chignola, R.; Peruffo, A.; Curioni, A.; Rizzi, C. Development of a new procedure for protein recovery and quantification in wine. Am. J. Enol. Vitic. 2005, 56, 182–187. [Google Scholar]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Milena, L. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef]
- Ratnayake, S.; Stockdale, V.; Grafton, S.; Munro, P.; Robinson, A.L.; Pearson, W.; McRae, J.M.; Bacic, A. Carrageenans as heat stabilisers of white wine. Aust. J. Grape Wine Res. 2019, 25, 439–450. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A recombinant Saccharomyces cerevisiae strain overproducing mannoproteins stabilizes wine against proteins haze. Appl. Environ. Microb. 2008, 77, 5533–5540. [Google Scholar] [CrossRef]
- Zoecklein, B. Bentonite Fining of Juice and Wine; Virginia Cooperative Extension Service: Blacksburg, VA, USA, 1988; Publication 463-014. [Google Scholar]
- Santoro, M. Fractionation and characterization of must and wine proteins. Am. J. Enol. Vitic. 1995, 46, 250–254. [Google Scholar]
- Anelli, G. The proteins of must. Am. J. Enol. Vitic. 1977, 28, 200–203. [Google Scholar]
- Yokotsuka, K.; Yoshii, M.; Aihara, T.; Kushida, T. Isolation and characterization of soluble glycoproteins in red wine. J. Ferment. Technol. 1977, 55, 510–515. [Google Scholar]
- Heatherbell, D.; Ngaba, P.; Fombin, B.; Watson, B., Jr.; Garcia, Z.; Flores, J.; Hsu, J. Recent developments in the application of ultrafiltration and protease enzymes to grape juice and wine processing. In Proceedings of the International Symposium on Cool Climate Viticulture and Enology, Eugene, OR, USA, 25–26 June 1984; Heatherbell, D.A., Lombard, P.B., Bodyfelt, F.W., Price, S.F., Eds.; Oregon State University: Eugene, OR, USA, 1985; pp. 418–445. [Google Scholar]
- Duncan, B. Varietal differences in white grape protein: Implications for bentonite fining. Aust. N. Z. Wine Ind. J. 1992, 7, 189–193. [Google Scholar]
- Dorrestein, E.; Ferreira, R.B.; Laureano, O.; Teixeira, A.R. Electrophorectic and FPLC analysis of soluble proteins in four Portuguese wines. Am. J. Enol. Vitic. 1995, 46, 235–242. [Google Scholar]
- Sarmento, M.R.; Oliveiraz, J.C.; Slatner, M.; Boulton, R.B. Effect of Ion-Exchange Adsorption on the Protein Profiles of White Wines. Rev. Agaroquimica y Tecnol. Aliment. 2001, 7, 217–224. [Google Scholar]
- Murphey, J.M.; Spayd, J.R.; Powers, J.R. Effect of grape maturation on soluble protein characteristics of Gewürztraminer and white Riesling juice and wine. Am. J. Enol. Vitic. 1989, 40, 199–207. [Google Scholar]
- Ough, C.S.; Anelli, G. Zinfandel grape juice fractions and their amino acid makeup as affected by crop level. Am. J. Enol. Vitic. 1979, 30, 8–10. [Google Scholar]
- Zoecklein, B. Protein Stability Determination in Juice and Wine; Virginia Cooperative Extension Service: Blacksburg, VA, USA, 1991; Publication 463-015. [Google Scholar]
- Pocock, K.F.; Hayasaha, Y.; McCarthy, M.G.; Waters, E.J. Thaumatin-like proteins and chitinases, the haze-forming proteins of wine, accumulate during ripening of grape (Vitis vinifera) berries and drought stress does not affect the final levels per very maturity. J. Agric. Food Chem. 2000, 48, 1637–1643. [Google Scholar] [CrossRef]
- Mesquita, P.R.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Effect of wine composition on protein stability. Am. J. Enol. Vitic. 2001, 52, 324–330. [Google Scholar]
- Vincenzi, S.; Polesani, M.; Curioni, A. Removal of specific protein components by chitin enhaces protein stability in a white wine. Am. J. Enol. Vitic. 2005, 56, 246–254. [Google Scholar]
- Mierczynska-Vasilev, A.; Boyer, P.; Vasilev, K.; Smith, P.A. A novel technology for the rapid, selective, magnetic removal of pathogenesis-related proteins from wines. Food Chem. 2017, 232, 508–514. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monteiro, S.; Piçarra-Pereira, M.A.; Teixeira, A.R. Engineering grapevine for increased resistance to fungal pathogens without compromising wine stability. Trends Biotechnol. 2004, 22, 168–173. [Google Scholar] [CrossRef]
- Moio, L.; Addeo, F. Focalizzazione isoelettrica delle proteine clouding. Vigne Vini 1989, 4, 53–57. [Google Scholar]
- Luguera, C.; Moreno-Arribas, V.; Pueyo, E.; Polo, C. Capillary electrophoretic analyses of wine proteins. Modifications during the manufacture of sparkling wines. J. Agric. Food Chem. 1997, 45, 3766–3770. [Google Scholar] [CrossRef]
- Okuda, T.; Fukui, M.; Takayanagi, T.; Yokotsuka, K. Characterization of major stable proteins in Chardonnay wine. Food Sci. Technol. Res. 2006, 12, 131–136. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and wine proteins: Their fractionation by hydrophobic interaction chromatography and identification by chromatographic and proteomic analysis. J. Agric. Food Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Somers, T.C.; Ziemelis, G. Direct determination of wine proteins. Am. J. Enol. Vitic. 1973, 24, 47–50. [Google Scholar]
- Correia, I.; Polo, M.C.; Amigo, L.; Ramos, M. Séparation des protéines des moûts de raisin au moyen de techniques électrophoretiques. Connaissance de la Vigne et du Vin 1988, 22, 1–9. [Google Scholar] [CrossRef]
- Gonzalez-Lara, R.; Correa, I.; Polo, M.C.; Martinalvarez, P.J.; Ramos, M. Classification of variety musts by statistical-analysis of their electrophoretic protein pattern. Food Chem. 1988, 34, 103–110. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Cabello, F.; Polo, M.C.; Martin-Alvarez, P.J.; Pueyo, E. Assessment of the native electrophoretic analysis of total grape must proteins for the characterization of Vitis vinifera L. cultivars. J. Agric. Food Chem. 1999, 47, 114–120. [Google Scholar] [CrossRef]
- Moretti, R.H.; Berg, H.W. Variability among wines to protein clouding. Am. J. Enol. Vitic. 1965, 16, 69–78. [Google Scholar]
- Pueyo, E.; Dizy, M.; Polo, M.C. Varietal differentiation of must and wines by means of protein fraction. Am. J. Enol. Vitic. 1993, 44, 255–260. [Google Scholar]
- Bayly, F.C.; Berg, H.W. Grape and wine proteins of white wine varietals. Am. J. Enol. Vitic. 1967, 24, 18–32. [Google Scholar]
- Yokotsuka, K.; Ebihara, T.; Sato, T. Comparasion of soluble proteins in juice and wine from koshu grapes. J. Ferment. Bioeng. 1991, 71, 248–253. [Google Scholar] [CrossRef]
- Santoro, M.; Faccia, M.; La Notte, E. La frazione proteica di mosti e vini. Nota I: Caratterizzazione elettroforetica. La Rivista di Scienza dell’Alimentazione 1994, 23, 75–80. [Google Scholar]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Characterization of natural haze protein in Sauvignon white wine. Food Chem. 2009, 113, 28–35. [Google Scholar] [CrossRef]
- Waters, E.J.; Wallace, W.; Williams, P.J. Peptidases in winemaking. In Proceedings of the Seventh Australian Wine Industry Technical Conference, Adelaide, Australia, 13–17 August 1990; Williams, P.J., Davidson, D.M., Lee, T.H., Eds.; pp. 186–191. [Google Scholar]
- Gonzáles-Lara, R.; Polo, M.C.; Correa, I.; Ramos, M. Características de las proteínas de mostos de uvas de variedades cultivadas en Espanã. Rev. Agroquim. Technol. 1989, 29, 332–339. [Google Scholar]
- Dawes, H.; Heatherbell, D.; Fisher, B. Some recent investigations into characterization and removal of unstable proteins in wine. In Proceedings of the 9th International Oenological Symposium, Cascais, Portugal, 24–26 May 1990; pp. 347–369. [Google Scholar]
- Lamikanra, O. The proteins of Muscadine grapes. J. Food Sci. 1987, 52, 483–484. [Google Scholar] [CrossRef]
- Lamikanra, O.; Inyang, I.D. Temperature influence on Muscadine wine protein characteristics. Am. J. Enol. Vitic. 1988, 39, 113–116. [Google Scholar]
- Hsu, J.-C.; Heatherbell, D.A. Isolation and characterization of soluble proteins in grapes, grape juice, and wine. Am. J. Enol. Vitic. 1987, 38, 6–10. [Google Scholar]
- Hsu, J.-C.; Heatherbell, D.A. Heat-unstable proteins in wine. I. Characterization and removal by bentonite fining and heat treatment. Am. J. Enol. Vitic. 1987, 38, 11–16. [Google Scholar]
- Dawes, H.; Boyes, S.; Keene, J.; Heatherbell, D.A. Protein instability of wines: Influence of protein isoelectric point. Am. J. Enol. Vitic. 1994, 45, 319–326. [Google Scholar]
- Ledoux, V.; Dulau, L.; Dubourdieu, D. Interprétation de l’amélioration de la stabilité protéique des vins au cours de l’élevage sur lies. J. Int. Sci Vigne Vin 1992, 26, 239–251. [Google Scholar] [CrossRef]
- Chabreyrie, D.; Chauvet, S.; Guyon, F.; Salagoity, M.H.; Antinelli, J.F.; Medina, B. Characterization and quantification of grape variety by means of shikimic acid concentration and protein fingerprint in still white wines. J. Agric. Food Chem. 2008, 56, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- Dubourdieu, D.; Canal-Liaubères, R.M. Estimation rapide des constituants macromolecullaires de mouts et des vins par chromatographie liquid haute pression (CLHP) de tamisage moleculaire. Connaissace de la Vigne et du Vin 1986, 20, 119–123. [Google Scholar]
- Marchal, R.; Bouquelet, S.; Maujean, A. Purification and partial biochemical characterization of glycoproteins in a champenois Chardonnay wine. J. Agric. Food Chem. 1996, 44, 1716–1722. [Google Scholar] [CrossRef]
- Canals, J.M.; Arola, L.; Zamora, F. Protein fraction analysis of white wine by FPLC. Am. J. Enol. Vitic. 1998, 49, 383–388. [Google Scholar]
- Monteiro, S.; Picarra-Pereira, M.A.; Tanganho, M.C.; Rente, J.P.; Loureiro, V.B.; Teixeira, A.; Ferreira, R.B. Preparation of polyclonal antibodies specific for wine proteins. J. Sci. Food Agric. 1999, 79, 772–778. [Google Scholar] [CrossRef]
- Kwon, S.W. Profiling of soluble proteins in wine by nano-highperformance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 7258–7263. [Google Scholar] [CrossRef]
- Baldwin, M.A. Protein identification by mass spectrometry: Issues to be considered. Mol. Cell. Proteom. 2004, 3, 1–9. [Google Scholar] [CrossRef]
- Carr, S.; Aebersold, R.; Baldwin, M.; Burlingame, A.; Clause, K.; Nesvizhskii, A. The need for guidelines in publication of peptide and protein identification data: Working Group on Publication Guidelines for Peptide and Protein Identification Data. Mol. Cell. Proteom. 2004, 3, 531–533. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M. Mass spectrometry in the analysis of grape and wine proteins. Expert Rev. Proteom. 2006, 3, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Miranda, J.D.; Igrejas, G.; Araujo, E.; Reboiro-Jato, M.; Capelo, J.L. Mass Spectrometry-Based Fingerprinting of Proteins & Peptides in Wine Quality Control: A Critical Overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 751–759. [Google Scholar]
- Monteiro, S.; Piçarra-Pereira, M.A.; Mesquita, P.R.; Loureiro, V.B.; Teixeira, A.; Ferreira, R.B. The wide diversity of structurally similar wine proteins. J. Agric. Food Chem. 2001, 49, 3999–4010. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, A.; Marino, G.; Amoresano, A. Rapid fingerprinting of red wines by MALDI mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 969–982. [Google Scholar] [CrossRef]
- Chambery, A.; del Monaco, G.; Di Maro, A.; Parente, A. Peptide fingerprint of high quality Campania white wines by MALDI-TOF mass spectrometry. Food Chem. 2009, 113, 1283–1289. [Google Scholar] [CrossRef]
- Cooper, H.J.; Marshall, A.G. Electrospray ionization Fourier transform mass spectrometric analysis of wine. J. Agric. Food Chem. 2001, 49, 5710–5718. [Google Scholar] [CrossRef]
- Catharino, R.R.; Cunha, I.B.S.; Fogaca, A.O.; Facco, E.M.P.; Godoy, H.T.; Daudt, C.E.; Eberlin, M.N.; Sawaya, A. Characterization of must and wine of six varieties of grapes by direct infusion electrospray ionization mass spectrometry. J. Mass Spectrom. 2006, 41, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C.; Ziemelis, B. The use of gel column analysis in evaluation of bentonite fining procedures. Am. J. Enol. Vitic. 1973, 24, 34–42. [Google Scholar]
- Koch, J.; Sajak, E. A review and some studies on grape protein. Am. J. Enol. Vitic. 1959, 18, 114–123. [Google Scholar]
- Waters, E.J.; Wallace, W.; Williams, P.J. Heat haze characteristics of fractionated wine proteins. Am. J. Enol. Vitic. 1991, 42, 123–127. [Google Scholar]
- Fusi, M.; Mainent, F.; Rizzi, C.; Zoccatelli, G.; Simonato, B. Wine hazing: A predictive assay based on protein and glycoprotein independent recovery and quantification. Food Control. 2010, 21, 830–834. [Google Scholar] [CrossRef]
- Esteruelas, M.; Kontoudakis, N.; Gil, M.; Fort, M.F.; Canals, J.M.; Zamora, F. Phenolic compounds present in natural haze protein of Sauvignon white wine. Food Res. Int. 2011, 44, 77–83. [Google Scholar] [CrossRef]
- Falconer, R.; Marangon, M.; Van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Waters, E.J. Thermal stability of thaumatin-like protein, chitinases, and invertase isolated from Sauvignon blanc and Semillon juice and their role in haze formation in wine. J. Agric. Food Chem. 2010, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A. Pathogenesis-Related proteins: Research progress in the last 15 years. Gen. Appl. Plant. Physiol. 2005, 31, 105–124. [Google Scholar]
- Odjakova, M.; Hadjiivanova, C. The complexity of pathogen defense in plants. Bulg. J. Plant. Physiol. 2001, 27, 101–109. [Google Scholar]
- Waters, E.J.; Hayasaka, Y.; Tattersall, D.B.; Adams, K.S.; Williams, P.J. Sequence analysis of grape (Vitis vinifera) berry chitinases that cause haze formation in wines. J. Agric. Food Chem. 1998, 46, 4950–4957. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.C.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Cilindre, C.; Jegou, S.; Hovasse, A.; Schaeffer, C.; Castro, A.J.; Clement, C.; Van Dorsselaer, A.; Jeandet, P.; Marchal, R. Proteomic approach to identify champagne wine proteins as modified by Botrytis cinerea infection. J. Proteome Res. 2008, 7, 1199–1208. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Waters, E.J.; Menz, R.I. Structure of haze forming proteins in white wines: Vitis vinifera thaumatin-like proteins. PLoS ONE 2014, 9, e113757. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Comparison of methods for estimating protein stability in white wines. Am. J. Enol. Vitic. 2009, 60, 302–311. [Google Scholar]
- Marangon, M.; Van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Haynes, P.A.; Waters, E.J.; Falconer, R.J. Roles of grape thaumatin-like protein and chitinase in white wine haze formation. J. Agric. Food Chem. 2011, 59, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Waters, E.J.; Lim, A.; O’Neill, B.K.; Colby, C.B. An alternative method for purification of a major thaumatin-like grape protein (VVTL1) responsible for haze formation in white wine. Asia-Pac. J. Chem. Eng. 2007, 2, 70–74. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monteiro, S.; Piçarra-Pereira, M.A.; Tanganho, M.C.; Loureiro, V.B.; Teixeira, A.R. Characterisation of the proteins from grapes and wines by immunological methods. Am. J. Enol. Vitic. 2000, 51, 22–28. [Google Scholar]
- Linthorst, H.J.M. Pathogenesis-related proteins of plants. Crit. Rev. Plant. Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Dufrechou, M.; Vernhet, A.; Roblin, P.; Sauvage, F.X.; Poncet-Legrand, C. White wine proteins: How does the pH affect their conformation at room temperature? Langmuir 2013, 29, 10475–10482. [Google Scholar] [CrossRef]
- Tattersall, D.B.; Pocock, K.F.; Hayasaka, Y.; Adams, K.; Van Heeswijck, R.; Waters, E.J.; Høj, P.B. Pathogenesis related proteins—Their accumulation in grapes during berry growth and their involvement in white wine heat instability. Current knowledge and future perspectives in relation to winemaking practices. Mol. Biol. Biotechnol. Grapevine 2001, 183–201. [Google Scholar] [CrossRef]
- Pocock, K.F.; Hayasaka, Y.; Peng, Z.; Williams, P.J.; Waters, E.J. The effect of mechanical harvesting and long-distance transport on the concentration of haze-forming proteins in grape juice. Aust. J. Grape Wine Res. 1998, 4, 23–29. [Google Scholar] [CrossRef]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef]
- Gazzola, D.; Van Sluyter, S.C.; Curioni, A.; Waters, E.J.; Marangon, M. Roles of proteins, polysaccharides, and phenolics in haze formation in white wine via reconstitution experiments. J. Agric. Food Chem. 2012, 60, 10666–10673. [Google Scholar] [CrossRef]
- D’Amato, A.; Fasoli, E.; Kravchuk, A.V.; Righetti, P.G. Mehercules, adhuc Bacchus! The Debate on Wine Proteomics Continues. J. Proteome Res. 2011, 10, 3789–3801. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Giribaldi, M.; Violetta, M.R.; Giuffrida, M.G. Heat-unstable protein removal by different bentonite labels in white wines. LWT-Food Sci. Technol. 2012, 46, 460–467. [Google Scholar] [CrossRef]
- Sarmento, M.R.; Oliveira, J.C.; Slatner, M.; Boulton, R.B. Influence of intrinsic factors on conventional wine protein stability tests. Food Control 2000, 11, 423–432. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Williams, P.J. Proteins in white wine, II: Their resistance to proteolysis is not due to either phenolic association or glycosylation. Aust. J. Grape Wine Res. 1995, 1, 94–99. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Singleton, V.L. Interactive precipitation between phenolic fractions and peptides in wine-like model solutions: Turbidity, particle size, and residual content as influenced by pH, temperature and peptide concentration. Am. J. Enol. Vitic. 1995, 46, 329–338. [Google Scholar]
- Besse, C.; Clark, A.; Scollary, G. Investigation of the role of total and free copper in protein in haze formation. Aust. Grapegrow. Winemak. 2000, 437, 19–20. [Google Scholar]
- Pellerin, P.; Waters, E.J.; Brillouet, J.-M.; Moutounet, M. Effet of polysaccharides sur la formation de trouble protéique dans un vin blanc. J. Int. Sci. Vigne Vin 1994, 24, 13–18. [Google Scholar] [CrossRef]
- Chagas, R.; Lourenço, A.M.; Monteiro, S.; Ferreira, R.B.; Ferreira, L.M. Is caffeic acid, as the major metabolite present in Moscatel wine protein haze hydrolysate, involved in protein haze formation? Food Res. Int. 2017, 98, 103–109. [Google Scholar] [CrossRef]
- Chagas, R.; Ferreira, L.M.; Laia, C.A.T.; Monteiro, S.; Ferreira, R.B. The challenging SO2-mediated chemical build-up of protein aggregates in wines. Food Chem. 2016, 192, 460–469. [Google Scholar] [CrossRef]
- de Bruijn, J.; Loyola, C.; Arumi, J.L.; Martinez, J. Effect of non-protein factors on heat stability of Chilean Sauvignon Blanc wines. Chil. J. Agric. Res. 2014, 74, 490–496. [Google Scholar] [CrossRef][Green Version]
- McRae, J.M.; Barricklow, V.; Pocock, K.F.; Smith, P.A. Predicting protein haze formation in white wines. Aust. J. Grape Wine Res. 2018, 24, 504–511. [Google Scholar] [CrossRef]
- Dupin, I.V.S.; McKinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Williams, P.J.; Waters, E.J. Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal for their mechanism of action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Jaeckels, N.; Meier, M.; Dietrich, H.; Will, F.; Decker, H.; Fronk, P. Influence of polysaccharides on wine protein aggregation. Food Chem. 2016, 200, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Toland, T.M.; Fugelsang, K.C.; Muller, C.J. Methods for estimating protein instability in white wines: A comparison. Am. J. Enol. Vitic. 1996, 47, 111–112. [Google Scholar]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Berg, H.W.; Akiyoshi, M. Determination of protein stability in wine. Am. J. Enol. Vitic. 1961, 12, 107–110. [Google Scholar]
- Pocock, K.; Rankine, B.C. Heat test for detecting protein instability in wine. Aust. Wine Brew. Spirit Rev. 1973, 91, 42–43. [Google Scholar]
- Rankine, B.C.; Pocock, K.F. A new method for detecting protein instability in white wines. Aust. Wine Brew. Spirit Rev. 1971, 89, 61. [Google Scholar]
- Mesrob, B.; Gorinova, N.; Tsakov, D. Characterization of the electrical properties and molecular weights of the proteins in white wines. Nahrung 1983, 27, 727–733. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Quiro, M.; Gonzalez, R. Three different targets for the genetic modification of wine yeast strains resulting in improved effectiveness of bentonite fining. J. Agric. Food Chem. 2009, 57, 8373–8378. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-Bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: Its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res. Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Meier, M.; Jaeckels, N.; Tenzer, S.; Stoll, M.; Decker, H.; Fronk, P.; Dietrich, H.; Will, F. Impact of drought stress on concentration and composition of wine proteins in Riesling. Eur. Food Res. Technol. 2016, 242, 1883–1891. [Google Scholar] [CrossRef]
- Vincenzi, S.; Marangon, M.; Tolin, S.; Curioni, A. Protein evolution during the early stages of white winemaking and its relations with wine stability. Aust. J. Grape Wine Res. 2011, 17, 20–27. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Serrano, M.; Vannier, A.C.; Ribéreau-Gayon, P. Étude comparée des tests de stabilité protéique. Connaiss. Vigne Vin 1988, 22, 261–273. [Google Scholar] [CrossRef]
- Gabrielli, M.; Fracassetti, D.; Tirelli, A. Release of phenolic compounds from corks toppers and its effect on protein-haze. Food Control 2016, 62, 330–336. [Google Scholar] [CrossRef]
- Giese, E.C.; Ocaña, M.C.; Simancas, N.B.; Briones Pérez, A.I.; Decker, R.F.H.; Barbosa, A.M. Evaluation of the components released by wine yeast strains on protein haze formation in white wine. Orbital Electron. J. Chem. 2016, 8, 307–313. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Nozaki, K.; Kushida, T. Turbidity formation caused by interaction of must proteins with wine tannins. J. Ferment. Technol. 1983, 61, 413–416. [Google Scholar]
- Lehninger, A.L. Biochemistry, 2th ed.; Worth Publishers: New York, NY, USA, 1981. [Google Scholar]
- Versari, A.; Laghi, L.; Thorngate, J.H.; Boulton, R.B. Prediction of colloidal stability in white wines using infrared spectroscopy. J. Food Eng. 2011, 104, 239–245. [Google Scholar] [CrossRef]
- Dulau, L. Recherches sur les protÉines Responsables de la Casse Protéique des vins Blancs Secs. Ph.D. Thesis, Universit’e de Bordeaux II, Bordeaux, France, 1990. [Google Scholar]
- Paetzold, M.; Dulau, L.; Dubourdieu, D. Fractionnement et caract´erisation des glycoproteins dans les moˆuts de raisins blancs. J. Int. Sci. Vigne Vin 1990, 24, 13–28. [Google Scholar]
- Kleijn, W.B.; Oster, J.D. A Model of Clay Swelling and Tactoid Formation. Clay Clay Miner. 1982, 30, 383–390. [Google Scholar] [CrossRef]
- Catarino, S.; Madeira, M.; Monteiro, F.; Rocha, F.; Curvelo-Gacia, A.S.; Bruno de Sousa, R. Effect of bentonite characteristics on the elemental composition of wine. J. Agric. Food Chem. 2008, 56, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Marchal, R.; Barret, J.; Maujean, A. Relations entre les caractéristiques physic-chimiques d’une bentonite et son pouvoir d’adsorption. J. Int. Sci. Vigne Vin 1995, 29, 27–42. [Google Scholar]
- Segad, M.; Jonsson, B.; Akesson, T.; Cabane, B. Ca/Na Montmorillonite: Structure, Forces and Swelling Properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Blade, W.; Boulton, R. Adsorption of protein by bentonite in a model wine solution. Am. J. Enol. Vitic. 1988, 39, 193–199. [Google Scholar]
- Lagace, L.S.; Bisson, L.F. Survey of yeast acid proteases for effectiveness of wine haze reduction. Am. J. Enol. Vitic. 1990, 41, 147–155. [Google Scholar]
- Jaeckels, N.; Tenzer, S.; Meier, M.; Will, F.; Dietrich, H.; Decker, H.; Fronk, P. Influence of bentonite fining on protein composition in wine. LWT-Food Sci. Technol. 2017, 75, 335–343. [Google Scholar] [CrossRef]
- Achaerandio, I.; Pachova, V.; Güell, C.; López, F. Protein adsorption by bentonite in a white wine model solution: Effect of protein molecular weight and ethanol concentration. Am. J. Enol. Vitic. 2001, 52, 122–126. [Google Scholar]
- Moio, L.; Ugliano, M.; Gambuti, A.; Genovese, A.; Piombino, P. Influence of clarification treatment on concentrations of selected free varietal aroma compounds and glycoconjugates in Falanghina (Vitis vinifera L.) must and wine. Am. J. Enol. Vitic. 2004, 55, 7–12. [Google Scholar]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of Bentonite Additions during Vinification on Protein Stability and Volatile Compounds of Albariño Wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Castro, E.; Ferreira, V.; Lopez, R. Effect of Bentonite Fining on Polyfunctional Mercaptans and Other Volatile Compounds in Sauvignon blanc Wines. Am. J. Enol. Vitic. 2017, 68, 30–38. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen compounds in must and volatile profile of white wine: Influence of clarification process before alcoholic fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Ewart, A.J.W.; Phipps, G.J.; Iland, P.G. Bentonite additions to wine: Before, during or after fermentation? Aust. N. Z. Grapegrow. Winemak. 1980, 196, 46–47. [Google Scholar]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci Technol. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Dordoni, R.; Colangelo, D.; Giribaldi, M.; Giuffrida, M.G.; De Faveri, D.M.; Lambri, M. Effect of Bentonite Characteristics on Wine Proteins, Polyphenols, and Metals under Conditions of Different pH. Am. J. Enol. Vitic. 2015, 66, 518–530. [Google Scholar] [CrossRef]
- Liu, A.; Nyavor, K.; Ankumah, R. Structural and adsorptive properties of Ba or Mg oxide modified zirconia. J. Colloid Interface Sci. 2005, 284, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.N.; Zamora, F.; Canals, J.M.; Lopez, F. Protein stabilization in sparkling base wine using zirconia and bentonite: Influence on the foam parameters and protein fractions. J. Int. Sci. Vigne Vin 2010, 44, 51–58. [Google Scholar]
- Wyss, C.; Cuénat, P. Stabilisation tartrique des vins par traitement aux zéolithes. Rev. Suisse Vitic. Arboric. Hortic. 2005, 37, 341–347. [Google Scholar]
- Powers, J.R.; Nagel, C.W.; Weller, K. Protein removal from a wine by immobilized grape proanthocyanidins. Am. J. Enol. Vitic. 1988, 39, 117–120. [Google Scholar]
- Gonçalves, F.; Heyraud, A.; Pinho, M.N.; Rinaudo, M. Characterization of white wine mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Influence of fining and tartaric Stabilisation Procedures on white wine mannoprotein content. S. Afr. J. Enol. Vitic. 2012, 33, 88–94. [Google Scholar] [CrossRef][Green Version]
- Strahl-Bolsinger, S.; Gentzsch, M.; Tanner, W. Protein O-mannosylation. Biochim. Biophys. Acta 1999, 1426, 297–307. [Google Scholar] [CrossRef]
- Waters, E.J.; Pellerin, P.; Brillouet, J.-M. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Hernández, L.M.; Ballou, L.; Alvarado, E.; Gillece-Castro, B.L.; Burlingame, A.L.; Ballou, C.E. A new Saccharomyces cerevisiae mnn mutant N-linked oligosaccharide structure. J. Biol. Chem. 1989, 264, 11849–11856. [Google Scholar]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representation of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- OIV. Codex Oenologique International; Internationale de la Vigne et du Vin: Paris, France, 2009. [Google Scholar]
- Peat, S.; Whelan, W.J.; Edwards, T.E. 6. Polysaccharide of baker’s yeast. Part IV. Mannan. J. Chem. Soc. 1961, 28–35. [Google Scholar] [CrossRef]
- Moine-Ledoux, V.; Dubourdieu, D. Rôle des mannoprotéines de levures vis-à-vis de la stabilisation tartrique des vins. Bulletin Organization de la vigne et du vin 2002, 75, 471–482. [Google Scholar]
- Gonzales-Ramos, D.; Gonzalez, R. Genetic determinants of the release of mannoproteins of enological interest by Saccharomyces cerevissiae. J. Agric Food Chem. 2006, 54, 9411–9416. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Pellerin, P.; Brillouet, J.M. A wine arabonogalactan-proteins that reduces heat-induced wine protein haze. Biosci. Biotechnol. Biochem. 1994, 58, 43–48. [Google Scholar] [CrossRef]
- Escot, S.; Feulliat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestarán, B. Yeast mannoproteins in red winemaking. Effect on polysaccharide, polyphenolic and colour composition. Am. J. Enol. Vitic. 2010, 61, 191–200. [Google Scholar]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and colour extraction in red wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar] [CrossRef]
- Moine-Ledoux, V.; Dubourdieu, D. An invertase fragment responsible for improving the protein stability of dry white wines. J. Sci. Food Agric. 1999, 79, 537–543. [Google Scholar] [CrossRef]
- Marangon, M.; Lucchetta, M.; Duan, D.; Stockdale, V.J.; Hart, A.; Rogers, P.J.; Waters, E.J. Protein removal from a Chardonnay juice by addition of carrageenan and pectin. Aust. J. Grape Wine Res. 2012, 18, 194–202. [Google Scholar] [CrossRef]
- Cabello-Pasini, A.; Victoria-Cota, N.; Macias-Carranza, V.; Hernandez-Garibay, E.; Muñiz-Salazar, R. Clarification of wines using polysaccharides extracted from seaweeds. Am. J. Enol. Vitic. 2005, 56, 52–59. [Google Scholar]
- Campo, V.L.; Kawano, F.F.; Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Guangling, J.; Guangli, Y.; Junzeng, Z.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar]
- Sime, W. The practical utilization of alginates in food gelling systems. In Gums and Stabilizers for the Food Industry; Phillips, G., Ed.; Pergamon Press: Oxford, UK, 1984; pp. 177–188. [Google Scholar]
- Usov, A.I.; Zelinski, N.D. Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Elsevier Science: Cambridge, UK, 2013; pp. 45–49. [Google Scholar]
- European Union (EU). Commission regulation (EU) 53/2011 of 21 January 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:019:0001:0006:EN:PDF (accessed on 22 January 2011).
- Arroyo, J.; Farkaš, V.; Sanz, A.B.; Cabib, E. Strengthening the fungal cell wall through chitin–glucan cross-links: Effects on morphogenesis and cell integrity. Cell Microbiol. 2016, 18, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, T.; Divol, B.; Bauera, F.F. Yeast Cell Wall Chitin Reduces Wine Haze Formation. Appl. Environ. Microbiol. 2018, 84, e00668-1. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Reducing the negative sensory impact of volatile phenols in red wine with different chitosans: Effect of structure on efficiency. Food Chem. 2018, 242, 591–600. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, C.; Pinho, M.N. White wine clarification by micro/ ultrafiltration: Effect of remover colloids in tartaric stability. Sep. Purif. Technol. 2001, 22–23, 423–429. [Google Scholar] [CrossRef]
- Miller, G.C.; Amon, J.M.; Gibson, R.L.; Simpson, R.F. Loss of wine aroma attributable to protein stabilization with bentonite or ultrafiltration. Aust. N. Z. Grapegrow. Winemak. 1985, 256, 46–50. [Google Scholar]
- Voilley, A.; Lamer, C.; Dubois, P.; Feuillat, M. Influence of macromolecules and tratments on the behavior of aroma compounds in a model wine. J. Agric. Food Chem. 1990, 38, 248–251. [Google Scholar] [CrossRef]
- Konja, G.; Clauss, E.; Kovacic, Z.; Pozderovic, A. The influence of ultrafiltration on the chemical composition and sensory characteristics of white and rose wine. Chem. Biochem. Eng. Q. 1988, 2, 235–241. [Google Scholar]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of free and immobilised stem bromelain on protein haze in white wine. Aust. J. Grape Wine Res. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of wine inhibitors on the proteolytic activity of papain from Carica papaya L. Latex. Biotechnol. Prog. 2015, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rehmanji, M.; Gopal, C.; Mola, A. Beer stabilization technology—Clearly a matter of choice. Master Brew. Assoc. Am. Technol. Q. 2005, 42, 332–338. [Google Scholar]
- Esti, M.; Benucci, I.; Lombardelli, C.; Liburdi, K.; Garzillo, A.M.V. Papain from papaya (Carica papaya L.) fruit and latex: Preliminary characterization in alcoholic-acidic buffer for wine application. Food Bioprod. Process. 2013, 91, 595–598. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Technol. 2016, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Mierczynskic, P.; Maniukiewiczc, W.; Visalakshanb, R.M.; Vasilevd, K.; Smith, P.A. Magnetic separation technology: Functional group efficiency in the removal of haze-forming proteins from wines. Food Chem. 2019, 275, 154–160. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Qi, G.; Smith, P.; Bindon, K.; Vasilev, K. Regeneration of Magnetic Nanoparticles Used in the Removal of Pathogenesis-Related Proteins from White Wines. Foods 2020, 9, 1. [Google Scholar] [CrossRef]

| Isoelectric Point (pI) | Molecular Weight (MW) | References | ||
|---|---|---|---|---|
| Grape | Wine | Grape | Wine | |
| 3.1–8.3 | [61] | |||
| 4.0–8.2 | 10.0–70.0 | [72] | ||
| 15.5–69.0 | [45] | |||
| 4.1–5.8 | 11.2–190.0 | [75] | ||
| 5.6–7.6 | 19.0–100.0 | [73] | ||
| 4.6–8.8 | 12.0–41.0 | [74] | ||
| 18.0–23.0 | [64] | |||
| 3.1–9.2 | 11.0–88.1 | [47] | ||
| 3.0–5.6 | 14.0–94.0 | [65] | ||
| 3.2–9.0 | [68] | |||
| 3.6–9.0 | 6.0–200.0 | [40] | ||
| 10.0–50.0 | [95] | |||
| 10.0–64.0 | [70] | |||
| 21.0–65.0 | [76] | |||
| 2.5–9.7 | [41,42] | |||
| Heating | Cooling | Reference |
|---|---|---|
| 60 °C for 4 days | 4 °C for 6 h | [29] |
| 80 °C for 2 h | 4 °C for 16 h | [134] |
| 80 °C for 2 h | 0 °C for 2 h | [5,127,141] |
| 80 °C for 2 h | 4 °C for 2 h | [128] |
| 80 °C for 2 h | 20 °C for 3 h | [37] |
| 80 °C for 3 h | 20 °C for 30 min | [131,142] |
| 80 °C for 6 h | 4 °C for 16 h | [9,137,143,144] |
| 80 °C for 30 min | No specified cooling time | [145,146] |
| 90 °C for 1 h | 4 °C for 18 h | [147] |
| 90 °C for 1 h | 4 °C for 6 h | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. https://doi.org/10.3390/beverages6010019
Cosme F, Fernandes C, Ribeiro T, Filipe-Ribeiro L, Nunes FM. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages. 2020; 6(1):19. https://doi.org/10.3390/beverages6010019
Chicago/Turabian StyleCosme, Fernanda, Conceição Fernandes, Tânia Ribeiro, Luís Filipe-Ribeiro, and Fernando M. Nunes. 2020. "White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview" Beverages 6, no. 1: 19. https://doi.org/10.3390/beverages6010019
APA StyleCosme, F., Fernandes, C., Ribeiro, T., Filipe-Ribeiro, L., & Nunes, F. M. (2020). White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages, 6(1), 19. https://doi.org/10.3390/beverages6010019