Optimization of Some Quality Parameters of Functional Pumpkin Puree Enriched with Banana Peel Powder Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Response Surface Methodology (RSM) Design
2.3. Preparation and Thermosonication of FPP
2.4. Determination of TPC and DPPH of FPP
2.5. pH, Titratable Acidity (TA), and Color Determination
2.6. Statistical Analysis
3. Results
3.1. Process Modeling of FPP
3.2. Total Phenolic Contents of FPP
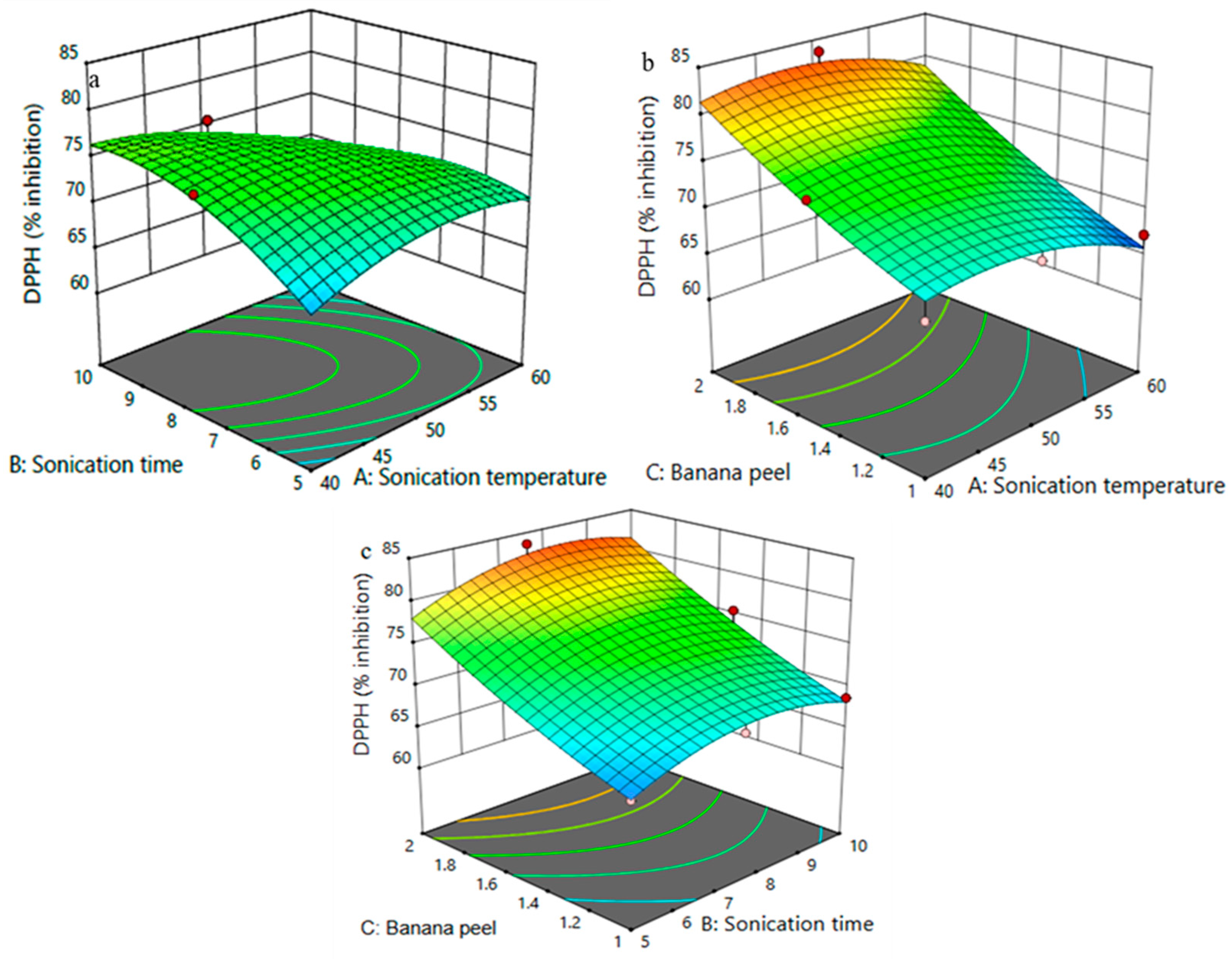
3.3. DPPH of FPP
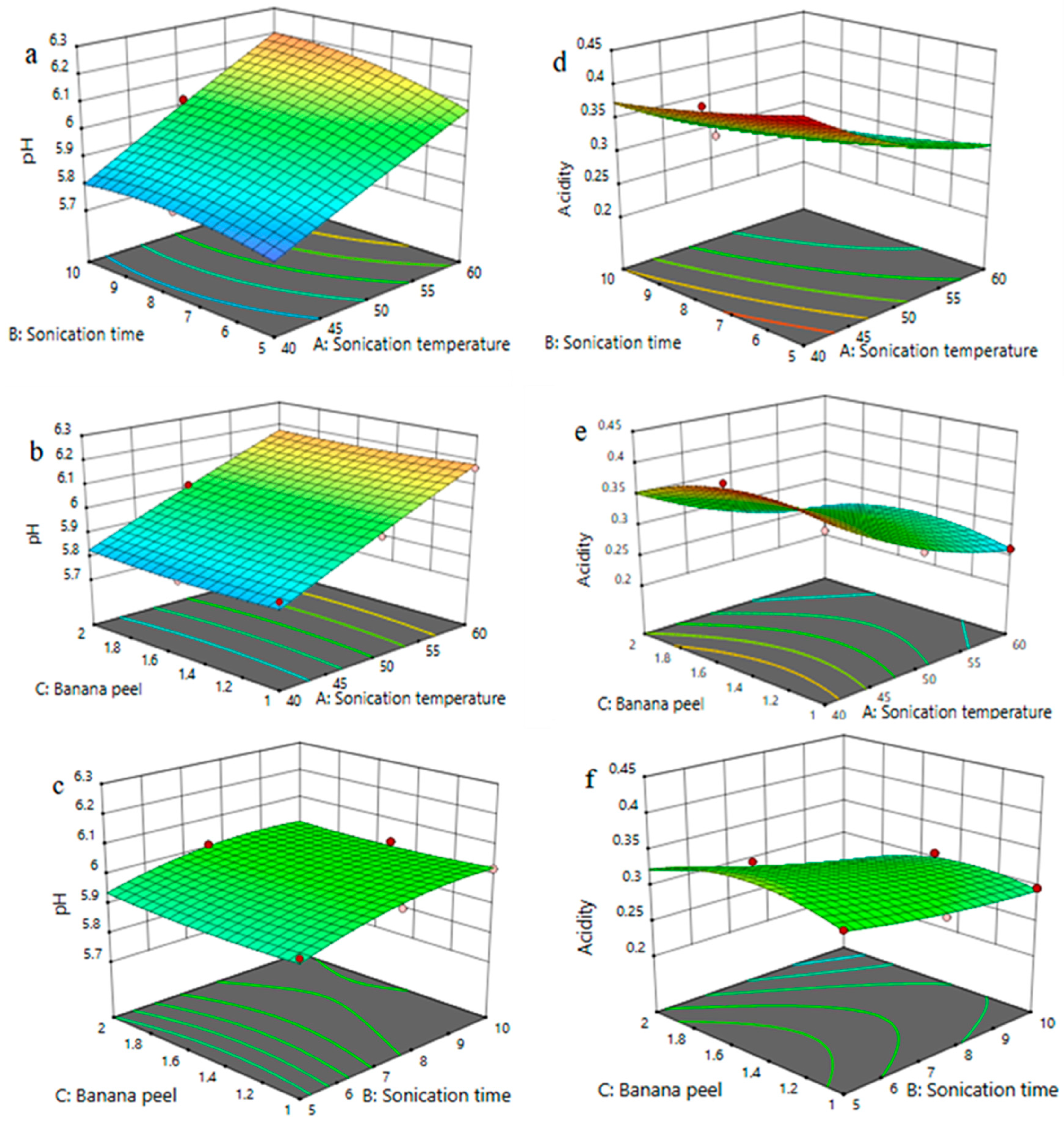
3.4. Effect of Process Variables on pH and Acidity of FPP
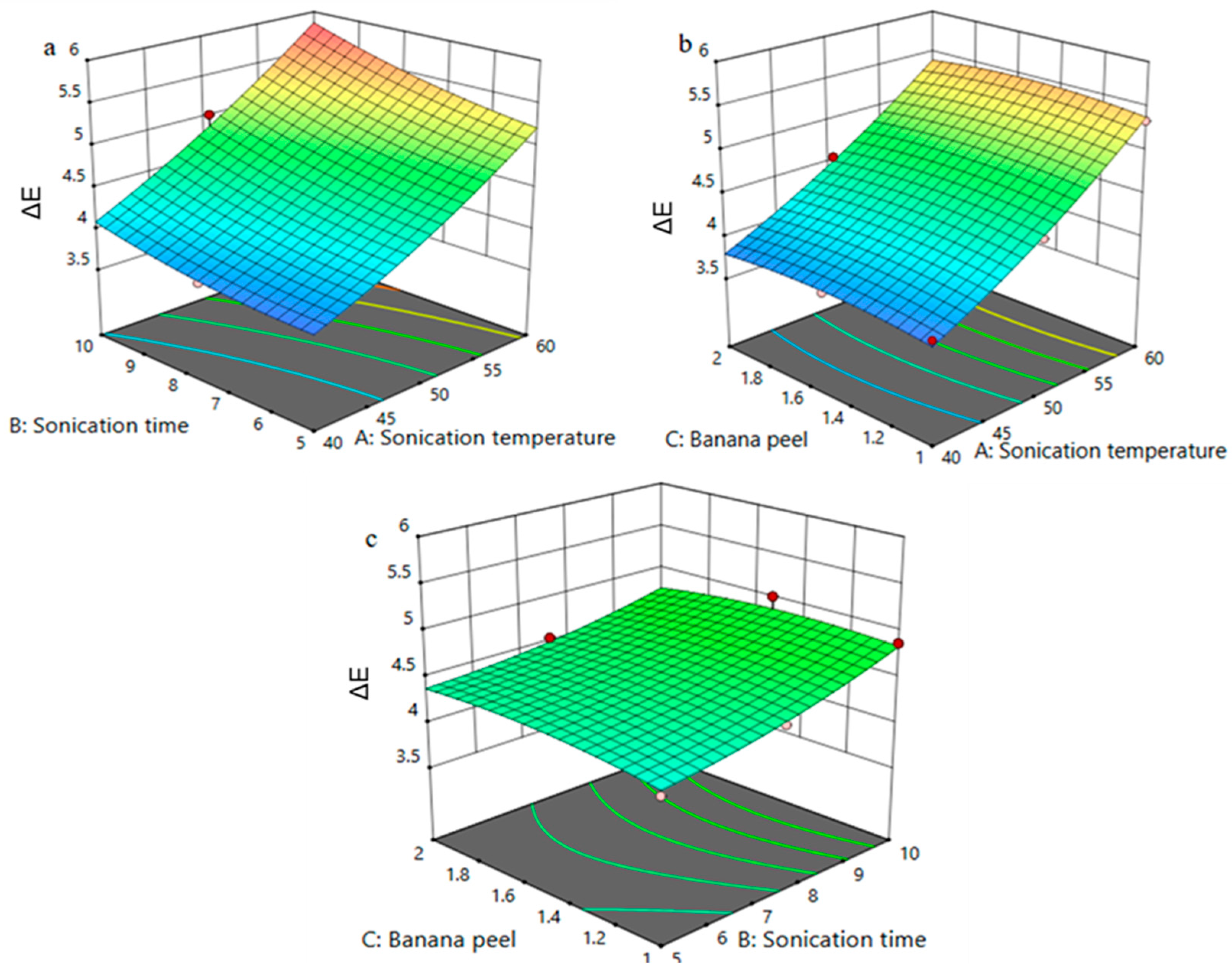
3.5. Effect of Process Variables on ΔE of FPP
3.6. Optimization of the Extraction Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit byproducts as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Olatunji, O.J.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2020, 50, 384–401. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, G.; Kehinde, B.A.; Chhikara, N.; Panghal, A.; Kaur, H. Pharmacological and biomedical uses of extracts of pumpkin and its relatives and applications in the food industry: A review. Int. J. Veg. Sci. 2020, 26, 79–95. [Google Scholar] [CrossRef]
- Asif, M.; Raza Naqvi, S.A.; Sherazi, T.A.; Ahmad, M.; Zahoor, A.F.; Shahzad, S.A.; Mahmood, N. Antioxidant, antibacterial and anti-proliferative activities of pumpkin (cucurbit) peel and puree extracts-an in vitro study. Pakistan J. Pharm. Sci. 2017, 30, 1327–1334. [Google Scholar]
- Bemfeito, C.M.; Carneiro, J.D.D.S.; Carvalho, E.E.N.; Coli, P.C.; Pereira, R.C.; Boas, E.V.D.B.V. Nutritional and functional potential of pumpkin (Cucurbita moschata) pulp and pequi (Caryocar brasiliense Camb.) peel flours. J. Food Sci. Technol. 2020, 57, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vieira, B.; Barbosa, C.; Pinheiro, R. Evaluation of mature banana peel flour on physical, chemical and texture properties of a gluten free Rissol. J. Food Proc. Pres. 2022, 46, e14441. [Google Scholar] [CrossRef]
- Pereira, A.; Maraschin, M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharm. 2015, 160, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Func. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Zafar, T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188. [Google Scholar] [CrossRef]
- Lepaus, B.M.; Valiati, B.S.; Machado, B.G.; Domingos, M.M.; Silva, M.N.; Silva, L.F.; de São José, J.F.B. Impact of ultrasound processing on the nutritional components of fruit and vegetable juices. Trends Food Sci. Technol. 2023, 138, 752–765. [Google Scholar] [CrossRef]
- da Rocha Cordeiro Dias, D.; Barros, Z.M.P.; de Carvalho, C.B.O.; Honorato, F.A.; Guerra, N.B.; Azoubel, P.M. Effect of sonication on soursop juice quality. LWT-Food Sci. Technol. 2015, 62, 883–889. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2023, 39, 3555–3577. [Google Scholar] [CrossRef]
- Juang, E.K.; De Koninck, L.H.; Vuong, K.S.; Gnanaskandan, A.; Hsiao, C.T.; Averkiou, M.A. Controlled hyperthermia with high-intensity focused ultrasound and ultrasound contrast agent microbubbles in porcine liver. Ultras. Med. Biol. 2023, 49, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.Y.; Yeon, S.J.; Hong, G.E.; Kim, J.H.; Ayush, C.T.; Lee, C.H. Antioxidant activity and quality characteristics of yogurt added green olive powder during storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.S.; Luyengi, L.; Games, E.J.C.; Mehta, R.G. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throu. Scr. 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L.U.; Shen, M.; Xu, T.; Zhan, W.; Wang, W. Extraction and purification of pumpkin polysaccharides and their hypoglycemic effect. Int. J. Biol. Macromol. 2017, 98, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Adiamo, O.Q.; Ghafoor, K.; Juhaimi, F.A.; Babiker, E.E.; Ahmed, I.A.M. Thermosonication process for optimal functional properties in carrot juice containing orange peel and pulp extracts. Food Chem. 2018, 245, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Maoto, M.M.; Jideani, A.I. Optimization of thermosonication conditions for critical quality parameters of watermelon juice using response surface methodology. Sci. Rep. 2024, 14, 13803. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Juhaimi, F.A.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Šic Žlabur, J.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of mango, apple and banana fruit peels as prebiotics and functional ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Oguntoyinbo, O.; Olumurewa, J.A.V.; Omoba, O.S. Chemical composition, dietary fiber and antioxidant activity of fermented ripe banana peel flour. J. Food Stab. 2020, 3, 27–42. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Juhaimi, F.A.; Mohamed Ahmed, I.A.; Babiker, E.E. Effects of thermosonication and orange byproducts extracts on quality attributes of carrot (Daucus carota) juice during storage. Int. J. Food Sci. Technol. 2017, 52, 2115–2125. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Lei, S.; Wu, T.; Zeng, X. Exploring the potential of thermosonication in carrot juice processing. J. Food Sci. Technol. 2015, 52, 7002–7013. [Google Scholar] [CrossRef]
- Devos, C.; Bampouli, A.; Brozzi, E.; Stefanidis, G.D.; Dusselier, M.; Van Gerven, T.; Kuhn, S. Ultrasound mechanisms and their effect on solid synthesis and processing: A review. Chem. Soc. Rev. 2024, 54, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and healthy yogurts fortified with probiotics and fruit peel powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Mahomud, M.S.; Islam, M.N.; Hossen, D.; Wazed, M.A.; Yasmin, S.; Sarker, M.S.H. Innovative probiotic yogurt: Leveraging green banana peel for enhanced quality, functionality, and sensory attributes. Heliyon 2024, 10, e38781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, L.; Liu, X.; Hasan, K.F.; Li, H.; Zhou, S.; Zhou, Y. Effect of thermosonication treatment on blueberry juice quality: Total phenolics, flavonoids, anthocyanin, and antioxidant activity. LWT-Food Sci. Technol. 2021, 150, 112021. [Google Scholar] [CrossRef]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of extraction solvent and temperature on polyphenol profiles, antioxidant and anti-inflammatory effects of red grape skin byproduct. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef] [PubMed]
- Baltacıoğlu, H. Thermosonication of peach juice: Investigation of PPO and POD activities, physicochemical and bioactive compounds changes, and development of FT-IR–based chemometric models for the evaluation of quality. Int. J. Food Sci. Technol. 2022, 57, 1688–1697. [Google Scholar] [CrossRef]
- Yildiz, G. Application of ultrasound and high-pressure homogenization against high temperature-short time in peach juice. J. Food Proc. Eng. 2019, 42, e12997. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, C.; Ma, L.; Su, W.; Jiang, J.; Hu, X. Influence of ultrasound on the microbiological, physicochemical properties, and sensory quality of different varieties of pumpkin juice. Heliyon 2024, 10, e27927. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.X.; Teh, L.K.; Tan, C.X. Effects of sonication and thermal pasteurization on the nutritional, antioxidant, and microbial properties of noni juice. Molecules 2022, 28, 313. [Google Scholar] [CrossRef] [PubMed]
- Chitgar, M.F.; Aalami, M.; Maghsoudlou, Y.; Milani, E. Comparative study on the effect of heat treatment and sonication on the quality of barberry (Berberis vulgaris) juice. J. Food Proc. Pres. 2017, 41, e12956. [Google Scholar] [CrossRef]
- Li, L.; Su, H.; Pang, L.; Pan, Y.; Li, X.; Xu, Q.; Qiao, L. Thermosonication enhanced the bioactive, antioxidant, and flavor attributes of freshly squeezed tomato juice. Ultras. Sonochem. 2025, 115, 107299. [Google Scholar] [CrossRef] [PubMed]

| Run | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | 1 | 2 | 3 | 4 | 5 | |
| Sonication Temperature | Sonication Time (min) | BPP (g/100 g) | TPC (mg/100 mL) | DPPH% | pH | Acidity (g/100 g) | Color | |
| 1 | 40 | 5 | 2 | 209.83 | 72.90 | 5.80 | 0.40 | 3.76 |
| 2 | 40 | 7.5 | 1 | 182.31 | 67.58 | 5.85 | 0.38 | 3.82 |
| 3 | 50 | 7.5 | 2 | 199.49 | 83.59 | 6.01 | 0.29 | 4.54 |
| 4 | 40 | 5 | 2 | 210.98 | 74.05 | 5.78 | 0.39 | 3.76 |
| 5 | 40 | 10 | 1 | 172.19 | 71.52 | 5.82 | 0.38 | 4.02 |
| 6 | 60 | 5 | 1 | 175.85 | 64.296 | 6.10 | 0.28 | 5.16 |
| 7 | 60 | 7.5 | 1 | 170.55 | 67.18 | 6.17 | 0.26 | 5.34 |
| 8 | 50 | 10 | 1.5 | 183.29 | 75.45 | 6.02 | 0.28 | 4.76 |
| 9 | 60 | 10 | 2 | 182.34 | 74.71 | 6.25 | 0.22 | 5.73 |
| 10 | 60 | 5 | 2 | 185.22 | 78.35 | 6.07 | 0.29 | 5.18 |
| 11 | 60 | 10 | 2 | 180.34 | 74.41 | 6.17 | 0.23 | 5.74 |
| 12 | 50 | 7.5 | 1 | 175.02 | 68.88 | 5.99 | 0.30 | 4.43 |
| 13 | 40 | 10 | 2 | 198.80 | 83.39 | 5.80 | 0.31 | 3.97 |
| 14 | 50 | 5 | 1 | 178.52 | 66.17 | 5.94 | 0.33 | 4.19 |
| 15 | 40 | 10 | 2 | 199.71 | 83.69 | 5.80 | 0.33 | 3.98 |
| 16 | 50 | 10 | 1 | 167.52 | 68.59 | 6.02 | 0.30 | 4.87 |
| 17 | 50 | 10 | 1.5 | 180.89 | 72.05 | 5.99 | 0.31 | 5.02 |
| 18 | 60 | 5 | 2 | 184.42 | 77.25 | 6.08 | 0.28 | 5.20 |
| 19 | 40 | 5 | 1 | 181.80 | 64.66 | 5.74 | 0.42 | 3.63 |
| 20 | 40 | 7.5 | 1.5 | 197.45 | 75.11 | 5.81 | 0.41 | 3.83 |
| Factors | TPC | DPPH | pH | Acidity | ΔE |
|---|---|---|---|---|---|
| Intercept | |||||
| β0 | 189.964 **** | 75.324 **** | 5.996 **** | 0.321 **** | 4.559 **** |
| Linear | |||||
| X1 (β1) | −7.521 **** | −1.555 ** | 0.177 **** | −0.057 **** | 0.805 **** |
| X2 (β2) | −4.945 **** | 1.318 * | 0.042 *** | −0.026 **** | 0.241 **** |
| X3 (β3) | 10.521 **** | 6.236 **** | −0.001 | −0.011 ** | 0.013 |
| Interaction | |||||
| X1X2 (β12) | 1.164 | −3.062 **** | 0.023 * | 0.002 | 0.079 ** |
| X1X3 (β13) | −3.353 *** | 0.363 | −0.004 | 0.007 | −0.007 |
| X2X3 (β23) | 0.802 | 0.358 | −0.006 | −0.009 * | 0.050 * |
| Quadratic | |||||
| X12 (β11) | −0.026 | −2.217 * | −0.005 | 0.019 * | 0.099 * |
| X22 (β22) | −3.420 ** | −2.670 ** | −0.032 * | 0.005 | 0.075 |
| X32 (β33) | −2.854 | 0.823 | 0.011 | −0.026 ** | −0.075 |
| Model F-value | 91.11 | 35.28 | 72.71 | 57.90 | 185.25 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean | 185.82 | 73.19 | 5.96 | 0.320 | 4.55 |
| C.V.% | 1.03 | 1.99 | 0.342 | 3.39 | 1.63 |
| Adeq. precision | 31.122 | 19.958 | 23.98 | 24.548 | 40.427 |
| R2 | 0.988 | 0.970 | 0.985 | 0.981 | 0.981 |
| Adjusted R2 | 0.977 | 0.942 | 0.971 | 0.964 | 0.989 |
| Predicted R2 | 0.934 | 0.868 | 0.941 | 0.926 | 0.978 |
| Std. Dev. | 1.910 | 1.450 | 0.026 | 0.011 | 0.074 |
| F-value (Lack of Fit) | 4.810 | 1.960 | 0.092 | 0.878 | 0.670 |
| p-value (Lack of Fit) | 0.055 | 0.239 | 0.544 | 0.555 | 0.665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhemaid, W.A.; Babiker, E.E.; Mohamed Ahmed, I.A.; Al Juhaimi, F.Y. Optimization of Some Quality Parameters of Functional Pumpkin Puree Enriched with Banana Peel Powder Using Response Surface Methodology. Beverages 2025, 11, 106. https://doi.org/10.3390/beverages11040106
Alhemaid WA, Babiker EE, Mohamed Ahmed IA, Al Juhaimi FY. Optimization of Some Quality Parameters of Functional Pumpkin Puree Enriched with Banana Peel Powder Using Response Surface Methodology. Beverages. 2025; 11(4):106. https://doi.org/10.3390/beverages11040106
Chicago/Turabian StyleAlhemaid, Weiam A., Elfadil E. Babiker, Isam A. Mohamed Ahmed, and Fahad Y. Al Juhaimi. 2025. "Optimization of Some Quality Parameters of Functional Pumpkin Puree Enriched with Banana Peel Powder Using Response Surface Methodology" Beverages 11, no. 4: 106. https://doi.org/10.3390/beverages11040106
APA StyleAlhemaid, W. A., Babiker, E. E., Mohamed Ahmed, I. A., & Al Juhaimi, F. Y. (2025). Optimization of Some Quality Parameters of Functional Pumpkin Puree Enriched with Banana Peel Powder Using Response Surface Methodology. Beverages, 11(4), 106. https://doi.org/10.3390/beverages11040106








