Functional Non-Alcoholic Beer Fermented with Potential Probiotic Yeasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of Yeast Inoculum
2.3. Saccharide Fermentation
2.4. β-Glucosidase Activity
2.5. Phenolic Off-Flavour (POF) Phenotype
2.6. Tolerances of Different Conditions
2.7. Fermentation and Maturation
2.8. Beer Analyses
2.8.1. Basic Beer Parameters
2.8.2. Organic Compound Analysis by HPLC-RID-DAD
2.8.3. Volatile Organic Compound Analysis by HS-SPME-GC-MS
3. Results and Discussion
3.1. Yeast Characterisation
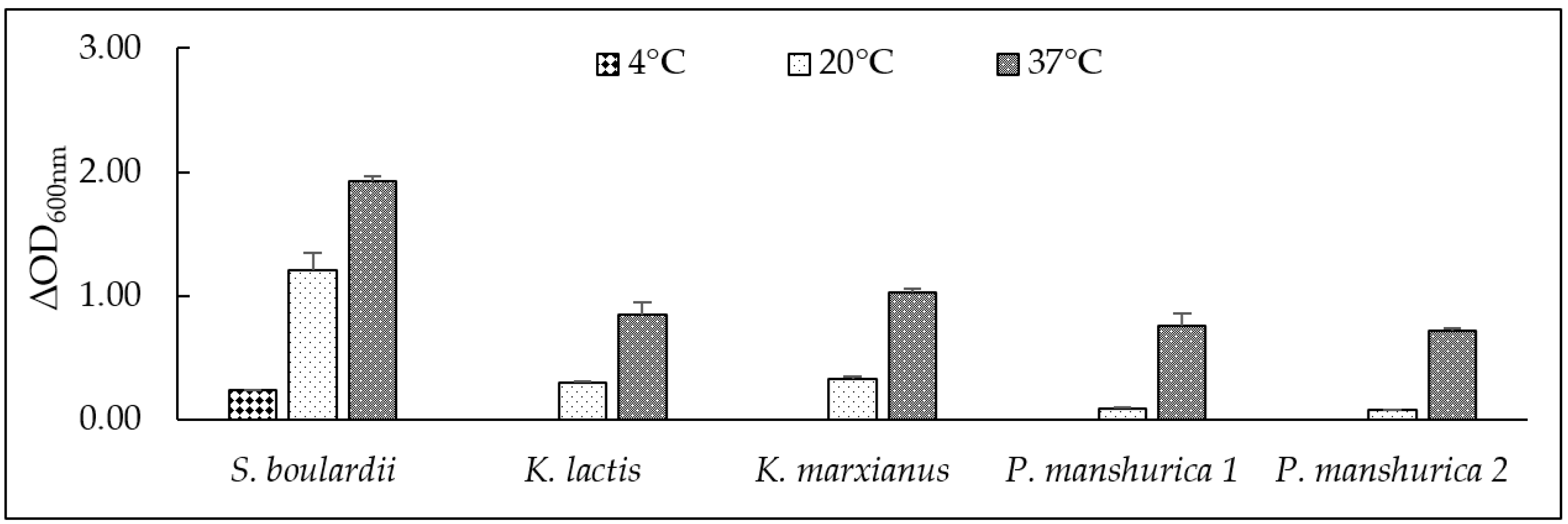
3.2. Tolerance at Different Temperatures
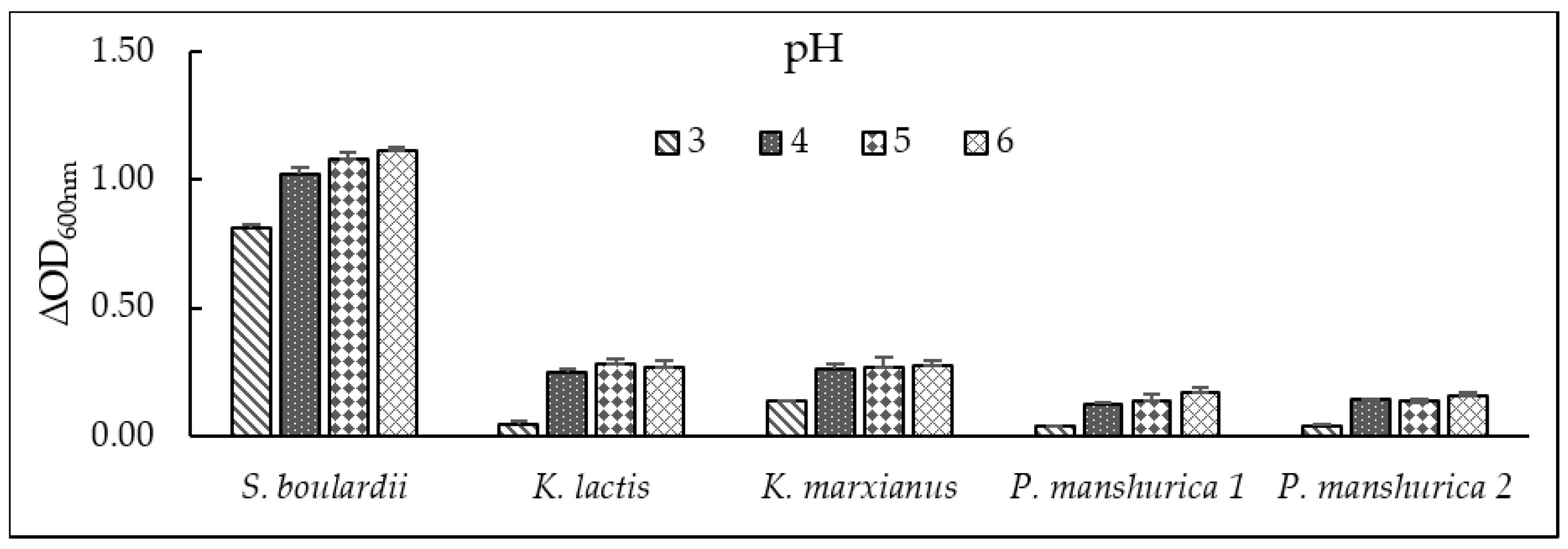
3.3. pH Tolerance
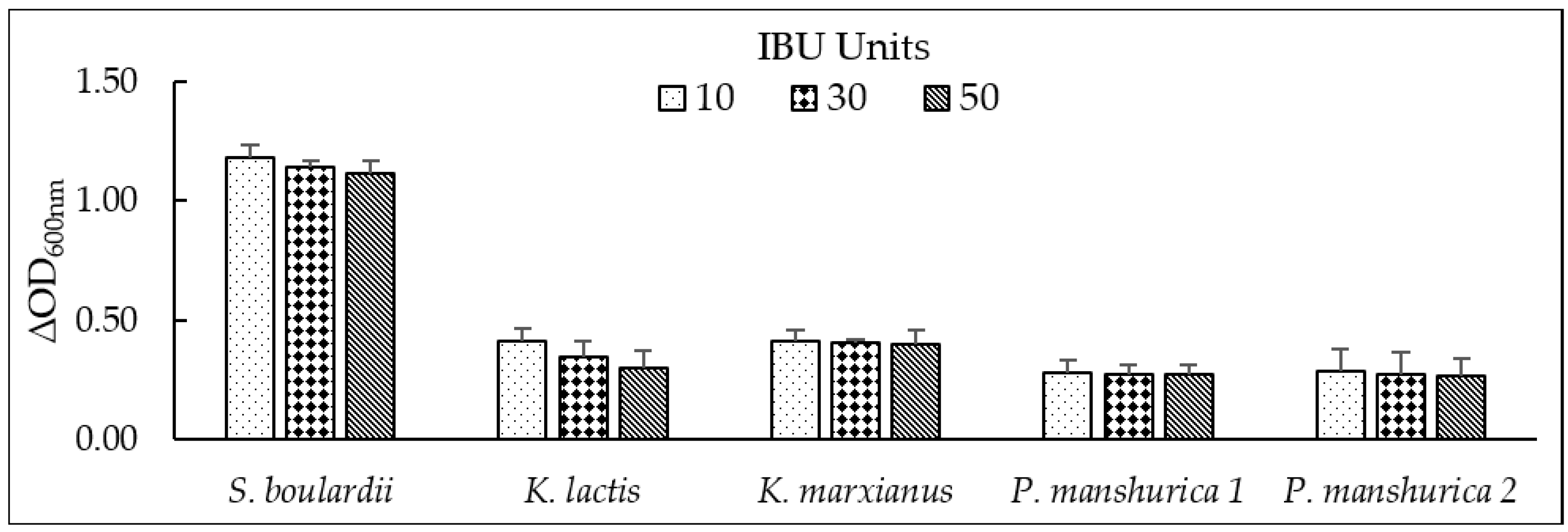
3.4. Tolerance to Iso-α-Bitter Acids
3.5. Basic Beer Parameter Analysis
3.6. Organic Compound HPLC Analysis
3.7. Volatile Organic Compound HS-SPME-GC-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, M.Z.A.; Toh, M.; Liu, S.Q. Beer with Probiotics and Prebiotics. In Probiotics and Prebiotics in Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–199. [Google Scholar]
- Statista. 2024. Available online: https://www.statista.com/outlook/cmo/alcoholic-drinks/beer/non-alcoholic-beer/worldwide (accessed on 30 April 2025).
- Habschied, K.; Živković, A.; Krstanović, V.; Mastanjević, K. Functional Beer—A Review on Possibilities. Beverages 2020, 6, 51. [Google Scholar] [CrossRef]
- Polaris Market Search. 2024. Available online: https://www.polarismarketresearch.com/industry-analysis/probiotics-market (accessed on 30 April 2025).
- Hinojosa-Avila, C.R.; García-Gamboa, R.; Chedraui-Urrea, J.J.T.; García-Cayuela, T. Exploring the potential of probiotic-enriched beer: Microorganisms, fermentation strategies, sensory attributes, and health implications. Food Res. Int. 2024, 175, 113717. [Google Scholar] [CrossRef]
- Johansson, L.; Nikulin, J.; Juvonen, R.; Krogerus, K.; Magalhães, F.; Mikkelson, A.; Nuppunen-Puputti, M.; Sohlberg, E.; de Francesco, G.; Perretti, G.; et al. Sourdough cultures as reservoirs of maltose-negative yeasts for low-alcohol beer brewing. Food Microbiol. 2021, 94, 103629. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. Defining No and Low (NoLo) Alcohol Products. Nutrients 2022, 14, 3873. [Google Scholar] [CrossRef]
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida e Silva, J.B. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; Atzler, J.J.; Hoehnel, A.; Lynch, K.M.; Arendt, E.K. Screening and Application of Cyberlindnera Yeasts to Produce a Fruity, Non-Alcoholic Beer. Fermentation 2019, 5, 103. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.J.; Steven Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications: Non-conventional yeast for beer fermentation. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Vaštík, P.; Rosenbergová, Z.; Furdíková, K.; Klempová, T.; Šišmiš, M.; Šmogrovičová, D. Potential of non-Saccharomyces yeast to produce non-alcoholic beer. FEMS Yeast Res. 2022, 22, foac039. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Senkarcinova, B.; Graça Dias, I.A.; Nespor, J.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). 2024. Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional (accessed on 30 April 2025).
- Del Valle, J.C.; Bonadero, M.C.; Fernández-Gimenez, A.V. Saccharomyces cerevisiae as probiotic, prebiotic, synbiotic, postbiotics and parabiotics in aquaculture: An overview. Aquaculture 2023, 569, 739342. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Shahryari, S.; Kharazmi, S.M.; Jafari, S.M. Food applications of probiotic yeasts; focusing on their techno-functional, postbiotic and protective capabilities. Trends Food Sci. Technol. 2022, 128, 278–295. [Google Scholar] [CrossRef]
- Koirala, S.; Anal, K. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Pereira De Paula, B.; De Souza Lago, H.; Firmino, L.; Wilson, J.F.L.J.; Mariana, F.D.C.; André, F.G.; Karen, S.P.; Maria, A.Z.C. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Fukuhara, H. Kluyveromyces lactis—A retrospective. FEMS Yeast Res. 2006, 6, 323–324. [Google Scholar]
- Varela, A.J.; Puricelli, M.; Ortiz-Merino, A.R.; Giacomobono, R.; Braun-Galleani, S.; Wolfe, H.K.; Morrissey, J.P. Origin of Lactose Fermentation in Kluyveromyces lactis by Interspecies Transfer of a Neo-functionalized Gene Cluster during Domestication. Curr. Biol. 2019, 29, 4284–4290. [Google Scholar] [CrossRef]
- González Siso, M.I.; Ramil, E.; Cerdán, M.E.; Freire-Picos, M.A. Respirofermentative metabolism in Kluyveromyces lactis: Ethanol production and the Crabtree effect. Enzym. Microb. Technol. 1996, 18, 585–591. [Google Scholar] [CrossRef]
- Bilal, M.; Ji, L.; Xu, Y.; Xu, S.; Lin, Y.; Iqbal, H.M.N.; Cheng, H. 2022. Bioprospecting Kluyveromyces marxianus as a Robust Host for Industrial Biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 851768. [Google Scholar]
- Toyotome, T.; Yamamoto, M.; Horie, M. Draft Genome Sequence of the Yeast Pichia manshurica YM63, a Participant in Secondary Fermentation of Ishizuchi-Kurocha, a Japanese Fermented Tea. Microbiol. Resour. Announc. 2019, 8, e00528-19. [Google Scholar]
- Saber, A.; Yari Khosroushahi, A.; Faghfoori, Z.; Seyyedi, M.; Alipour, B. Molecular identification and probiotic characterization of isolated yeasts from Iranian traditional dairies. Prog. Nutr. 2019, 21, 445–457. [Google Scholar]
- Zhang, Q.; Huo, N.; Wang, Y.; Zhang, Y.; Wang, R.; Hou, H. Aroma-enhancing role of Pichia manshurica isolated from Daqu in the brewing of Shanxi Aged Vinegar. Int. J. Food Prop. 2017, 20, 2169–2179. [Google Scholar] [CrossRef]
- Vaštík, P.; Sulo, P.; Rosenbergová, Z.; Klempová, T.; Dostálek, P.; Šmogrovičová, D. Novel Saccharomyces cerevisiae × Saccharomyces mikatae Hybrids for Non-alcoholic Beer Production. Fermentation 2023, 9, 221. [Google Scholar] [CrossRef]
- Karaoglan, S.Y.; Jung, R.; Gauthier, M.; Kinčl, T.; Dostálek, P. Maltose-Negative Yeast in Non-Alcoholic and Low-Alcoholic Beer Production. Fermentation 2022, 8, 273. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444521491. [Google Scholar]
- Gao, P.; Peng, S.; Sam, F.E.; Zhu, Y.; Liang, L.; Li, M.; Wang, J. Indigenous Non-Saccharomyces Yeasts With β-Glucosidase Activity in Sequential Fermentation with Saccharomyces cerevisiae: A Strategy to Improve the Volatile Composition and Sensory Characteristics of Wines. Front. Microbiol. 2022, 13, 845837. [Google Scholar] [CrossRef]
- Mertens, S.; Steensels, J.; Gallone, B.; Souffriau, B.; Malcorps, P.; Verstreppen, K.J. Rapid Screening Method for Phenolic Off-Flavor (POF) Production in Yeast. J. Am. Soc. Brew. Chem. 2017, 75, 318–323. [Google Scholar] [CrossRef]
- Montini, N.; Doughty, T.W.; Domenzain, I.; Fenton, D.A.; Baranov, P.V.; Harrington, R.; Nielsen, J.; Siewers, V.; Morrissey, J.P. Identification of a novel gene required for competitive growth at high temperature in the thermotolerant yeast Kluyveromyces marxianus. Microbiology 2022, 168, 001148. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and Growth Characterization of a Novel Strain of Saccharomyces boulardii Isolated from Soya Paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef]
- Gilliland, R.B. Determination of yeast viability. J. Inst. Brew. 1959, 65, 424–429. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Żyła, K.; Tuszyński, T. Optimization of Fermentation Parameters in a Brewery: Modulation of Yeast Growth and Yeast Cell Viability. Processes 2025, 13, 906. [Google Scholar] [CrossRef]
- Moradi, R.; Nosrati, R.; Zare, H.; Tahmasebi, T.; Saderi, H.; Owlia, P. Screening and characterization of in-vitro probiotic criteria of Saccharomyces and Kluyveromyces strains. Iran. J. Microbiol. 2018, 10, 123–131. [Google Scholar]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing Science and Practice; Woodhead Publishing Limited: Cambridge, UK, 2004. [Google Scholar]
- Yang, X.; Wang, Z.; Weizhe, S.; Yingjia, L.; Meizi, P.; Yang, D. Characterization and formation mechanisms of viable, but putatively non-culturable brewer’s yeast induced by isomerized hop extract. LWT 2022, 155, 112974. [Google Scholar]
- Michel, M.; Cocuzza, S.; Biendl, M.; Peifer, F.; Hans, S.; Methner, Y.; Pehl, F.; Back, W.; Jacob, F.; Hutzler, M. The impact of different hop compounds on the growth of selected beer spoilage bacteria in beer. J. Inst. Brew. 2020, 126, 354–361. [Google Scholar]
- Hazelwood, L.A.; Walsh, M.C.; Pronk, J.T.; Daran, J.M. Involvement of Vacuolar Sequestration and Active Transport in Tolerance of Saccharomyces cerevisiae to Hop Iso-α-Acids. Appl. Environ. Microbiol. 2010, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Decree 2014. No 30/2014 of the Ministry of Agriculture and Rural Development of the Slovak Republic of 31 January 2014 on Requirements for Beverages. Available online: https://www.slov-lex.sk/ezbierky/pravne-predpisy/SK/ZZ/2014/30/ (accessed on 30 April 2025).
- Díaz, A.B.; Durán-Guerrero, E.; Valiente, S.; Castro, R.; Lasanta, C. Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast. Foods 2023, 12, 2912. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K. The Effect of Beer pH on Colloidal Stability and Stabilization—A Review and Recent Findings. Tech. Q. Master Brew. Assoc. Am. 2010, 47, 1–5. [Google Scholar] [CrossRef]
- Basařová, G.; Šavel, J.; Basař, P.; Lejsek, T. Pivovarství: Teorie a Praxe Výroby Piva; VŠCHT: Prague, Czech Republic, 2010; pp. 1–863. ISBN 978-80-7080-734-7. [Google Scholar]
- Van Landschoot, A. Saccharides and sweeteners in beer. Cerevisia 2009, 34, 19–25. [Google Scholar]
- Fukuhara, H. The Kluyver effect revisited. FEMS Yeast Res. 2003, 3, 327–331. [Google Scholar] [CrossRef]
- Merico, A.; Galafassi, S.; Piskur, J.; Compagno, C. The oxygen level determines the fermentation pattern in Kluyveromyces lactis. FEMS Yeast Res. 2009, 9, 749–756. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Liu, F. Changes in Organic Acids during Beer Fermentation. J. Am. Soc. Brew. Chem. 2015, 73, 275–279. [Google Scholar] [CrossRef]
- de Carvalho, B.T.; Subotić, A.; Vandecruys, P.; Deleu, S.; Vermeire, S.; Thevelein, J.M. Enhancing probiotic impact: Engineering Saccharomyces boulardii for optimal acetic acid production and gastric passage tolerance. Appl. Environ. Microbiol. 2024, 90, e0032524. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, A.; De Vuyst, L. Acetic Acid Bacteria in Sour Beer Production: Friend or Foe? Front. Microbiol. 2022, 13, 957167. [Google Scholar] [CrossRef] [PubMed]
- Van Oevelen, D.; Delescaille, F.; Verachtert, H. Synthesis of aroma components during spontaneous fermentation of lambic and gueuze. J. Inst. Brew. 1976, 82, 322–326. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Kodama, Y.; Omura, F.; Miyajima, K.; Toshihiko, A. Control of Higher Alcohol Production by Manipulation of the BAP2 Gene in Brewing Yeast. J. Am. Soc. Brew. Chem. 2001, 59, 157–162. [Google Scholar]
- Landaud, S.; Latrille, E.; Corrieu, G. Top pressure and temperature control the fusel alcohol/ester ratio through yeast growth in beer fermentation. J. Inst. Brew. 2001, 107, 107–117. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar]
- Bennis, N.X.; Bieseman, J.; Daran, J.M.G. Unlocking lager’s flavour palette by metabolic engineering of Saccharomyces pastorianus for enhanced ethyl ester production. Metab. Eng. 2024, 85, 180–193. [Google Scholar] [PubMed]
- Nešpor, J.; Andrés-Iglesias, C.; Karabin, M.; Montero, O.; Blanco, C.; Dostálek, P. Volatile Compound Profiling in Czech and Spanish Lager Beers in Relation to Used Production Technology. Food Anal. Methods 2019, 12, 2293–2305. [Google Scholar] [CrossRef]
- Arellano-Plaza, M.; Noriega-Cisneros, R.; Clemente-Guerrero, M.; González-Hernández, J.C.; Robles-Herrera, P.D.; Manzo-Ávalos, S.; Saavedra-Molina, A.; Gschaedler-Mathis, A. Fermentative capacity of Kluyveromyces marxianus and Saccharomyces cerevisiae after oxidative stress. J. Inst. Brew. 2017, 123, 519–526. [Google Scholar] [CrossRef]
| Yeast | Abbreviation | Characterisation |
|---|---|---|
| Pichia manshurica 1 CCY * 039-063-001 | PM1 | Potential probiotic strain |
| Pichia manshurica 2 CCY * 039-063-004 | PM2 | Potential probiotic strain |
| Kluyveromyces lactis CCY * 026-012-002 | KL | Potential probiotic strain |
| Kluyveromyces marxianus CCY * 029-008-010 | KM | Potential probiotic strain |
| Saccharomyces cerevisiae var. boulardii HANSEN CBS ** 5926 (syn. S. boulardii) | SBL | Control probiotic strain |
| Yeast (Abbreviation) | * Saccharide Fermentation | ** β-Glucosidase Activity | *** POF Phenotype | ||
|---|---|---|---|---|---|
| Glucose | Maltose | Lactose | |||
| Saccharomyces boulardii (SBL) | + | + | − | positive | POF+ |
| Pichia manshurica 1(PM1) | + | − | − | w/d | POF+ |
| Pichia manshurica 2 (PM2) | + | − | − | w/d | POF+ |
| Kluyveromyces lactis (KL) | + | − | + | positive | POF+ |
| Kluyveromyces marxianus (KM) | + | − | + | positive | POF+ |
| Yeast | S. boulardii (SBL) | P. manshurica 1 (PM1) | P. manshurica 2 (PM2) | K. lactis (KL) | K. marxianus (KM) |
|---|---|---|---|---|---|
| Viability at 4 °C | 83% | 27% | 30% | 35% | 37% |
| Viability at 20 °C | 97% | 82% | 83% | 80% | 85% |
| Viability at 37 °C | 98% | 97% | 98% | 96% | 96% |
| Yeast | S. boulardii (SBL) | P. manshurica 1 (PM1) | P. manshurica 2 (PM2) | K. lactis (KL) | K. marxianus (KM) |
|---|---|---|---|---|---|
| Viability at pH 3 | 69% | 17% | 16% | 21% | 18% |
| Viability at pH 6 | 97% | 84% | 86% | 83% | 87% |
| Sample | ||||||
|---|---|---|---|---|---|---|
| Basic Parameters | 8 °P Wort | SBL | PM1 | PM2 | KL | KM |
| Ethanol % (v/v) | n.d. | 1.52 ± 0.06 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.02 | 0.14 ± 0.01 |
| pH | 6.00 ± 0.06 | 4.83 ± 0.02 | 5.80 ± 0.01 | 5.73 ± 0.02 | 5.41 ± 0.01 | 5.36 ± 0.03 |
| Sample | ||||||
|---|---|---|---|---|---|---|
| Compound (g·L−1) | 8 °P Wort | SBL | PM1 | PM2 | KL | KM |
| Glucose | 7.3 ± 0.2 | n.d. | 5.3 ± 0.2 | 5.4 ± 0.1 | 5.4 ± 0.1 | 5.4 ± 0.1 |
| Maltose | 36.8 ± 0.5 | 24.1 ± 0.3 | 35.5 ± 0.3 | 35.1 ± 0.3 | 34.3 ± 0.3 | 35.5 ± 0.6 |
| Glycerol | n.d. | 1.2 ± 0.0 | 0.3 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Citric acid | n.d. | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Acetic acid | n.d. | 0.1 ± 0.0 | n.d. | n.d. | n.d. | n.d. |
| Lactic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Beer Sample | |||||
|---|---|---|---|---|---|
| Compound (μg·L−1) | SBL | PM1 | PM2 | KL | KM |
| Ethyl acetate | 530.5 ± 85.8 | 18.6 ± 6.7 | 24.4 ± 7.9 | 212.0 ± 6.3 | 373.8 ± 54.1 |
| 2-Phenylethyl acetate | 73.1 ± 18.4 | n.d. | n.d. | 358.1 ± 7.1 | 166.7 ± 11.2 |
| 3-Methylbutyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Methyl-1-propanol | 981.4 ± 199 | 160.6 ± 26.5 | 983.5 ± 87.2 | 371.1 ± 92.7 | 212.4 ± 46.8 |
| 2-Methyl-1-butanol | 2867.3 ± 66.7 | 363.5 ± 35.7 | 828.2 ± 13.1 | 803.5 ± 42.4 | 488.2 ± 50.0 |
| 3-Methyl-1-butanol | 6125.8 ± 21.5 | 615.0 ± 57.1 | 1195.5 ± 38.5 | 992.8 ± 20.2 | 775.2 ± 48.9 |
| 2-Phenylethanol | 7955.3 ± 163.1 | 1730.0 ± 220.4 | 2377.2 ± 78.9 | 1372.9 ± 93.2 | 1197.5 ± 112.1 |
| Ethyl hexanoate | 274.2 ± 21.6 | 162.9 ± 18.9 | 146.0 ± 7.6 | 139.0 ± 3.0 | 149.4 ± 7.6 |
| Ethyl octanoate | 307.5 ± 44.3 | n.d. | n.d. | n.d. | n.d. |
| Ethyl decanoate | 366.1 ± 91.6 | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid | 3855.8 ± 488.0 | 332.4 ± 30.3 | 304.3 ± 51.6 | 239.1 ± 15.0 | 274.7 ± 16.2 |
| Octanoic acid | 2736.1 ± 484.2 | 827.3 ± 140.6 | 524.4 ± 50.7 | 381.9 ± 23.8 | 448.1 ± 15.2 |
| Decanoic acid | 1420.8 ± 273.5 | n.d. | n.d. | n.d. | n.d. |
| 4-Vinylguaiacol | 3033.9 ± 48.3 | 650.11 ± 46.4 | 628.10 ± 36.6 | 629.65 ± 44.7 | 681.30 ± 50.1 |
| Butane-2,3-dione | n.d. | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaštík, P.; Brunner, J.; Jung, R.; Klempová, T.; Furdíková, K.; Šmogrovičová, D.; Dostálek, P. Functional Non-Alcoholic Beer Fermented with Potential Probiotic Yeasts. Beverages 2025, 11, 140. https://doi.org/10.3390/beverages11050140
Vaštík P, Brunner J, Jung R, Klempová T, Furdíková K, Šmogrovičová D, Dostálek P. Functional Non-Alcoholic Beer Fermented with Potential Probiotic Yeasts. Beverages. 2025; 11(5):140. https://doi.org/10.3390/beverages11050140
Chicago/Turabian StyleVaštík, Peter, Ján Brunner, Rudolf Jung, Tatiana Klempová, Katarína Furdíková, Daniela Šmogrovičová, and Pavel Dostálek. 2025. "Functional Non-Alcoholic Beer Fermented with Potential Probiotic Yeasts" Beverages 11, no. 5: 140. https://doi.org/10.3390/beverages11050140
APA StyleVaštík, P., Brunner, J., Jung, R., Klempová, T., Furdíková, K., Šmogrovičová, D., & Dostálek, P. (2025). Functional Non-Alcoholic Beer Fermented with Potential Probiotic Yeasts. Beverages, 11(5), 140. https://doi.org/10.3390/beverages11050140








