Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region
Abstract
:1. Introduction
2. Materials and Methods
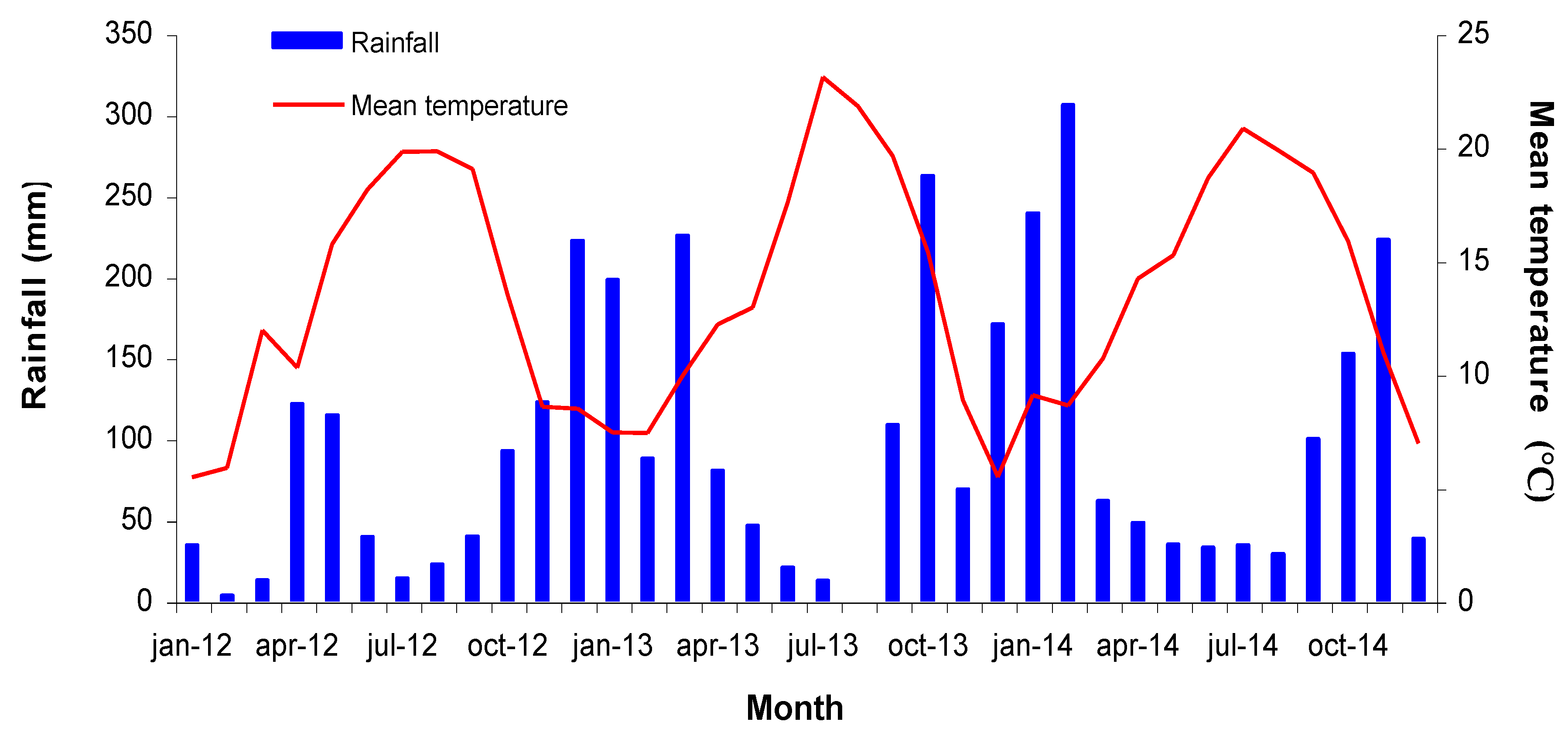
2.1. Description of the Study Site
2.2. Experimental Design
- Soil tillage (ST);
- native vegetation (NV);
- English ryegrass (Lolium perenne L.) sown at 40 kg ha−1 (ER); and
- subterranean clover (Trifolium subterraneum L.) sown at 30 kg ha−1 (SC).
2.3. Sampling and Winemaking
2.4. Basic Parameters of Musts and Wines
2.5. Analytical Methods
2.5.1. Chemical Reagents
2.5.2. Quantification of Amino Acids in Musts and Wines
2.5.3. Determination of Volatile Compounds
2.5.4. Odor Activity Values
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physiological, Vegetative Growth, and Yield Performance
3.2. General Attributes of Musts and Wines
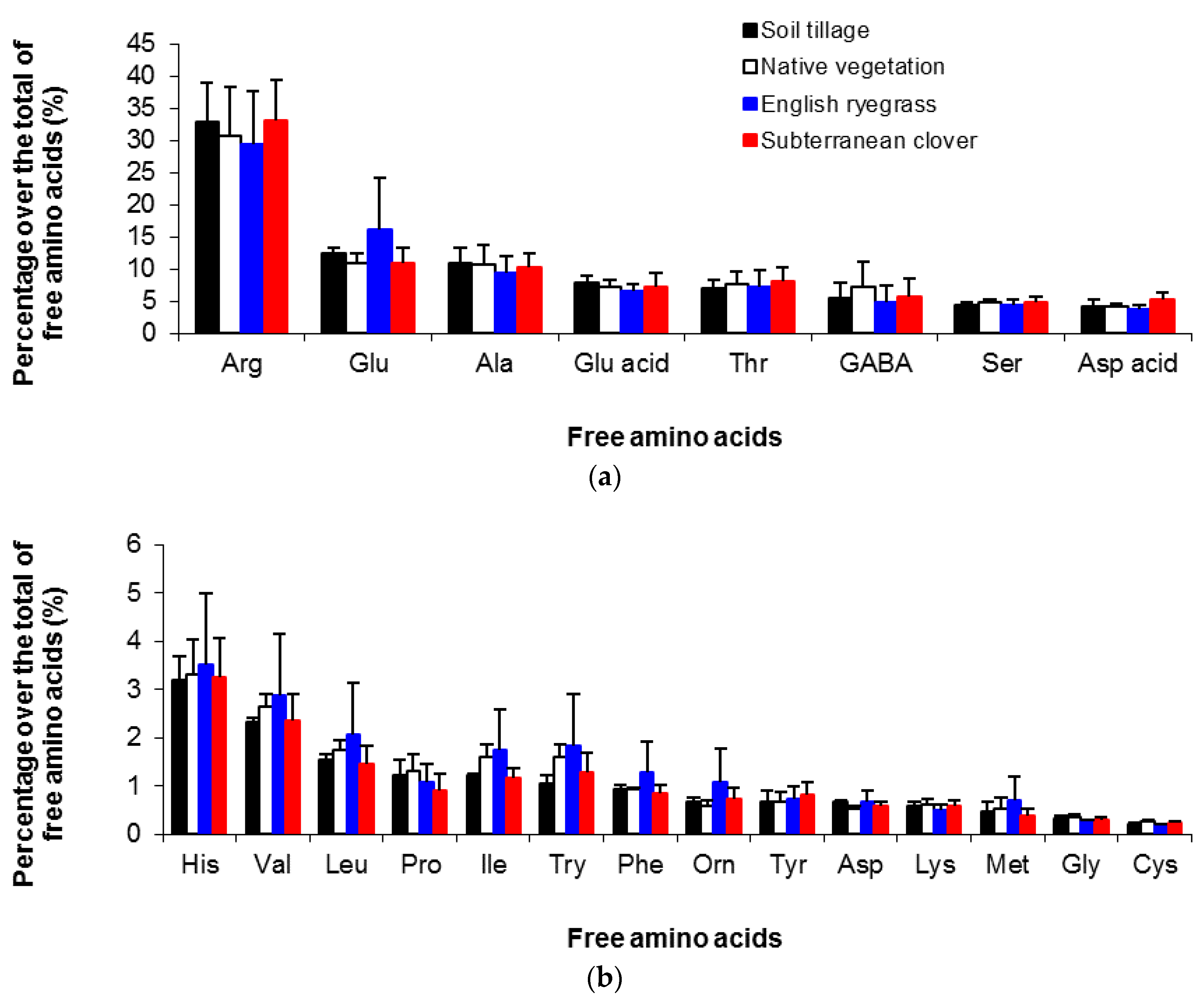
3.3. Amino Acid Composition of Musts and Wines
3.4. Volatile Composition of Mencía Wines
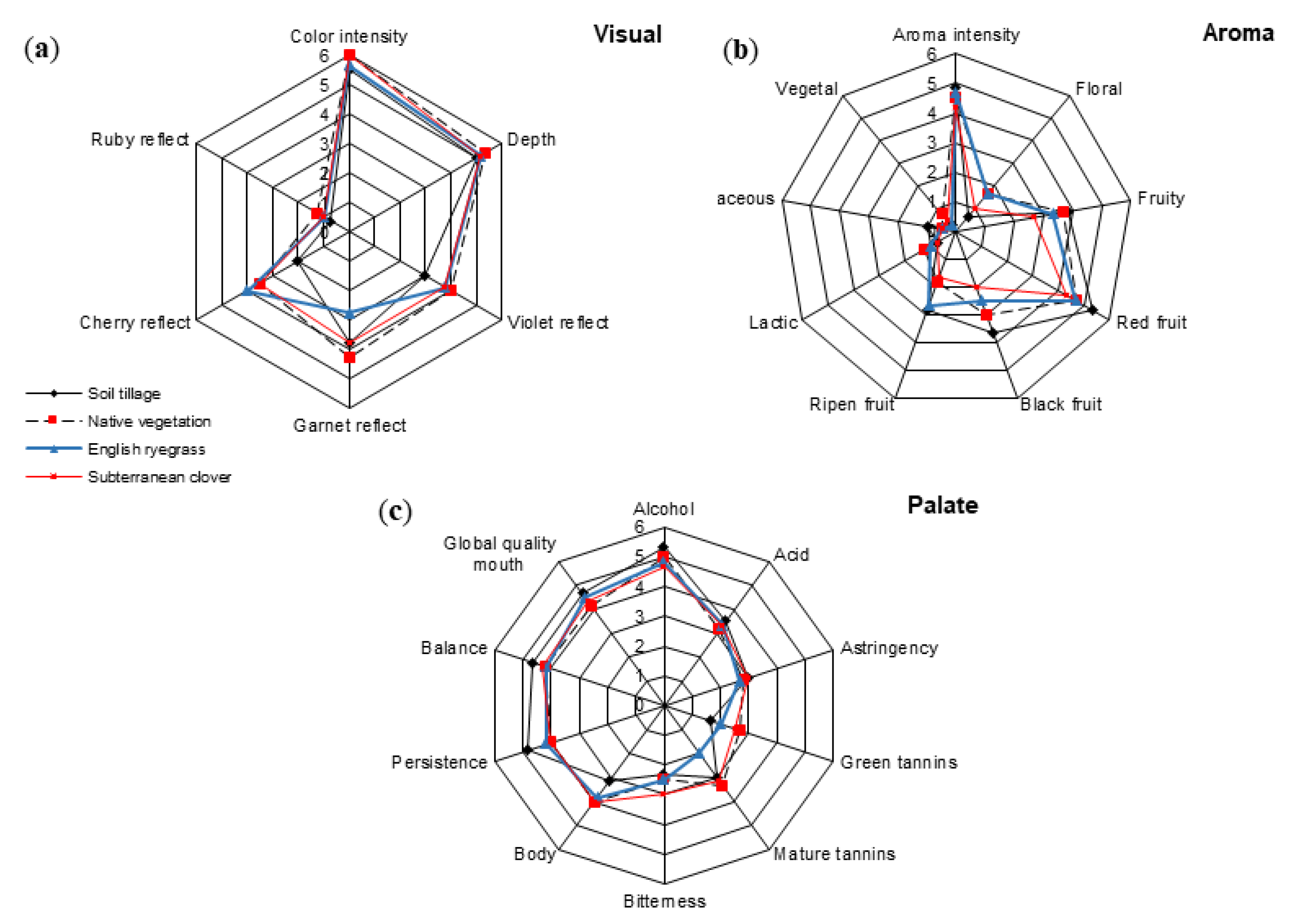
3.5. Sensory Profiles of Mencía Wines
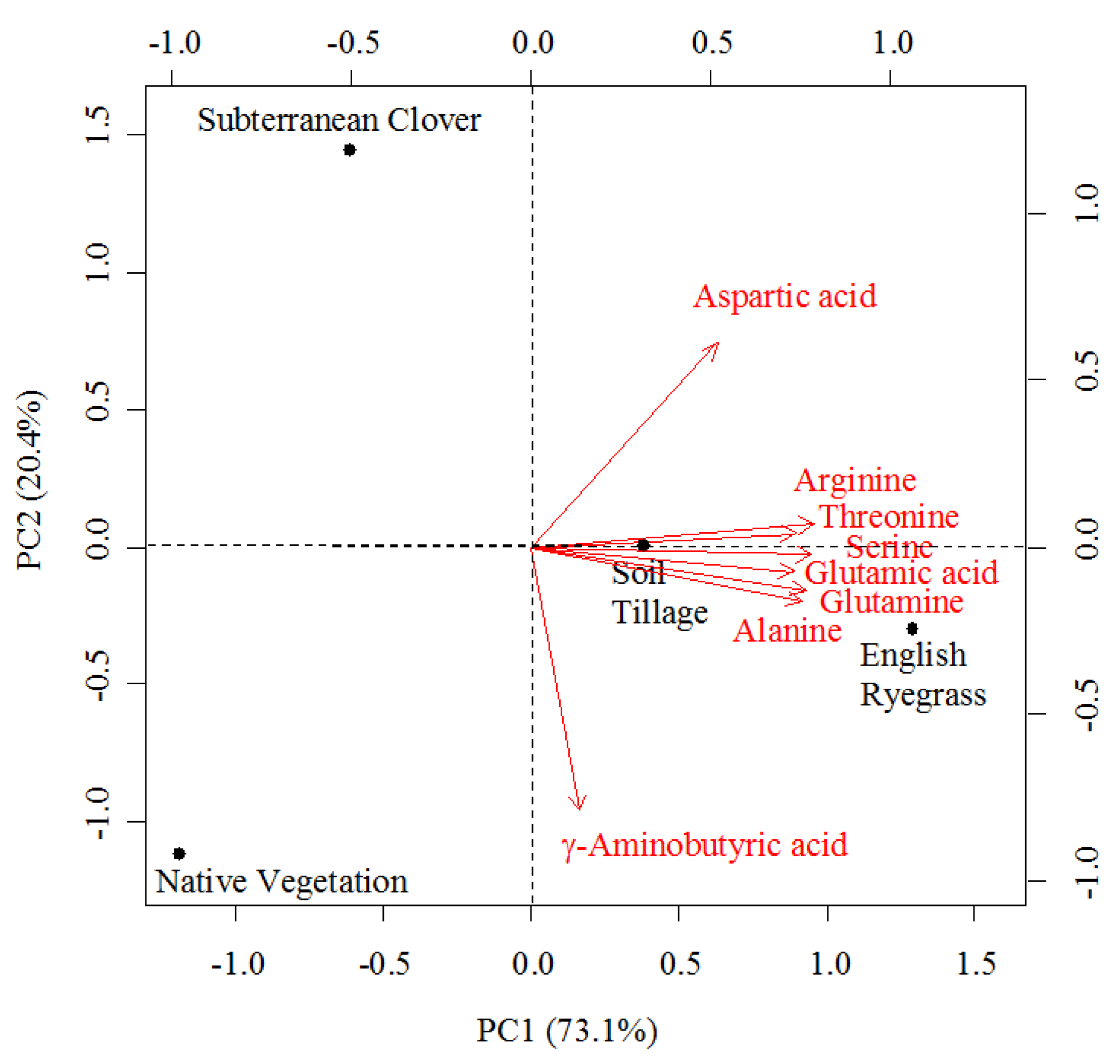
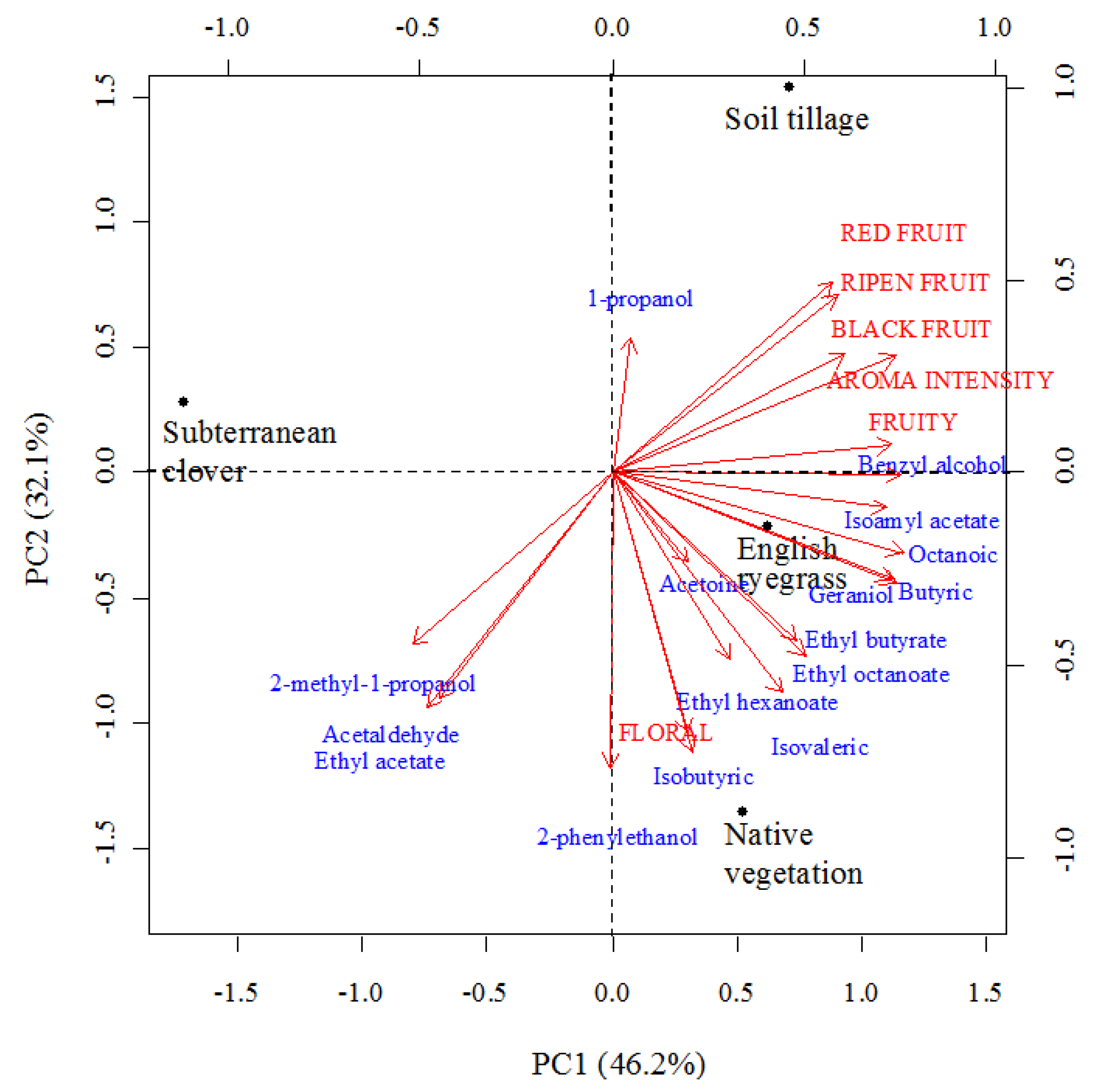
3.6. Relationships among Amino Acids, Volatile Compounds, and Sensory Descriptors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Guerra, B.; Steenwerth, K. Influence of floor management technique on grapevine growth, disease pressure, and juice and wine composition: A review. Am. J. Enol. Vitic. 2012, 63, 149–164. [Google Scholar] [CrossRef]
- Celette, F.; Findeling, A.; Gary, C. Competition for nitrogen in an unfertilzed intercropping system: The case of an association of grapevine and grass cover in a Mediterranean climate. Eur. J. Agron. 2009, 30, 41–51. [Google Scholar] [CrossRef]
- Ripoche, A.; Celette, F.; Cinna, J.P.; Gary, C. Design of intercrop management plans to fulfill production and environmental objectives in vineyards. Eur. J. Agron. 2010, 32, 30–39. [Google Scholar] [CrossRef]
- Afonso, J.M.; Monteiro, A.M.; Lopes, C.M.; Lourenço, J. Enrelvamento do solo em vinha na região dos Vinhos Verdes. Três anos de estudo na casta ‘Alvarinho’. Ciência e Técnica Vitivinícola 2003, 18, 47–63. [Google Scholar]
- Wheeler, S.J.; Black, A.S.; Pickering, G.J. Vineyard floor management improves wine quality in highly vigorous Vitis vinifera ‘Cabernet Sauvignon’ in New Zealand. N. Z. J. Crop Hortic. Sci. 2005, 33, 317–328. [Google Scholar] [CrossRef]
- Nazrala, J.B. Influencia del manejo del suelo y las coberturas vegetales en el microclima de la canopia de la vid, la composición de la uva y el vino. Rev. FCA UNCuyo 2008, XL, 85–104. [Google Scholar]
- Lopes, C.M.; Monteiro, A.; Machado, J.P.; Fernandes, N.; Araujo, A. Cover cropping in a sloping non-irrigated vineyard: II—Effects on vegetative growth, yield, berry and wine quality of ‘Cabernet Sauvignon’ grapevines. Ciência e Técnica Vitivinícola 2008, 23, 37–43. [Google Scholar]
- Celette, F.; Gary, C. Dynamics of water and nitrogen stress along the grapevine cycle as affected by cover cropping. Eur. J. Agron. 2013, 45, 142–152. [Google Scholar] [CrossRef]
- Ruiz-Colmenero, M.; Bienes, R.; Marqués, M.J. Soil and water conservation dilemas associated with the use of green cover in steep vineyards. Soil Tillage Res. 2011, 117, 211–223. [Google Scholar] [CrossRef]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Urrutia, I.; Enrique, A.; Bescansa, P. Soil quality evaluation following the implementation of permanent cover crops in semiarid vineyards. Organic matter, physical and biological soil properties. Span. J. Agric. Res. 2012, 10, 1121–1132. [Google Scholar] [CrossRef]
- Coniberti, A.; Ferrari, V.; Disegna, E.; Garcia Petillo, M.; Lakso, A.N. Under-trellis cover crop and planting density to achieve vine balance in a humid climate. Sci. Hortic. 2018, 227, 65–74. [Google Scholar] [CrossRef]
- Ibáñez Pascual, S. Gestión del suelo en viñedo mediante cubiertas vegetales. Incidencia sobre el control del rendimiento y el vigor. Aspectos ecofisiológcos, nutricionales, microclimáticos y de calidad del mosto y del vino. Ph.D. Thesis, Universidad de La Rioja, Logroño, Spain, 2013. [Google Scholar]
- Smart, R.E.; Robinson, M. Sunlight into Wine. A Hand-Book for Winegrape Canopy Management; Winetitles: Adelaide, Australia, 1991; p. 88. ISBN 978-1875130108. [Google Scholar]
- Albers, E.; Larsson, C.; Liden, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [PubMed]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Pérez Álvarez, E.P.; García Escudero, E.; Peregrina, F. Soil nutrient availability under cover crops: Effects on vines, must and wine in a Tempranillo vineyard. Am. J. Enol. Vitic. 2015, 66, 311–320. [Google Scholar] [CrossRef]
- Lee, J.; Steenwerth, K.L. Rootstock and vineyard floor management influence on ‘Cabernet Sauvignon’ grape yeast assimilable nitrogen (YAN). Food Chem. 2011, 127, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.M.; Tao, Y.S.; Zhang, L.; Li, H. Impact of cover crops in vineyard on the aroma compounds of Vitis vinifera L. cv. Cabernet Sauvignon wine. Food Chem. 2011, 127, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Crupi, P.; Gambacorta, G.; Dipalmo, T.; Antonacci, D.; Coletta, A. Free and bound aroma compounds characterization by GC-MS of Negroamaro wine as affected by soil management. J. Mass Spectrom. 2012, 47, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Trigo-Córdoba, E.; Bouzas-Cid, Y.; Orriols-Fernández, I.; Díaz-Losada, E.; Mirás-Avalos, J.M. Influence of cover crop treatments on the performance of a vineyard in a humid region. Span. J. Agric. Res. 2015, 13, e0907. [Google Scholar] [CrossRef]
- Calleja, A.; Falqué, E. Volatile composition of Mencía wines. Food Chem. 2005, 90, 357–363. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Quantitative determination and characterisation of the main odourants of Mencía monovarietal wines. Food Chem. 2009, 117, 473–484. [Google Scholar] [CrossRef]
- Añón, A.; López, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revilla, E.; Losada, M.M.; Gutiérrez, E. Phenolic composition and color of single cultivar young red wines made with Mencia and Alicante-Bouschet grapes in AOC Valdeorras (Galicia, NW Spain). Beverages 2016, 2, 18. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Cardoso, R.M.; Soares, P.M.M.; Cancela, J.J.; Pinto, J.G.; Santos, J.A. Integrated analysis of climate, soil, topography and vegetative growth in Iberian viticultural regions. PLoS ONE 2014, 9, e108708. [Google Scholar] [CrossRef] [PubMed]
- OIV. Recueil des Méthodes Internationales d’Analyses des Vins et des Moûts; Office International de la Vigne et du Vin: Paris, France, 2009; p. 544. ISBN 979-10-91799-82-9. [Google Scholar]
- Zamora, F. Elaboración y Crianza del Vino Tinto: Aspectos Científicos y Prácticos; Mundi-Prensa: Madrid, Spain, 2003; p. 225. ISBN 978-84-89922-88-4. [Google Scholar]
- Gómez Alonso, S.; Hermosín-Gutiérrez, I.; García Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Falqué, E.; Orriols-Fernández, I.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Treixadura (Vitis vinifera L.) musts and wines, and on the aromatic composition and sensory profiles of its wines. Food Chem. 2018, 240, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Influence of supplementary irrigation on the amino acid and volatile composition of Godello wines from the Ribeiro Designation of Origin. Food Res. Int. 2018, 111, 715–723. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, C.; Bollaín, M.H.; Moser, S.; Orriols, I. Characterization and differentiation of monovarietal grape pomace distillate from native varieties of Galicia. J. Agric. Food Chem. 2010, 58, 9657–9665. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Ferreira, V. Volatile aroma compounds and wine sensory attributes. In Managing Wine Quality: Viticulture and Wine Quality, 1st ed.; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 3–28. ISBN 978-18-45694-84-5. [Google Scholar]
- Vilanova, M.; Campo, E.; Escudero, A.; Graña, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from North West Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org (accessed on 9 July 2017).
- Monteiro, A.; Lopes, C.M. Influence of cover crop on water use and performance of vineyard in Mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Response of grapevine cv. ‘Tempranillo’ to timing and amount of irrigation: Water relations, vine growth, yield and berry and wine composition. Irrig. Sci. 2010, 28, 113–125. [Google Scholar] [CrossRef]
- Maigre, D.; Aerny, J. Enherbement permanent et fumure azotée sur cv. ‘Gamay’ dans le Valais Central. Rev. Suis. Vitic. Arboric. Hortic 2001, 114, 255–258. [Google Scholar]
- Delalande, M.; Forguet, C.; Cazals, G.; Tauzin, D. Enherbement du vignoble méditerranéen avec des luzemes annuelles. Prog. Agric. Vitic. 2009, 126, 135–148. [Google Scholar]
- De Pascali, S.A.; Coletta, A.; Del Coco, L.; Basile, T.; Gambacorta, G.; Fanizzi, F.P. Viticultural practice and winemaking effects on metabolic profile of Negroamaro. Food Chem. 2014, 161, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Rodríguez, I.; Canosa, P.; Otero, I.; Gamero, E.; Moreno, D.; Talaverano, I.; Valdés, E. Variability in chemical composition of Vitis vinifera cv. Mencía from different geographic areas and vintages in Ribeira Sacra (NW Spain). Food Chem. 2015, 169, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Pérez Álvarez, E.P.; Garde Cerdán, T.; Santamaría, P.; García Escudero, E.; Peregrina, F. Influence of two different cover crops on soil N availability, N nutritional status, and grape yeast-assimilable N (YAN) in a cv. Tempranillo vineyard. Plant Soil 2015, 390, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Schreiner, R.P. Free amino acid profiles from ‘Pinot noir’ grapes are influenced by vine N-status and sample preparation method. Food Chem. 2010, 119, 484–489. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, M.R. Study of the evolution of nitrogen compounds during grape ripening. Application to differentiate grape varieties and cultivated systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Díaz-Losada, E.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Garde-Cerdán, T.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Albariño (Vitis vinifera L.) musts and wines in two different terroirs. Sci. Hortic. 2018, 227, 313–325. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Volatile compounds formation in alcoholic fermentation from grapes collected at 2 maturation stages: Influence of nitrogen compounds and grape variety. J. Food Sci. 2012, 71, C71–C79. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Heras, M.; Pérez-Magariño, S.; Del-Villar-Garrachón, V.; González-Huerta, C.; Moro González, L.C.; Guadarrama Rodríguez, A.; Villanueva Sánchez, S.; Gallo González, R.; Martín de la Helguera, S. Study of the effect of vintage, maturity degree, and irrigation on the amino acid and biogenic amine content of a white wine from the Verdejo variety. J. Sci. Food Agric. 2014, 94, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Soufleros, E.H.; Bouloumpasi, E.; Zotou, A.; Loukou, Z. Determination of biogenic amines in Greek wines by HPLC and ultraviolet detection after dansylation and examination of factors affecting their presence and concentration. Food Chem. 2007, 101, 704–716. [Google Scholar] [CrossRef]
- Martínez-Pinilla, O.; Guadalupe, Z.; Hernández, Z.; Ayestarán, B. Amino acids and biogenic amines in red varietal wines: The role of grape variety, malolactic fermentation and vintage. Eur. Food Res. Technol. 2013, 237, 887–895. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Bouloumpasi, E.; Tsarchopoulos, C.; Bilianderis, C.G. Primary amino acid profiles of Greek white wines and their use in classification according to variety, origin and vintage. Food Chem. 2003, 80, 261–273. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma: A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Shinohara, T. Gas chromatographic analysis of volatile fatty acids in wines. Agric. Biol. Chem. 1985, 49, 2211–2212. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Hopfer, H.; Heymann, H. Judging wine quality: Do we need experts, consumers or trained panelists? Food Qual. Prefer. 2014, 32, 221–233. [Google Scholar] [CrossRef]
| Attribute | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|
| Total soluble solids (° Brix) | 22.8 ± 0.7 | 22.4 ± 0.5 | 22.2 ± 0.7 | 21.1 ± 1.1 | ns |
| Total acidity (g L−1 tartaric acid) | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.5 ± 0.2 | 4.8 ± 0.6 | ns |
| pH | 3.85 ± 0.06 | 3.79 ± 0.04 | 3.74 ± 0.05 | 3.65 ± 0.10 | ns |
| Tartaric acid (g L−1) | 5.0 ± 0.1 | 5.0 ± 0.0 | 5.1 ± 0.1 | 5.2 ± 0.1 | ns |
| Malic acid (g L−1) | 3.4 ± 0.2 | 3.1 ± 0.2 | 3.4 ± 0.1 | 3.3 ± 0.3 | ns |
| Attribute | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|
| Alcohol content (% vol.) | 13.2 ± 0.5 | 12.9 ± 0.6 | 12.7 ± 0.5 | 12.1 ± 0.4 | ns |
| Total acidity (g L−1 tartaric acid) | 4.0 ± 0.7 | 4.0 ± 0.7 | 4.1 ± 0.4 | 3.9 ± 0.2 | ns |
| Volatile acidity (g L−1 acetic acid) | 0.30 ± 0.07 | 0.23 ± 0.04 | 0.23 ± 0.04 | 0.33 ± 0.06 | ns |
| pH | 4.42 ± 0.14 | 4.37 ± 0.10 | 4.32 ± 0.07 | 4.31 ± 0.05 | ns |
| Tartaric acid (g L−1) | 1.8 ± 0.4 | 2.0 ± 0.4 | 1.7 ± 0.4 | 1.9 ± 0.2 | ns |
| Malic acid (g L−1) | 1.5 ± 0.8 | 1.4 ± 0.6 | 1.4 ± 0.3 | 1.7 ± 0.6 | ns |
| Lactic acid (g L−1) | 1.6 ± 0.4 | 1.2 ± 0.3 | 1.5 ± 0.1 | 1.7 ± 0.3 | ns |
| Dry extract (g L−1) | 32.2 ± 3.3 | 29.9 ± 2.4 | 28.2 ± 1.5 | 28.0 ± 1.0 | ns |
| Absorbance 420 nm | 0.349 ± 0.032 ab | 0.380 ± 0.070 ab | 0.327 ± 0.012 a | 0.431 ± 0.063 b | * |
| Absorbance 520 nm | 0.388 ± 0.047 ab | 0.394 ± 0.087 ab | 0.387 ± 0.004 a | 0.502 ± 0.102 b | * |
| Absorbance 620 nm | 0.152 ± 0.018 | 0.146 ± 0.029 | 0.132 ± 0.005 | 0.168 ± 0.021 | ns |
| Color intensity | 9.5 ± 0.9 a | 10.1 ± 1.6 ab | 9.1 ± 0.7 a | 10.8 ± 1.3 b | * |
| Color Hue (%) | 86.9 ± 4.6 b | 87.1 ± 9.4 b | 77.9 ± 7.2 a | 80.5 ± 8.0 ab | * |
| Total Polyphenol Index | 54.3 ± 3.8 | 53.0 ± 7.4 | 53.8 ± 3.8 | 54.3 ± 4.6 | ns |
| Total anthocyanins (mg L−1) | 570 ± 100 | 571 ± 127 | 606 ± 112 | 562 ± 111 | ns |
| Total tannins (g L−1) | 2.0 ± 0.1 b | 1.8 ± 0.2 a | 2.1 ± 0.1 b | 2.1 ± 0.1 b | * |
| Compound | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|
| Aspartic acid | 43.5 ± 9.7 | 34.8 ± 4.4 | 45.3 ± 9.2 | 45.8 ± 10.1 | ns |
| Glutamic acid | 81.1 ± 10.7 | 59.8 ± 10.1 | 80.5 ± 12.9 | 62.4 ± 19.4 | ns |
| Asparagine | 6.9 ± 0.4 | 4.5 ± 0.4 | 8.1 ± 3.1 | 5.2 ± 0.6 | ns |
| Serine | 44.9 ± 5.2 | 39.5 ± 4.8 | 53.2 ± 12.5 | 42.0 ± 7.7 | ns |
| Glutamine | 128.1 ± 9.7 | 91.7 ± 12.3 | 198.8 ± 98.3 | 96.1 ± 20.4 | ns |
| Histidine | 33.0 ± 5.0 | 27.9 ± 6.0 | 43.1 ± 18.2 | 28.6 ± 7.0 | ns |
| Glycine | 3.3 ± 0.6 | 3.0 ± 0.4 | 3.1 ± 0.4 | 2.7 ± 0.4 | ns |
| Threonine | 71.2 ± 13.9 | 64.9 ± 15.1 | 87.8 ± 32.0 | 70.7 ± 19.4 | ns |
| Arginine | 339.4 ± 61.7 | 258.0 ± 62.8 | 361.9 ± 99.1 | 291.0 ± 55.7 | ns |
| Alanine | 111.4 ± 25.5 | 90.4 ± 25.8 | 114.7 ± 31.9 | 90.1 ± 18.4 | ns |
| γ-Aminobutyric acid (GABA) | 56.8 ± 24.1 | 60.7 ± 32.8 | 58.9 ± 31.0 | 49.3 ± 25.5 | ns |
| Proline | 12.6 ± 3.3 | 11.0 ± 3.1 | 13.2 ± 4.7 | 7.9 ± 3.1 | ns |
| Tyrosine | 6.9 ± 2.5 | 5.7 ± 1.7 | 8.9 ± 3.1 | 7.3 ± 2.3 | ns |
| Ammonium ion | 174.3 ± 21.4 | 162.5 ± 18.2 | 182.8 ± 19.2 | 183.0 ± 23.3 | ns |
| Valine | 24.0 ± 1.0 | 22.2 ± 2.1 | 35.5 ± 15.7 | 20.8 ± 4.8 | ns |
| Methionine | 4.9 ± 2.0 | 4.4 ± 1.9 | 8.7 ± 5.9 | 3.4 ± 1.2 | ns |
| Cysteine | 2.1 ± 0.3 | 2.2 ± 0.3 | 2.2 ± 0.3 | 2.1 ± 0.3 | ns |
| Isoleucine | 12.5 ± 0.4 | 13.4 ± 2.1 | 21.2 ± 10.6 | 10.2 ± 1.9 | ns |
| Tryptophan | 10.8 ± 1.8 | 13.3 ± 2.3 | 22.6 ± 13.0 | 11.3 ± 3.6 | ns |
| Leucine | 15.9 ± 1.3 | 14.7 ± 1.6 | 25.3 ± 13.1 | 12.9 ± 3.2 | ns |
| Phenylalanine | 9.6 ± 1.0 | 7.8 ± 0.3 | 15.9 ± 7.8 | 7.6 ± 1.5 | ns |
| Ornithine | 7.0 ± 0.8 | 4.9 ± 1.1 | 13.3 ± 8.3 | 6.3 ± 2.0 | ns |
| Lysine | 5.9 ± 0.7 | 5.2 ± 0.8 | 6.1 ± 1.5 | 5.2 ± 0.9 | ns |
| Sum of amino acids | 1031.8 ± 118.9 | 839.7 ± 148.5 | 1228.3 ± 401.8 | 878.8 ± 162.6 | ns |
| Compound | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|
| Aspartic acid | 53.6 ± 10.7 | 35.2 ± 5.1 | 40.7 ± 8.0 | 38.6 ± 0.7 | ns |
| Glutamic acid | 26.9 ± 9.9 | 13.8 ± 4.3 | 12.9 ± 5.9 | 13.9 ± 6.8 | ns |
| Asparagine | 9.0 ± 4.2 | 6.8 ± 3.3 | 14.3 ± 7.9 | 10.3 ± 5.1 | ns |
| Serine | 13.6 ± 1.7 | 8.8 ± 1.9 | 10.7 ± 3.1 | 10.8 ± 2.6 | ns |
| Glutamine | 3.9 ± 1.3 | 2.4 ± 0.7 | 4.4 ± 2.3 | 3.5 ± 1.3 | ns |
| Histidine | 18.9 ± 3.2 | 12.5 ± 1.5 | 16.5 ± 5.9 | 13.3 ± 2.8 | ns |
| Glycine | 26.4 ± 2.6 | 16.9 ± 3.2 | 22.5 ± 6.8 | 20.0 ± 4.6 | ns |
| Threonine | 12.7 ± 1.5 | 8.1 ± 2.1 | 10.7 ± 3.5 | 11.2 ± 3.3 | ns |
| Arginine | 44.3 ± 18.1 | 15.7 ± 3.3 | 36.3 ± 21.2 | 20.0 ± 11.8 | ns |
| Alanine | 43.8 ± 6.6 | 25.4 ± 5.9 | 41.7 ± 17.3 | 35.2 ± 9.4 | ns |
| γ-Aminobutyric acid (GABA) | 31.7 ± 2.0 | 19.7 ± 4.0 | 34.9 ± 13.1 | 27.7 ± 6.4 | ns |
| Proline | 4833.8 ± 1347.2 | 4144.6 ± 1087.3 | 4377.5 ± 1673.9 | 3892.8 ± 1207.9 | ns |
| Tyrosine | 8.1 ± 3.3 | 6.2 ± 2.5 | 7.6 ± 3.7 | 3.3 ± 2.7 | ns |
| Ammonium ion | 25.6 ± 0.8 | 20.8 ± 4.0 | 22.7 ± 4.8 | 25.1 ± 5.0 | ns |
| Valine | 31.2 ± 6.1 | 17.8 ± 3.5 | 23.8 ± 9.6 | 23.4 ± 5.5 | ns |
| Methionine | 5.9 ± 0.7 | 4.0 ± 1.3 | 5.1 ± 1.9 | 4.5 ± 1.2 | ns |
| Cysteine | 0.9 ± 0.6 | 0.8 ± 0.6 | 1.0 ± 0.6 | 0.7 ± 0.6 | ns |
| Isoleucine | 10.4 ± 1.7 | 6.3 ± 1.0 | 7.5 ± 2.3 | 7.8 ± 1.1 | ns |
| Tryptophan | 4.9 ± 2.1 | 3.8 ± 1.7 | 4.7 ± 2.2 | 3.3 ± 1.4 | ns |
| Leucine | 22.8 ± 5.9 | 14.0 ± 4.9 | 19.1 ± 7.4 | 19.1 ± 5.4 | ns |
| Phenylalanine | 13.0 ± 2.7 | 8.3 ± 2.8 | 10.9 ± 4.2 | 11.1 ± 3.2 | ns |
| Ornithine | 25.6 ± 5.8 | 22.4 ± 11.8 | 28.8 ± 13.7 | 34.9 ± 17.6 | ns |
| Lysine | 33.8 ± 3.5 | 21.3 ± 6.1 | 27.3 ± 8.9 | 26.5 ± 6.0 | ns |
| Sum of amino acids | 441.5 ± 54.1 | 270.1 ± 46.5 | 381.4 ± 126.3 | 338.9 ± 68.7 | ns |
| Compound | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|
| Methanol (mg L−1) | 171 ± 10 c | 126 ± 14 a | 136 ± 12 ab | 150 ± 13 b | ** |
| Ethyl acetate (mg L−1) | 50 ± 1 | 53 ± 8 | 51 ± 7 | 52 ± 11 | ns |
| Acetaldehyde (mg L−1) | 6 ± 3 | 14 ± 6 | 8 ± 2 | 13 ± 9 | ns |
| Higher alcohols (mg L−1) | |||||
| 1-propanol | 39 ± 6 | 37 ± 8 | 33 ± 7 | 36 ± 9 | ns |
| 2-methyl-1-propanol | 43 ± 2 | 50 ± 2 | 43 ± 2 | 50 ± 7 | ns |
| 2-methyl-1-butanol | 215 ± 10 | 235 ± 6 | 210 ± 13 | 203 ± 22 | ns |
| 3-methyl-1-butanol | 57 ± 4 | 66 ± 6 | 56 ± 6 | 54 ± 6 | ns |
| Σ Higher alcohols | 355 ± 15 | 389 ± 7 | 342 ± 18 | 344 ± 39 | ns |
| Other alcohols (mg L−1) | |||||
| 1-hexanol | 1.7 ± 0.6 | 1.8 ± 0.6 | 1.7 ± 0.6 | 1.9 ± 0.6 | ns |
| trans-3-hexen-1-ol | 0.11 ± 0.04 | 0.11 ± 0.02 | 0.09 ± 0.02 | 0.10 ± 0.04 | ns |
| cis-3-hexen-1-ol | 0.13 ± 0.00 | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.05 | ns |
| Benzyl alcohol | 3.91 ± 0.26 | 3.89 ± 0.63 | 3.79 ± 0.51 | 3.53 ± 0.63 | ns |
| 2-phenylethanol | 25 ± 8 | 34 ± 6 | 24 ± 3 | 23 ± 4 | ns |
| Other compounds (mg L−1) | |||||
| Ethyl lactate | 31 ± 5 | 26 ± 9 | 33 ± 14 | 29 ± 10 | ns |
| Acetoine | 12 ± 6 | 13 ± 9 | 10 ± 3 | 11 ± 3 | ns |
| Acetol | 38 ± 4 | 34 ± 7 | 31 ± 5 | 37 ± 8 | ns |
| 2,3-butanediol levo | 875 ± 126 | 806 ± 67 | 687 ± 57 | 688 ± 112 | ns |
| 2,3-butanediol meso | 293 ± 94 | 269 ± 71 | 224 ± 67 | 227 ± 76 | ns |
| Methionol | 1.23 ± 0.22 | 1.02 ± 0.11 | 1.08 ± 0.31 | 1.23 ± 0.42 | ns |
| γ-butyrolactone | 58 ± 16 | 50 ± 19 | 47 ± 15 | 50 ± 10 | ns |
| Acetates of higher alcohols (mg L−1) | |||||
| Isoamyl acetate | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.2 | ns |
| 2-phenylethyl acetate | 0.25 ± 0.08 | 0.26 ± 0.09 | 0.22 ± 0.07 | 0.23 ± 0.08 | ns |
| Σ Acetates of higher alcohols | 0.85 ± 0.12 | 0.86 ± 0.14 | 0.72 ± 0.08 | 0.78 ± 0.23 | ns |
| Esters (mg L−1) | |||||
| Ethyl butyrate | 0.09 ± 0.03 | 0.12 ± 0.05 | 0.15 ± 0.05 | 0.12 ± 0.06 | ns |
| Ethyl hexanoate | 0.11 ± 0.04 | 0.15 ± 0.09 | 0.19 ± 0.07 | 0.18 ± 0.08 | ns |
| Ethyl octanoate | 0.15 ± 0.05 | 0.18 ± 0.09 | 0.20 ± 0.10 | 0.21 ± 0.09 | ns |
| Ethyl decanoate | 0.03 ± 0.02 | 0.05 ± 0.03 | 0.26 ± 0.20 | 0.03 ± 0.02 | ns |
| Σ Ethyl esters C6-C10 | 0.38 ± 0.13 | 0.48 ± 0.26 | 0.71 ± 0.36 | 0.54 ± 0.25 | ns |
| Volatile fatty acids (mg L−1) | |||||
| Isobutyric acid | 1.77 ± 0.23 | 2.59 ± 0.56 | 1.91 ± 0.27 | 2.73 ± 1.13 | ns |
| Butyric acid | 1.41 ± 0.23 | 1.60 ± 0.39 | 1.52 ± 0.35 | 1.41 ± 0.53 | ns |
| Isovaleric acid | 1.04 ± 0.14 | 1.40 ± 0.16 | 1.09 ± 0.13 | 1.37 ± 0.50 | ns |
| Σ Volatile fatty acids C4-C5 | 4.22 ± 0.56 | 5.60 ± 1.11 | 4.52 ± 0.72 | 5.51 ± 1.56 | ns |
| Hexanoic acid | 2.06 ± 0.43 | 2.46 ± 0.58 | 2.34 ± 0.42 | 2.15 ± 0.78 | ns |
| Octanoic acid | 1.55 ± 0.36 | 1.67 ± 0.36 | 1.80 ± 0.33 | 1.43 ± 0.50 | ns |
| Decanoic acid | 0.31 ± 0.06 | 0.33 ± 0.07 | 0.32 ± 0.10 | 0.27 ± 0.10 | ns |
| Σ Volatile fatty acids C6-C10 | 3.93 ± 0.84 | 4.46 ± 1.01 | 4.46 ± 0.82 | 3.85 ± 0.67 | ns |
| Free terpenes (μg L−1) | |||||
| trans-linalool oxide (furan) a | 5.8 ± 0.4 | 5.6 ± 0.9 | 6.5 ± 1.6 | 4.8 ± 1.9 | ns |
| cis-linalool oxide (furan) a | 1.9 ± 0.8 | 0.8 ± 0.3 | 1.1 ± 0.4 | 0.9 ± 0.3 | ns |
| trans-linalool oxide (pyran) a | 3.6 ± 0.1 b | 3.3 ± 0.4 ab | 1.9 ± 0.4 a | 2.5 ± 0.9 ab | * |
| cis-linalool oxide (pyran) a | 1.4 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.3 | 0.7 ± 0.4 | ns |
| Linalool (L) | 3.8 ± 1.3 | 4.5 ± 1.4 | 6.4 ± 2.1 | 3.9 ± 1.5 | ns |
| Hotrienol (3,7-dimethyl-1,5,7-octatriene-3-ol) a | 5.3 ± 4.1 | 3.9 ± 2.9 | 2.7 ± 1.5 | 6.3 ± 2.7 | ns |
| α-terpineol (αT) | 3.9 ± 0.6 | 3.0 ± 0.6 | 4.0 ± 0.3 | 4.7 ± 1.6 | ns |
| Citronellol (C) | 16.6 ± 0.8 | 17.8 ± 0.1 | 14.9 ± 3.0 | 20.0 ± 7.2 | ns |
| Nerol (N) | 17.2 ± 5.8 | 10.8 ± 4.1 | 19.4 ± 6.5 | 17.3 ± 6.0 | ns |
| Geraniol (G) | 30.7 ± 3.3 | 34.0 ± 1.5 | 36.1 ± 8.0 | 32.9 ± 11.0 | ns |
| Σ Free terpenes (L + αT + C + N + G) | 83.8 ± 13.8 | 80.9 ± 6.9 | 86.8 ± 11.1 | 94.0 ± 31.4 | ns |
| Hodiol I (trans-3,7-dimethyl-1,5-octadiene-3,7-diol) a | 4.9 ± 2.0 | 4.9 ± 2.5 | 4.2 ± 2.1 | 3.2 ± 1.3 | ns |
| 2,7-dimethyloctane-4,5-diol a | 119.9 ± 9.7 | 112.0 ± 5.0 | 110.3 ± 18.5 | 99.5 ± 38.2 | ns |
| Compound | Odor Threshold (μg L−1) | Descriptor | Soil Tillage | Native Vegetation | English Ryegrass | Subterranean Clover | Statistical Significance |
|---|---|---|---|---|---|---|---|
| Ethyl acetate | 7500 | Pineapple | 6.6 ± 0.1 | 7.0 ± 1.0 | 6.8 ± 1.0 | 7.0 ± 1.4 | ns |
| Acetaldehyde | 500 | Fruity | 13.0 ± 5.5 | 27.4 ± 12.8 | 16.0 ± 4.2 | 25.4 ± 17.6 | ns |
| Higher alcohols | |||||||
| 1-propanol | 750 | Alcohol | 52.2 ± 7.5 | 49.6 ± 11.2 | 43.9 ± 9.8 | 48.2 ± 11.3 | ns |
| 2-methyl-1-propanol | 40,000 | Alcohol | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.0 | 1.3 ± 0.2 | ns |
| Other alcohols | |||||||
| Benzyl alcohol | 620 | Blackberry | 6.3 ± 0.4 | 6.3 ± 1.0 | 6.1 ± 0.8 | 5.7 ± 1.0 | ns |
| 2-phenylethanol | 14,000 | Rose | 1.8 ± 0.1 | 2.4 ± 0.4 | 1.7 ± 0.2 | 1.7 ± 0.3 | ns |
| Other compounds | |||||||
| Acetoine | 10,000 | Butter, almond | 1.2 ± 0.6 | 1.3 ± 0.9 | 1.0 ± 0.3 | 1.1 ± 0.3 | ns |
| Acetates of higher alcohols | |||||||
| Isoamyl acetate | 30 | Banana | 19.1 ± 3.9 | 19.6 ± 4.2 | 16.6 ± 1.7 | 18.4 ± 1.6 | ns |
| 2-phenylethyl acetate | 250 | Rose, honey | 1.0 ± 0.0 | 1.1 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | ns |
| Esters | |||||||
| Ethyl butyrate | 20 | Fruity | 4.7 ± 1.3 | 5.9 ± 2.6 | 7.3 ± 2.3 | 6.2 ± 2.7 | ns |
| Ethyl hexanoate | 14 | Fruity | 7.5 ± 3.2 | 10.4 ± 6.7 | 13.8 ± 4.8 | 12.7 ± 5.9 | ns |
| Ethyl octanoate | 5 | Fruity | 30.7 ± 9.8 | 36.9 ± 17.9 | 40.7 ± 19.1 | 41.3 ± 18.5 | ns |
| Volatile fatty acids | |||||||
| Isobutyric acid | 2300 | Cheese | 0.8 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.4 | ns |
| Butyric acid | 173 | Cheese | 8.1 ± 1.3 | 9.2 ± 2.3 | 8.8 ± 2.0 | 8.1 ± 2.0 | ns |
| Isovaleric acid | 33 | Cheese | 31.7 ± 4.1 | 42.5 ± 4.8 | 32.9 ± 3.9 | 41.4 ± 8.3 | ns |
| Hexanoic acid | 3000 | Cheese | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.1 | ns |
| Octanoic acid | 500 | Rancid | 3.1 ± 0.7 | 3.3 ± 0.7 | 3.6 ± 0.7 | 2.9 ± 0.4 | ns |
| Free terpenes | |||||||
| Geraniol | 30 | Flower | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.1 | ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E.; Mirás-Avalos, J.M. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages 2018, 4, 76. https://doi.org/10.3390/beverages4040076
Bouzas-Cid Y, Trigo-Córdoba E, Orriols I, Falqué E, Mirás-Avalos JM. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages. 2018; 4(4):76. https://doi.org/10.3390/beverages4040076
Chicago/Turabian StyleBouzas-Cid, Yolanda, Emiliano Trigo-Córdoba, Ignacio Orriols, Elena Falqué, and José Manuel Mirás-Avalos. 2018. "Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region" Beverages 4, no. 4: 76. https://doi.org/10.3390/beverages4040076
APA StyleBouzas-Cid, Y., Trigo-Córdoba, E., Orriols, I., Falqué, E., & Mirás-Avalos, J. M. (2018). Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages, 4(4), 76. https://doi.org/10.3390/beverages4040076







