Abstract
The main diterpenes found in coffee, kahweol and cafestol, possess anti-inflammatory, anti-diabetic, and anticancer properties but are also reported to cause hypercholesterolemic effects. Their concentrations are known to be variable in coffee. This review aimed to discuss the concentrations of kahweol and cafestol from green coffee beans to brewed coffee. The results showed that the average concentrations of kahweol and cafestol in Arabica green beans were higher than in roasted and brewed coffee. The decrease in kahweol from green beans to roasted beans was 14.83%. In brewed coffee, kahweol was reduced by 90.26% and cafestol by 88.28%, compared to green beans. The changes in kahweol and cafestol levels were found to be influenced by various factors, including roasting methods and brewing techniques. The ratio of kahweol to cafestol in Arabica green beans was 1.7; in green coffee oil and roasted coffee oil, 1.2; in roasted beans, 1.3; and in brewed coffee, 1.1. In addition to their health-related functional properties, kahweol and cafestol concentrations and their ratio are suggested to be relevant markers in distinguishing between coffee species at various processing stages.
1. Introduction
Coffee is the world’s second most frequently consumed drink, surpassed only by water [1]. Based on data from the International Coffee Organization [2], worldwide annual coffee consumption reached 10.7 million tons in 2022/2023, showing an increase of 1.7% compared to the previous year. About 124 coffee species have been identified, and four species are currently traded: Arabica coffee (C. arabica), Robusta coffee (C. canephora var. robusta), Liberica coffee (C. liberica var. liberica), and Excelsa coffee (C. liberica var. dewevrei) [3,4]. Arabica coffee is the most popular and dominates the world coffee trade, with a share of 57.5%. The remaining 47.4% is Robusta, while Liberica and Excelsa coffee contribute less than 1% [5]. Arabica coffee is organoleptically superior to other types and thus commands a higher price in the global market. Brewed Arabica coffee has a fragrant, sweet, smooth, slightly sour flavor with a complex aroma [6].
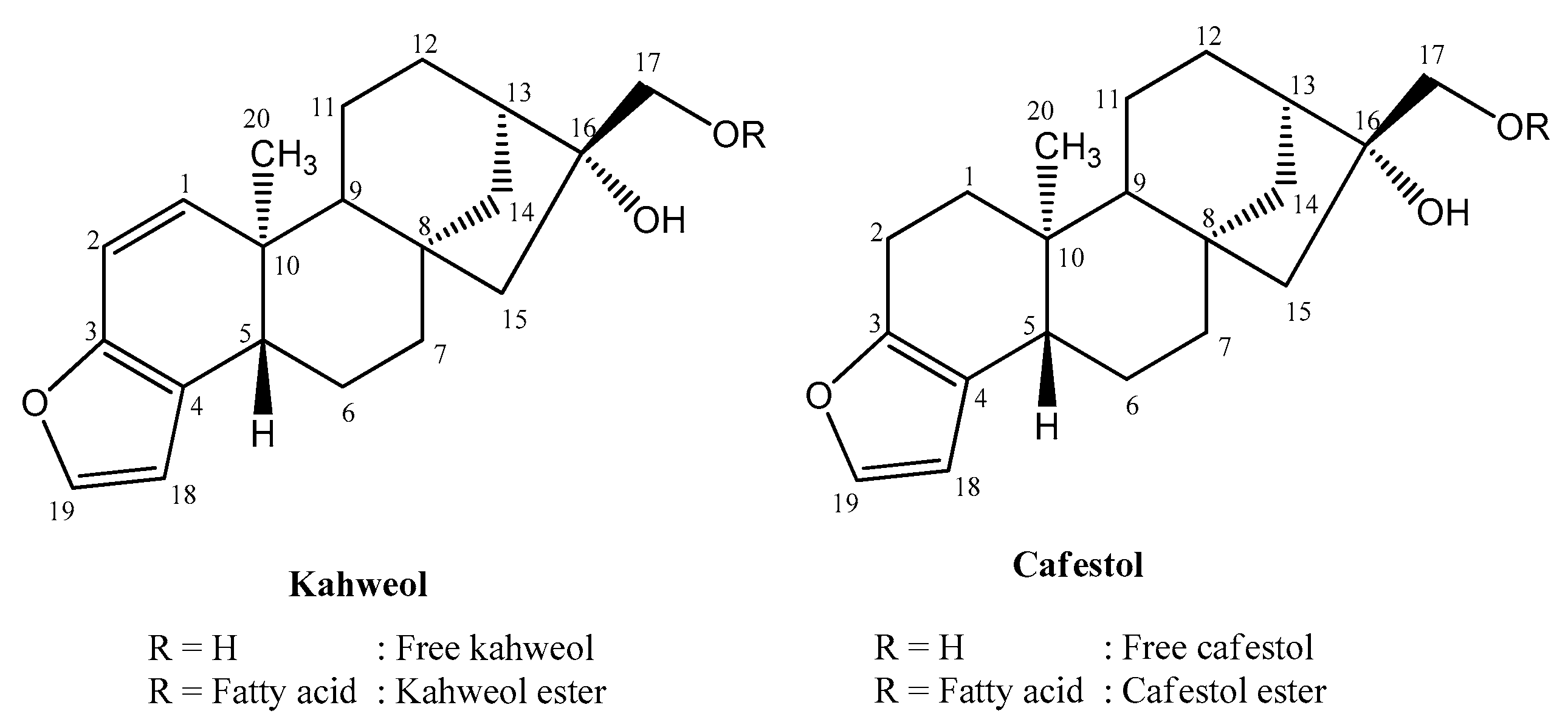
Coffee is favored for its distinctive taste, scent, and health advantages [7]. It contains many bioactive compounds such as caffeine, trigonelline, chlorogenic acid, melanoidin, and diterpenes [8,9]. The main diterpenes in coffee are kahweol and cafestol [10]. Kahweol and cafestol, found only in coffee [11,12,13], belong to the terpenoid group known as diterpenes because they are composed of four isoprene units (C5). The structure of kahweol and cafestol is C-20 polycyclic with a kaurene skeleton [14]. According to the International Union of Pure and Applied Chemistry (IUPAC), kahweol, with the molecular formula C20H26O3, is (1S,4S,12S,13R,16R,17R)-17-(hydroxymethyl)-12-methyl-8-oxapentacyclo [14.2.1.01,13.04,12.05,9]nonadeca-5(9),6,10-trien-17-ol, while, cafestol, with the molecular formula C20H28O3, is (1S,4S,12S,13R,16R,17R)-17-(hydroxymethyl)-12-methyl-8-oxapentacyclo [14.2.1.01,13.04,12.05,9]nonadeca-5(9),6-dien-17-ol [15]. Kahweol and cafestol differ in the double bond at C atom no. 1-2 present in kahweol. This double bond forms a conjugation with the furan ring, resulting in different properties (Figure 1). Data from the UV spectrum illustrates this. While kahweol exhibits maximum absorbance at 290 nm, cafestol’s peak absorption is at 230 nm [16].
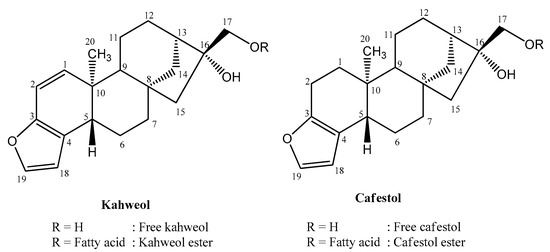
Figure 1.
Structure of kahweol and cafestol. Adopted from Moeenfard et al. [3]. Redrawn with ChemDraw Ultra 12.0.
Kahweol and cafestol are present in both free and esterified forms in the lipid fraction of coffee [10]. Free kahweol exists as kahweol alcohol, while esterified kahweol forms esters with fatty acids. The fatty acids that form esters with kahweol and cafestol can vary, but the most common is palmitic acid (36–49%), which is followed by linoleic acid (22–69%). Other fatty acids such as oleic and stearic acids also form esters with kahweol and cafestol but in smaller percentages [12,17].
Diterpenes in the esterified form constitute about 18% of the total coffee lipids, whereas diterpenes in the free form constitute only 0.4% [13]. Therefore, in the analysis of diterpenes, diterpene esters are first converted into diterpene alcohols through a saponification reaction with potassium hydroxide in ethanol or methanol [12].
The kahweol and cafestol contents were reported to vary between coffee species, cultivars, and harvest periods, with Arabica coffee being characterized by kahweol ranging from 371 to 986 mg/100 g and cafestol ranging from 221 to 604 mg/100 g [18]. In Robusta, kahweol was shown to reach 5–8 mg/100 g, and cafestol was shown to reach 239–250 mg/100 g. In Liberica coffee, kahweol amounted to 152–154 mg/100 g and cafestol to 273–283 mg/100 g, while in Excelsa coffee, kahweol content was 54–95 mg/100 g and cafestol content was 334–616 mg/100 g [19]. In Coffea canephora coffees, which are natural intervarietal hybrids of Conilon and Robusta, the cafestol content ranged from 96 to 457 mg/100 g, while the kahweol content ranged from absent to 36.9 mg/100 g [20].
In recent years, diterpenes have attracted considerable attention due to their various pharmacological functionalities, such as anti-inflammatory, hepatoprotective, anticancer, anti-diabetic, and anti-osteoclastogenic potential [21], but they were also reported to have hypercholesterolemic and hyperlipidemic effects. The consumption of five cups of unfiltered coffee per day was estimated to provide an intake of approximately 17.5 mg of cafestol and 6 mg of kahweol, showing a potential for increasing serum cholesterol levels by 0.15 mmol/L for every 10 mg of cafestol consumed [19,22]. However, another study did not confirm such a hypercholesterolemic effect after the moderate consumption of coffee brews from commercial capsules [23].
Kahweol and cafestol have similar structures but differ in biological activity, which may be due to the double bonds conjugated with furan rings [21]. The presence of double bonds at C1-C2 allows kahweol to undergo faster biotransformation into other compounds [3]. The administration of 10 mg of cafestol for four weeks was shown to increase serum cholesterol by 0.13 mmol/L, while kahweol increased it by only 0.02 mmol/L [24,25]. At the same time, cafestol is more effective in increasing serum triglyceride levels. While cafestol was shown to increase serum triglycerides by 86%, the combination of kahweol and cafestol caused only a 7% triglyceride increase [21,25]. The presence of double bonds in kahweol can accelerate its conversion into other compounds that have less impact on cholesterol levels (hyperlipidemia). In addition, the double bonds in kahweol may facilitate its excretion from the body [3]. The difference between kahweol and cafestol can also be seen from the fact that kahweol can inhibit lipid accumulation through the activation of AMP-activated protein kinase (AMPK), while cafestol does not exhibit a similar effect. As a result, kahweol has the potential to be an anti-obesity agent, while cafestol does not [26]. However, the study conducted by Mellbye et al. (2024) [27] showed that while cafestol did not improve insulin sensitivity or glucose tolerance, the administration of 6 mg twice daily led to significant reductions in body weight, visceral fat, and gamma-glutamyl transferase levels. Thus, cafestol administration contributed to the inverse relationship between coffee consumption and type 2 diabetes [27].
Surma and Oparil [28] reported that the regular consumption of traditional brewed coffee (125 mL/serving) or espresso (25 mL/serving), containing 0.0003 mg of kahweol/mL and 0.034 mg of cafestol/mL, in the amount of 1–3 cups of coffee per day, has no negative impact on blood pressure, including in people with hypertension. Conversely, occasional coffee consumption might elevate the risk of arterial hypertension.
Kahweol and cafestol also show activity as anticancer [29] and anti-inflammatory agents [30]. The key mechanisms of their action include reducing inflammatory mediators, boosting glutathione (GSH) levels, promoting tumor cell apoptosis, and inhibiting angiogenesis. Kahweol acts as a protective agent in traumatic brain injury (TBI) by reducing secondary injury and improving neurobehavioral outcomes through the attenuation of immune responses, including pro-inflammatory cytokine secretion and microglia activation [31]. While cafestol and kahweol exhibit comparable biological activities in this respect, they are not identical. This difference may stem from the presence of a conjugated double bond on the furan ring in kahweol [21].
In addition to their functional properties, kahweol and cafestol have also gained attention for their use in distinguishing between coffee species [32]. De Souza and Benassi [33] used kahweol and cafestol to distinguish Arabica and Robusta species. Kahweol/cafestol and caffeine/kahweol ratios are claimed to be new markers for distinguishing coffee species. Data show that Arabica coffee has a kahweol/cafestol ratio ranging from 1.73 to 3.40, while the caffeine/cafestol ratio is 1.18–1.91. Robusta coffee has a caffeine/kahweol ratio of >4.00. Thus, adding Robusta coffee to Arabica coffee will lower the kahweol/cafestol ratio and raise the caffeine/kahweol ratio. The kahweol/cafestol relationship can also be used to distinguish Arabica cultivars. The kahweol/cafestol ratio ranges from 1.1 to 3.5 for HKI cultivars, 0.6 to 1.1 for catuai cultivars, and 0.9 for Icatu cultivars [18].
The high consumption of coffee, especially Arabica, around the world, the variety of coffee beverage preparation techniques that affect the diterpene content, and the debate about the concentration and stability of diterpenes make it important to evaluate the contents of diterpenes (kahweol and cafestol) in Arabica coffee during processing from green bean to brew. This review also discusses the percent reduction in kahweol and cafestol, as well as the kahweol/cafestol (K/C) ratio, at each processing stage to understand the degradation process of kahweol and cafestol during Arabica coffee processing.
2. Materials and Methods
2.1. Literature Search
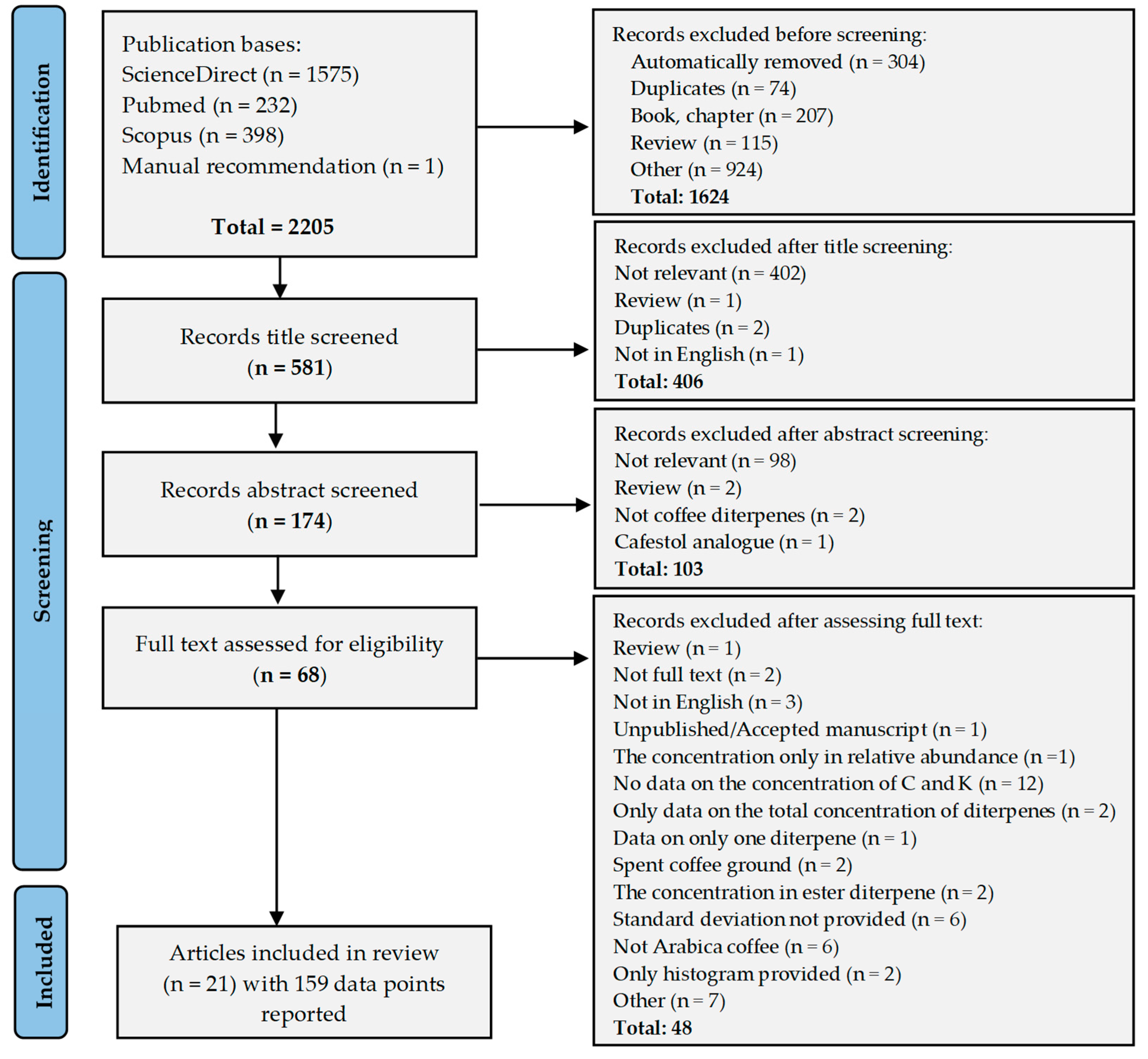
This review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [34] with precisely specified keywords, inclusion criteria, and exclusion criteria. The screening process resulted in the inclusion of 21 articles, as outlined in the PRISMA flowchart (Figure 2).
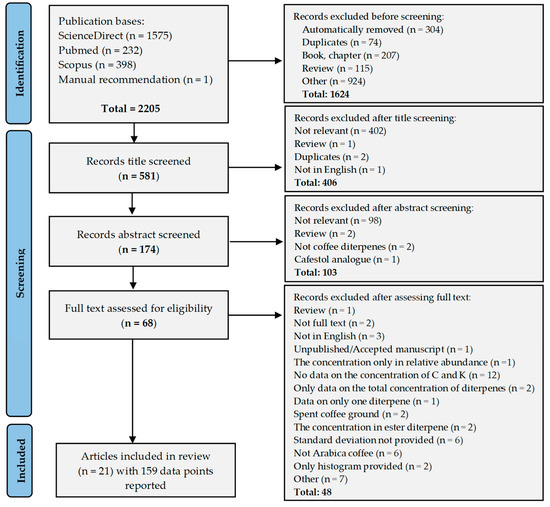
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
The 21 articles included in the review presented 159 data points on the kahweol and cafestol content of Arabica coffee. These data are presented in the Supplementary Materials, Tables S1–S4. The list of articles included in the review is presented in Supplementary Materials, Table S5.
The data was further processed using Microsoft Excel 2019. All included studies presented data on kahweol and cafestol in Arabica coffee. The unit used for the processed data was mg/100 g, with some studies requiring unit conversion. Publications searches were conducted using the search engines, such as Science Direct, PubMed, and Scopus, with the following keywords: kahweol AND cafestol OR “coffee diterpene”, coffee AND roasted AND kahweol AND cafestol, coffee AND brewed AND kahweol AND cafestol, roasted AND brewed AND kahweol AND cafestol OR “coffee diterpene”. Inclusion and exclusion criteria are presented in Table 1.

Table 1.
Inclusion and exclusion criteria applied in this study.
2.2. Kahweol and Cafestol Concentrations: Data Processing
The kahweol and cafestol concentration data were grouped by coffee processing stages into green bean (GB) for kahweol (K) and cafestol (C) analyzed in green coffee beans, green coffee oil (GO) for K and C analyzed in oil extracted from green coffee beans, roasted coffee oil (RO) for K and C analyzed in oil extracted from roasted coffee beans, roasted bean (RB) for K and C analyzed in roasted coffee beans, and brewed (B) for K and C analyzed in brewed Arabica coffee. The K and C concentrations in the studies included in the review were presented in various units, including mg/g, mg/mL, mg/kg, mg/kg of oil, mg/100 g of oil, and mg/cup. Unit conversion into mg/100 g was done by obtaining data such as coffee mass, brewed coffee volume, and oil yield. Specifically, mg/100 g of green beans was used for green beans, mg/100 g of roasted coffee for roasted and brewed coffee, and mg/100 g of oil for green and roasted coffee oils. Converted data was processed using Microsoft Excel 2019, and the K and C data were presented in boxplot form. The percent reduction in the kahweol and cafestol levels was obtained from the difference and comparison using the following formula:
3. Results and Discussion
3.1. Number of Publications and Coffee Origin
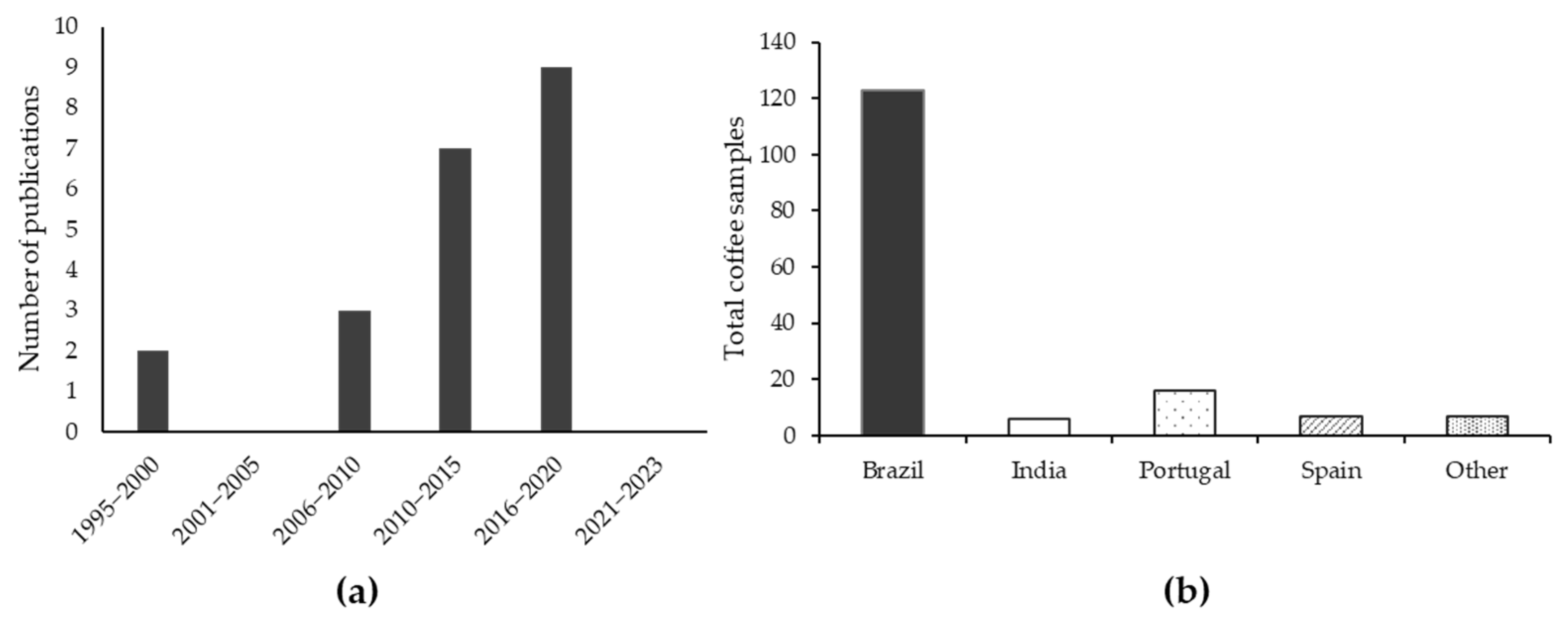
The publications search was conducted without limiting the initial search year to November 2023. The search results that met the inclusion criteria included references from 1995 to 2020. Some references discussing kahweol and cafestol, including the latest from 2020 to 2023, were not considered in this review because they did not meet the inclusion requirements. Moreover, the screening process led to the exclusion of publications where the data on kahweol and cafestol content could not be quantified. In the end, the literature search and screening resulted in the inclusion of 21 articles. The number of articles published in certain periods and countries of samples’ origins is presented in Figure 3a,b, respectively.
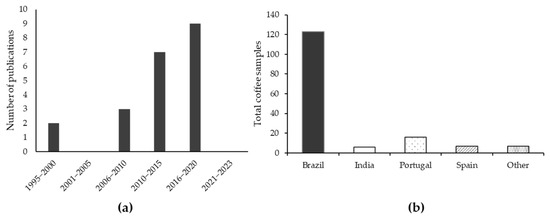
Figure 3.
Number of publications in the period of 1995–2023 (a). Country of origin of Arabica coffee samples in the studies included in the review (b).
Figure 3a shows that the number of publications on cafestol and kahweol has been consequently increasing over the years, confirming a growing scientific interest in these coffee compounds. It began in 2006 due to many studies investigating their health benefits and pharmacological properties. Figure 3b shows the origin of the coffee samples analyzed for kahweol and cafestol in the studies included in the review. The majority (77%) of the studied coffee samples came from Brazil. Brazil is the largest coffee-producing country in the world [2]. Some recent articles are not shown in the graphs because they did not meet the inclusion criteria (i.e., did not present standard deviation values or discussed only one compound—either kahweol or cafestol) and thus could not be included in the quantitative data processing.
3.2. Kahweol and Cafestol in Green Coffee Beans
As previously mentioned, kahweol, cafestol, and their derivatives are diterpene compounds found exclusively in coffee species [13]. Arabica coffee contains both kahweol and cafestol, while Robusta coffee contains cafestol, kahweol, and distinctive diterpenes known as 16-OMC (16-O-methyl cafestol) and 16-OMK (16-O-methyl kahweol), which are not found in other coffee species [6,35,36]. However, Gunning et al. [37] reported the presence of 16-OMC in Arabica coffee, albeit in very small amounts.
The coffee plant produces the diterpenes kahweol and cafestol mostly in the coffee bean’s endosperm. The biosynthesis of kahweol and cafestol starts from the cyclization of geranylgeranyl diphosphate (GGDP) into cafestol synthase and kahweol synthase. They catalyze the formation of intermediate compounds, which are then converted into the final end products by a series of enzymatic reactions. The type of coffee bean, genetic background, the stage at which the fruit develops, and environmental factors, like temperature and light intensity, all have an impact on these compounds’ biosynthesis [3,38].
Thus, the concentration of kahweol and cafestol in Arabica coffee green beans and their proportions show considerable variability. Some studies report higher cafestol content compared to kahweol [39,40], while others indicate the opposite, with higher kahweol content [18,41,42]. For instance, an analysis of kahweol and cafestol in four Arabica coffee cultivars (Catuai, IPR 100, IPR 102, and IPR 106) from two different harvest years (2009 and 2010) showed, in most cases, higher kahweol content compared to cafestol, except for the Catuai cultivar in the 2009 harvest, where cafestol content (604 mg/100 g) exceeded that of kahweol (371 mg/100 g) [18]. Rendon et al. [39] analyzed the Catuai cultivar and found kahweol at 585.01 mg/100 g and cafestol at 906.04 mg/100 g, while Dias et al. [32] reported higher kahweol content at 589 mg/100 g compared to cafestol at 304 mg/100 g. This finding aligns with Kitzberger et al. [43], who found kahweol at 843.19 mg/100 g and cafestol at 380.7 mg/100 g.
In another study of 13 Brazilian Arabica cultivars over three years, with consistent conditions (a 650 m altitude and an average temperature of 22–23 °C), Kitzberger et al. [43] observed that kahweol levels were highest in cultivars with Robusta gene introgression, such as Timor Hybrid and Villa Sarchi crosses (Iapar 59, IPR 97, IPR 98, IPR 99, and IPR 104 Timor). These cultivars showed stable high kahweol and low cafestol levels over the three years.
3.3. Kahweol and Cafestol in Roasted Bean
As mentioned, diterpene content in coffee beans is influenced by various factors. This includes post-harvest processing [3]. The amount of cafestol and kahweol in roasted coffee beans varies greatly depending on the roasting method and coffee species. Roasting is an important process that enhances the flavor of coffee while simultaneously reducing its bioactive properties [44]. Several studies have reported the effect of roasting on the kahweol and cafestol content of Arabica coffee [41,45]. In Arabica coffee, kahweol concentrations range from 661 to 923 mg/100 g, while cafestol ranges from 360 to 478 mg/100 g [41]. The debate on the impact of roasting on kahweol and cafestol values is ongoing. According to some authors [3,35], diterpene compounds are relatively stable against temperature changes. At the same time, the study of Sridevi et al. [45] showed that increased roasting temperatures and extended roasting time substantially affect the diterpene profile in roasted coffee. Roasting for 8 min at 230 °C was reported to lead to a loss of 60 to 75% of kahweol and cafestol [46]. Studies show that lighter roasting results in higher amounts of kahweol and cafestol, but darker roasting degrades these compounds due to increased heat exposure [38]. The effect of roasting on diterpene content continues to be debated, with variability arising, i.e., from different coffee varieties.
Extraction methods and analytical techniques also affect the reported data on kahweol and cafestol content in roasted coffee beans. Two commonly used diterpene analysis methods include analysis with lipid pre-extraction followed by saponification and diterpene separation [47], and direct saponification without lipid extraction [18,48]. Direct saponification can prevent the degradation of diterpene compounds that can occur during the lipid extraction process. Of the 159 data reports, only 3.2% did not use the saponification process because what would be determined are free diterpenes naturally present in the sample. Compounds were separated with gel permeation columns, followed by analysis using liquid chromatography (LC) and gas chromatography (GC) instruments. Instruments commonly used to analyze coffee kahweol and cafestol levels included HPLC (89%), GC-MS (4%), and LC-MS (3%).
Dias et al. [16] compared four methods for extracting kahweol and cafestol: Direct Hot Saponification (DHS), Direct Cold Saponification (DCS), Bligh and Dyer (BD), and Soxhlet extraction (SO) with saponification. DHS was the most efficient, fastest, and most economical, producing kahweol and cafestol levels nearly 15% higher than DCS and up to 88% higher than SO and BD. Analysis methods using the direct saponification of coffee beans or roasted coffee without prior lipid extraction have been reported [18,39,40,49,50], with KOH in methanol or ethanol used for saponification, followed by extraction with diethyl ether or tBME and analysis by HPLC-DAD/UV.
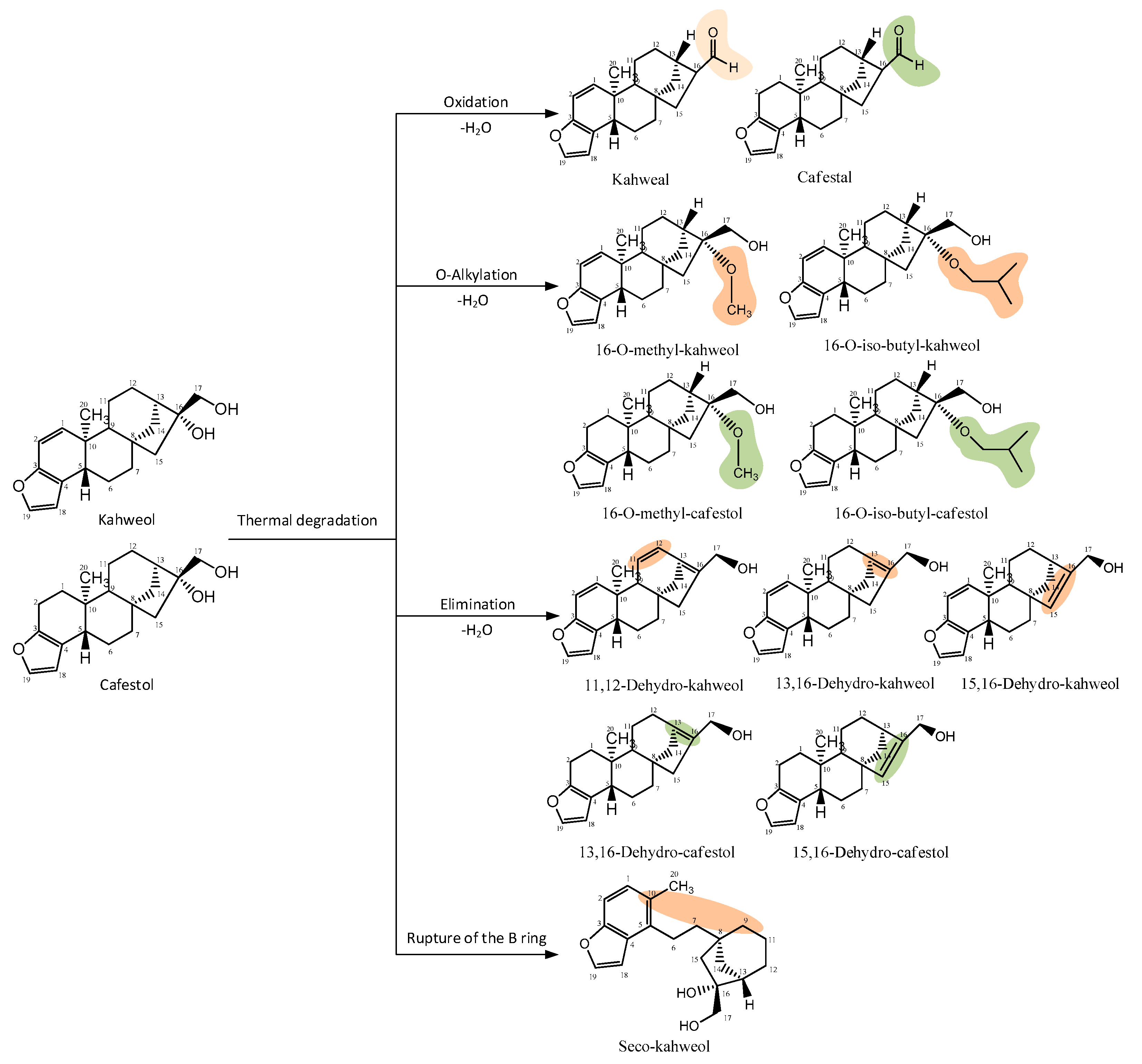
Kahweol and cafestol undergo degradation through oxidation and inter- and intramolecular elimination reactions during roasting. Novaes et al. [51] identified sixteen derivatives of kahweol and cafestol, with ten derived from kahweol and six from cafestol, indicating that cafestol is more stable at high temperatures. These derivatives generated from pyrolysis due to high roasting temperatures include aldehydes, ethers, and anhydrous derivatives (Figure 4).
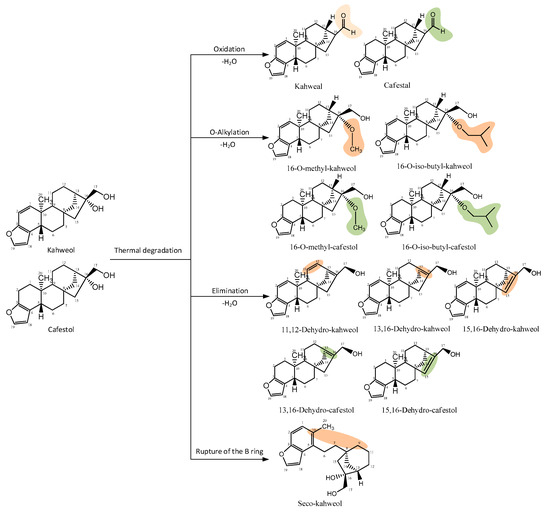
Figure 4.
Thermal degradation products of kahweol and cafestol (modified from Novaes et al. [52]). Drawn with ChemDraw Ultra 12.0.
As shown in Figure 4, the degradation of kahweol and cafestol is influenced by the Maillard reaction and other thermal processes that occur during roasting, which not only diminish the diterpene concentration but also convert it into various molecules with distinct characteristics [42]. Dehydration reactions are significant, leading to the formation of dehydro-derivatives such as dehydrocafestol and dehydrokahweol, especially noticeable in dark roast samples [46]. Although the degradation of these beneficial compounds is significant, it is important to consider that some roasting methods can retain higher diterpene levels. Thus, the optimization of roasting techniques is important to retain diterpene levels and their health-related properties.
3.4. Kahweol and Cafestol in Brewed Coffee
The brewing method has a crucial impact on the levels of kahweol and cafestol in the final coffee beverage. French press coffee showed the highest diterpene content, with concentrations of kahweol and cafestol reaching 17.2 mg/cup and 19.7 mg/cup, respectively. At the same time, the filter paper method of brewing resulted in very low levels of both diterpenes [45]. The tubruk technique was shown to provide larger quantities of the diterpenes than the V60 drip and hanging drip bag methods, with tubruk Arabica Gayo yielding 7.86 mg/L of kahweol and 3.03 mg/L of cafestol [52]. Instant coffee had the least kahweol and no cafestol, while espresso and paper-filtered coffee had similar diterpene levels. Cloth-filtered coffee contained fewer solids but higher diterpene concentrations per gram, with kahweol ranging from 0.05 to 0.16 mg and cafestol from 0.11 to 0.14 mg per 50 mL. Paper-filtered coffee has less kahweol and cafestol because the paper filter retains these lipid components. Cloth-filtered coffee, however, maintains more diterpenes because of the greater pore size, which allows more lipids to flow through [53]. Moeenfard et al. [11] found that the way espresso is prepared significantly impacts the levels of diterpenes in the coffee. Factors like the coffee-to-water ratio, grind size, and brewing temperature all play a role. Using very fine coffee grounds and larger serving sizes led to more efficient extraction, increasing the amount of these compounds. On the other hand, brewing at lower temperatures, such as 70 °C, reduced the extraction of kahweol and cafestol, highlighting the importance of brewing conditions for their levels. Espresso contains moderate amounts of kahweol and cafestol. High pressure and short brewing time result in lower concentrations of these compounds in the brew, though not as low as noted in unfiltered coffee [53].
3.5. Kahweol and Cafestol in Coffee Bean Oil
The diterpene content is also often analyzed in the oil extracted from green or roasted Arabica coffee beans. D’Amelio et al. [54] reported the kahweol and cafestol contents of green Arabica coffee from four countries: Brazil, Kenya, Colombia, and Guatemala. The kahweol values ranged from 10.0 to 36.3 g/kg of oil, while cafestol levels ranged from 17.7 to 57.0 g/kg of oil. De Oliveira et al. [55] reported the kahweol content of Brazilian green Arabica coffee at 63.8 g/kg of oil and cafestol at 50.2 g/kg of oil. Coffee oil was extracted using the Soxhlet method for 4 and 6 h. Belandria et al. [56] analyzed the kahweol and cafestol content of Brazilian Arabica coffee by extracting the coffee oil first using the PFE (pressurized-fluid extraction) method, with a pressure of 20 bar, a flow rate of 0.5–3 mL/min, and temperatures of 60, 80, and 100 °C. The results showed kahweol values of 325.3–689.7 and cafestol values of 185.6–461.40 mg/100 g of green bean.
Tsukui et al. [10] extracted green coffee oil from 13 different green Arabica coffee beans with microwave-assisted extraction (MAE) and Soxhlet extraction. The diterpene content of coffee oil extracted by the Soxhlet method was higher than MAE. MAE resulted in diterpene levels ranging from 4.5 to 8.9 g/kg of oil, while Soxhlet extraction resulted in levels ranging from 6.9 to 9.8 g/kg of oil. Nevertheless, the microwave-assisted extraction of green coffee oil makes it possible to develop a robust methodology, as it takes less time (only 10 min at 45 °C) compared to the traditional Soxhlet extraction method. Microwave-assisted extraction utilizes microwave radiation to warm a solvent that contacts a sample matrix for extracting desired chemicals. In contrast, conventional heating starts by heating the sample next to the equipment’s surface and then transfers heat inward through conduction, radiation, and convection [57].
3.6. Changes in Kahweol and Cafestol from Coffee Bean to Brew
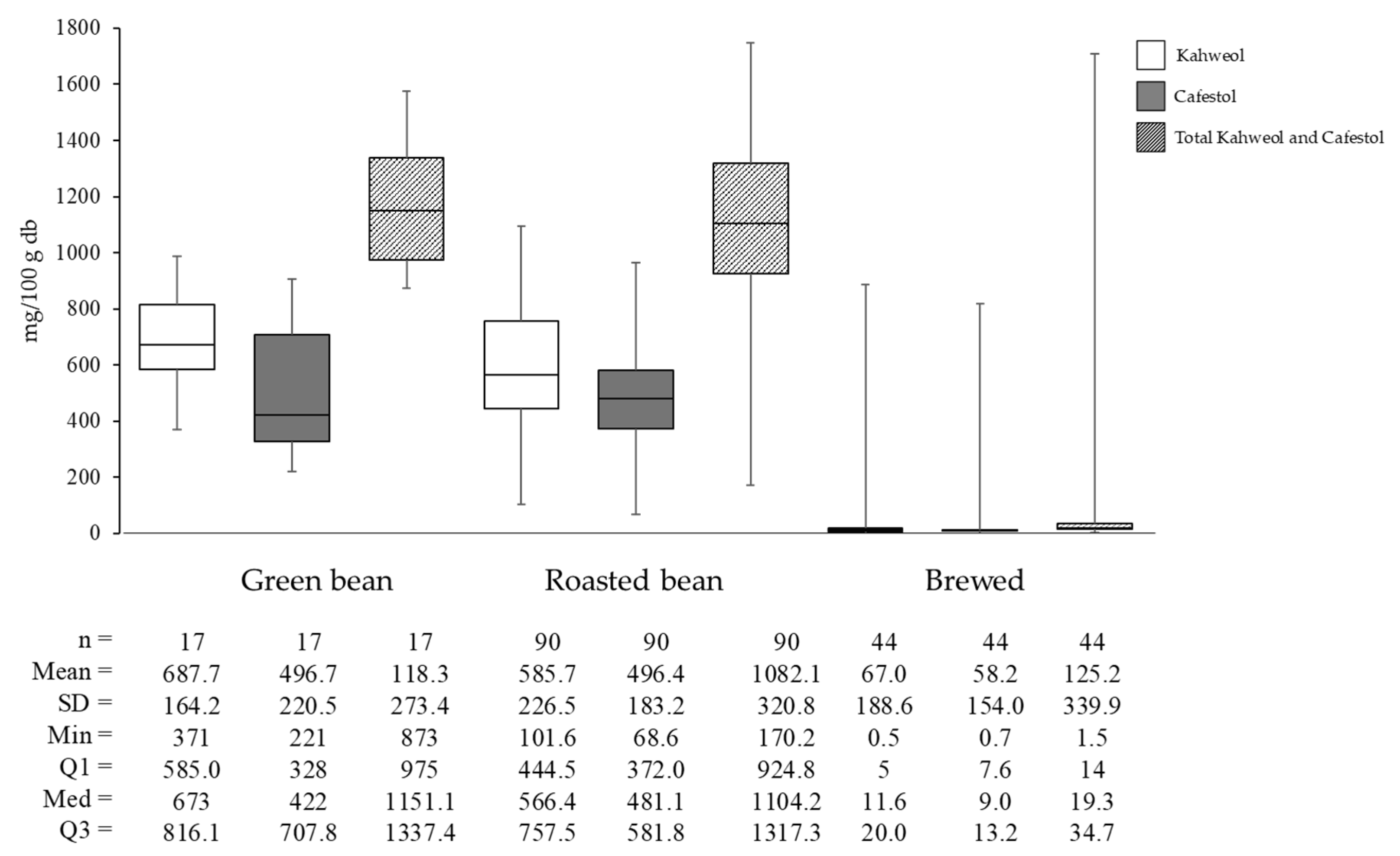
The change of cafestol and kahweol concentrations from green coffee beans to brewed coffee involves several factors, including brewing techniques and conditions and the chemical properties of these diterpenes. Figure 5 illustrates the levels of kahweol and cafestol in Arabica coffee green beans, roasted beans, and brewed coffee. Diterpene levels decrease from green beans to roasted beans and further to brewed coffee.
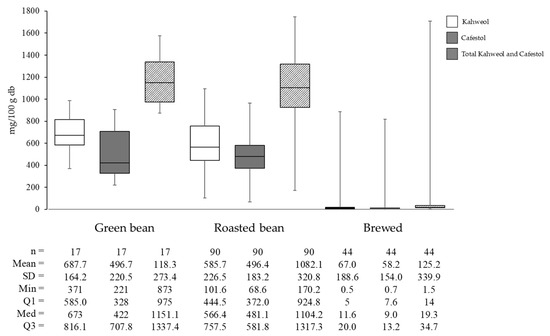
Figure 5.
Kahweol, cafestol, and total diterpene content of Arabica coffee: green bean, roasted bean, and brewed.
Based on the analyzed literature included in the review, the average total diterpenes level was 1184.33 mg/100 g in green beans, 1082.12 mg/100 g in roasted beans, and 125.19 mg/100 g in brewed coffee. Specifically, the decline in kahweol concentration from green beans to roasted coffee is 14.83%, while cafestol decreases by only 0.05%. This indicates that kahweol degrades faster than cafestol during roasting. The rapid degradation of kahweol is due to the presence of a double bond at the C atom positions 1-2, making it more susceptible to reactions such as addition, oxidation, or substitution [39].
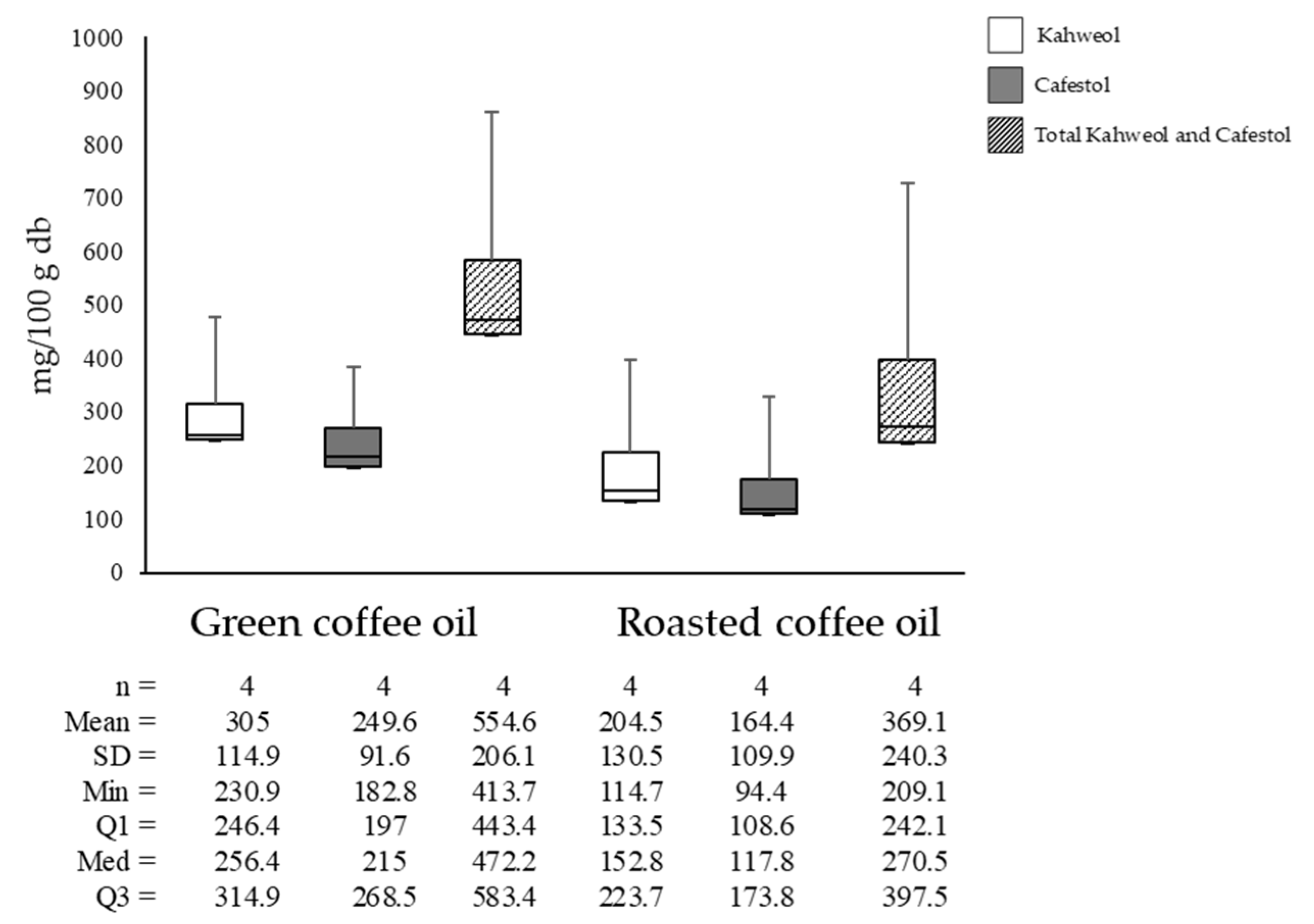
Figure 6 shows that kahweol concentrations in green coffee oil averaged 304.95 mg/100 g and cafestol averaged 249.6 mg/100 g. In roasted coffee oil, kahweol averaged 204.5 mg/100 g and cafestol averaged 164.6 mg/100 g. This represented differences of 32.94% and 41.56% for kahweol and cafestol, respectively, attributed to the compounds’ degradation during lipid extraction due to increased temperature and pressure.
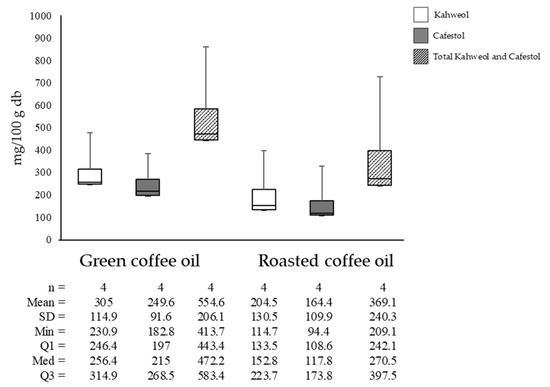
Figure 6.
Kahweol, cafestol, and total diterpene concentrations in green and roasted coffee oils.
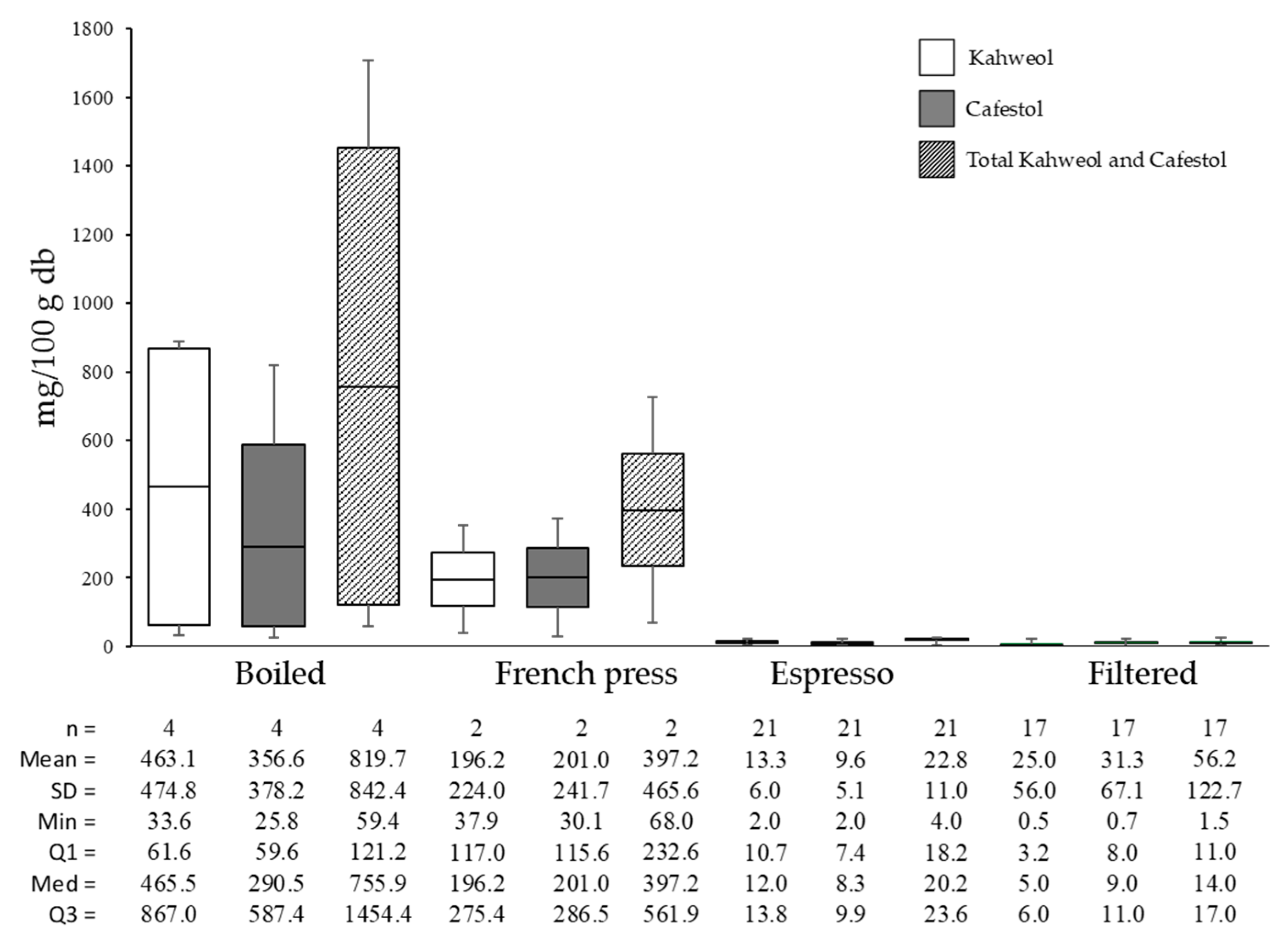
Figure 7 displays the results on the kahweol and cafestol levels across various brewing methods. Boiled brewing resulted in the highest total diterpenes (819.75 mg/100 g), followed by French press (397.25 mg/100 g), filtered (56.23 mg/100 g), and espresso (22.81 mg/100 g).
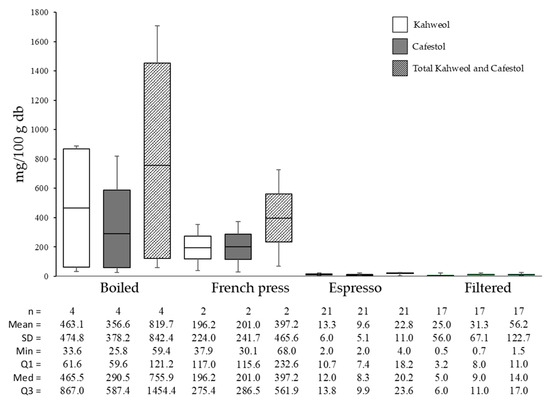
Figure 7.
Kahweol and cafestol concentrations in coffee of different brewing types.
The average total diterpene content in roasted coffee was 1082.12 mg/100 g, with the decrease in diterpene content from roasted coffee to brewed coffee being 24.25% for boiled, 63.29% for French press, 97.89% for espresso, and 94.80% for the filter method. At the same time, in the research conducted by Wuerges et al. [53], kahweol and cafestol content in paper-filtered coffee was lower than in espresso.
Brewed coffee contains suspended particles, including kahweol and cafestol. Filtration, pressurization, and coffee powder particle size all impact their concentration [58]. The variations in cafestol and kahweol concentrations across different coffee brewing methods are primarily influenced by the extraction process and the filtration system used. As lipid-derived diterpenes are present in coffee, their concentrations are affected by the brewing technique, which modulates both solubility and retention. Consequently, differences in brewing parameters lead to varying levels of these compounds in the final coffee beverage.
3.7. Kahweol/Cafestol Ratio of Arabica Coffee
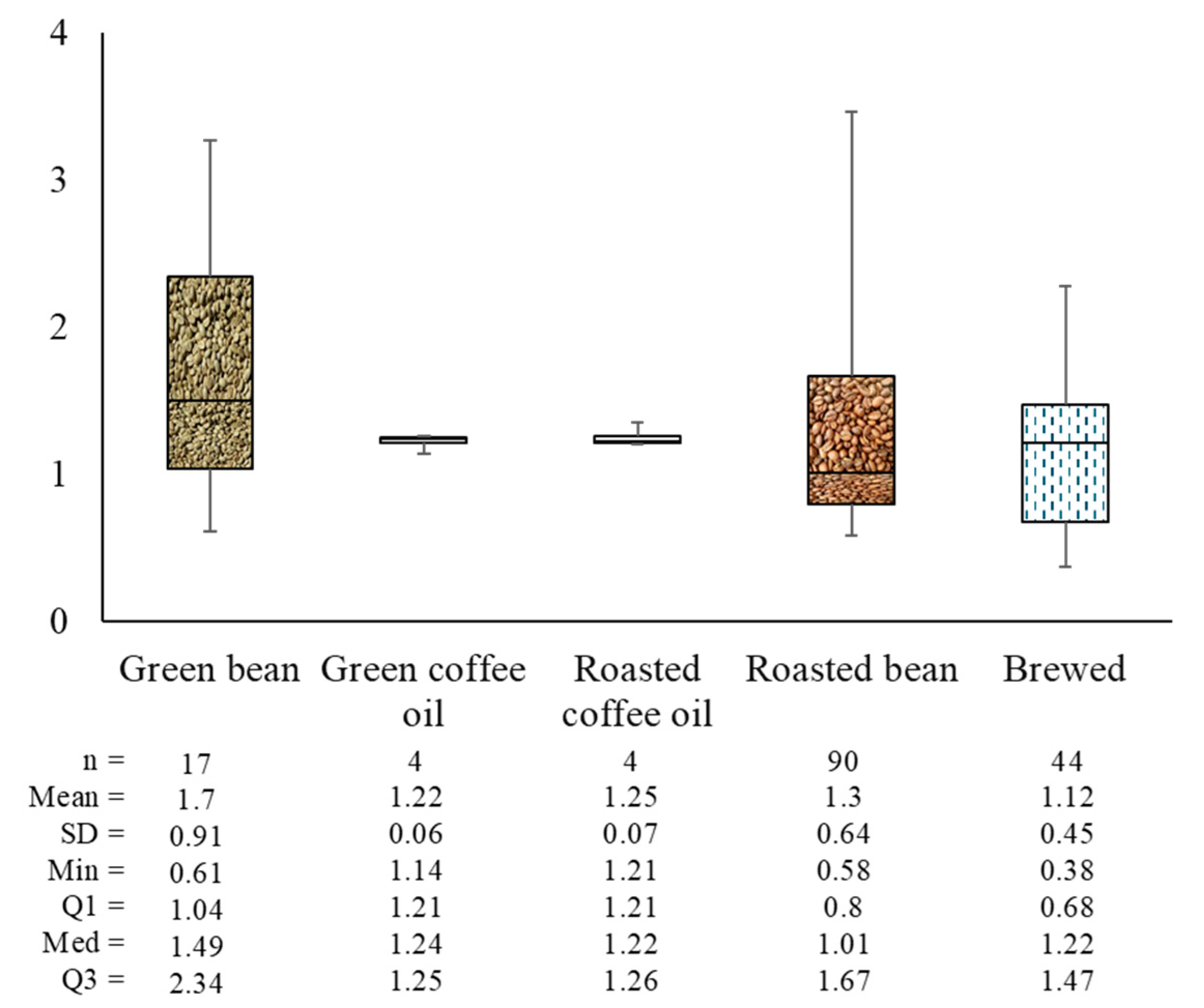
Kitzberger et al. [18] investigated the kahweol/cafestol (K/C) ratio across various Arabica cultivars. They found that the K/C ratio for IPR cultivars ranged from 1.70 to 3.07 in both green and roasted coffee beans, whereas the Catuaí and Icatu cultivars showed a K/C ratio of 0.61 to 0.93 in both forms. Notably, the roasting process did not affect the K/C ratio, suggesting that this ratio can be used to characterize coffee cultivars irrespective of the roasting levels.
The identification of coffee species in commercial coffee products based on kahweol/cafestol and caffeine/cafestol ratios was suggested by De Souza and Benassi [33]. Pure Arabica coffee typically has a K/C ratio ranging from 1 to 3, while Robusta coffee has a K/C ratio between 0 and 0.06. A higher proportion of Robusta in commercial coffee results in a lower K/C ratio and a higher caffeine/cafestol ratio. Zanin et al. [59] reported that the K/C ratio for Arabica coffee ranged from 0.63 to 2.77. In addition to the K/C ratio, the caffeine/total diterpenes ratio is also used to characterize Arabica coffee. In another study, a lower K/C ratio in the green coffee beans was associated with higher scores for the coffee brews [60]. Figure 8 illustrates the K/C ratio across various coffee forms: green beans, green coffee oil, roasted coffee oil, roasted beans, and brewed coffee. The average ratios observed were 1.7 for green beans, 1.2 for both green and roasted coffee oil, 1.3 for roasted beans, and 1.1 for brewed coffee.
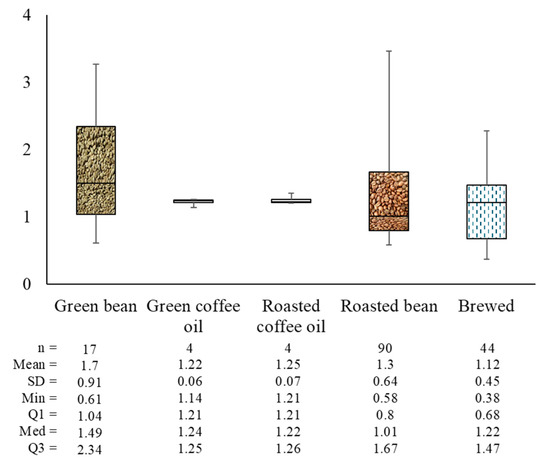
Figure 8.
Kahweol/cafestol ratio of Arabica coffee.
The decrease in the K/C ratio with processing indicates a more significant reduction in kahweol compared to cafestol. This is because kahweol is more readily degraded into its derivatives than cafestol. As previously mentioned, cafestol and kahweol degradation in coffee beans depends on the roasting temperature and time. In a light roast (230 °C for 12 min), the degradation is more limited, but it becomes more noticeable in a medium roast (240 °C for 14 min) and most significant in a dark roast (250 °C for 17 min). The longer and hotter the roasting process, the greater the degradation of diterpenes, reducing their concentration in the coffee [51].
4. Conclusions
This review has shown differences in kahweol and cafestol concentrations in Arabica coffee depending on various factors, including roasting methods and brewing techniques. The average concentrations of kahweol and cafestol in Arabica green beans were higher than in roasted and brewed coffee. The decrease in kahweol from green beans to roasted beans was, on average, 14.83%. In brewed coffee, kahweol was reduced by 90.26%, and cafestol by 88.28% compared to green beans. The ratio of kahweol to cafestol in Arabica coffee also differed depending on the processing stage, reaching 1.7 in green beans, 1.2 in green coffee oil and roasted coffee oil, 1.3 in roasted beans, and 1.1 in brewed coffee. In addition to their health-related functional properties, kahweol and cafestol concentrations and their ratio are suggested to be relevant markers in distinguishing between Arabica coffee at various processing stages. The management of kahweol and cafestol concentrations, as well as their ratio, plays a crucial role in creating healthier coffee products. Choosing the right brewing methods, such as using paper filters to reduce the concentrations of these compounds or using French press and espresso methods to maintain them, can significantly impact coffee quality. With proper management, the resulting coffee products can better align with the preferences of health-conscious consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages11040105/s1, Table S1: Cafestol and kahweol concentrations in green Arabica coffee beans; Table S2: Cafestol and kahweol concentrations in green beans oil of Arabica coffee; Table S3: Cafestol and kahweol concentrations in roasted Arabica coffee beans; Table S4: Cafestol and kahweol concentrations in brewed Arabica coffee; Table S5: Articles included in the review: [11,16,18,23,32,33,39,40,41,42,43,45,47,48,49,53,59,60,61,62,63].
Author Contributions
Conceptualization, A.I.J., D.N.F., D.H., N.A., R.K. and D.Ś.-T.; methodology, A.I.J., D.N.F., D.H. and N.A.; validation, A.I.J., D.N.F., D.H. and N.A.; formal analysis, A.I.J., D.N.F., D.H. and N.A.; investigation, A.I.J., D.N.F., D.H. and N.A.; resources, A.I.J., D.N.F., D.H. and N.A.; data curation, A.I.J., D.N.F., D.H., N.A., R.K. and D.Ś.-T.; writing—original draft preparation, A.I.J., D.N.F., D.H., N.A. and D.Ś.-T.; writing—review and editing, A.I.J., D.N.F., D.H., N.A., R.K. and D.Ś.-T.; visualization, A.I.J., D.N.F., D.H., N.A. and D.Ś.-T.; supervision, A.I.J., D.N.F., D.H. and N.A.; project administration, A.I.J., D.N.F., D.H. and N.A.; funding acquisition, A.I.J., D.N.F., D.H., N.A., R.K. and D.Ś.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by The Ministry of Education, Culture, Research, and Technology (Indonesia), with funding for the doctoral dissertation program (grant number 027/E5/PG.02.00.PL/2024 and 22156/IT3.D10/PT.01.03/P/B/2024), and by The Polish Ministry of Science and Higher Education, with funds of the Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS) for scientific research.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available upon request by the author Nuri Andarwulan.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| C. arabica | Coffea arabica |
| C. canephora var. robusta | Coffea canephora var. robusta |
| C. liberica var. liberica | Coffea liberica var. liberica |
| C. liberica var. dewevrei | Coffea liberica var. dewevrei |
| ICO | International Coffee Organization |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| K | Kahweol |
| C | Cafestol |
| GB | Green Bean |
| GO | Green Coffee Oil |
| RO | Roasted Coffee Oil |
| RB | Roasted Bean |
| B | Brewed Coffee |
| SD | Standard Deviation |
| DHS | Direct Hot Saponification |
| DCS | Direct Cold Saponification |
| BD | Bligh and Dyer (method) |
| SO | Soxhlet Extraction (method) |
| HPLC | High-Performance Liquid Chromatography |
| UPLC | Ultra-Performance Liquid Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| SFE | Supercritical Fluid Extraction |
| K/C | Kahweol-to-cafestol ratio |
| AMPK | AMP-activated protein kinase |
| IUPAC | International Union of Pure and Applied Chemistry |
| UV | Ultraviolet |
References
- Novaes, F.J.M.; Bayan, F.C.; Neto, F.R.A.; Rezende, C.M. The Occurrence of Cafestol and Kahweol Diterpenes in Different Coffee Brews. Coffee Sci. 2019, 14, 265–280. [Google Scholar] [CrossRef]
- International Coffee Organization (ICO). Coffee Report and Outlook; Volume April; International Coffee Organization: London, UK, 2023; Available online: https://icocoffee.org/documents/cy2023-24/cy2022-23/Coffee_Report_and_Outlook_April_2023_-_ICO.pdf (accessed on 8 January 2024).
- Moeenfard, M.; Alves, A. New Trends in Coffee Diterpenes Research from Technological to Health Aspects. Food Res. Int. 2020, 134, 109207. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, N.A.; Zhu, F. Coffee Bean Processing: Emerging Methods and Their Effects on Chemical, Biological and Sensory Properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef] [PubMed]
- Portaluri, V.; Thomas, F.; Guyader, S.; Jamin, E.; Bertrand, B.; Remaud, G.S.; Schievano, E.; Mammi, S.; Guercia, E.; Navarini, L. Limited Genotypic and Geographic Variability of 16-O-Methylated Diterpene Content in Coffea Arabica Green Beans. Food Chem. 2020, 329, 127129. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.; BK, A.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of Processing on Bioaccessibility and Bioavailability of Bioactive Compounds in Coffee Beans. Food Biosci. 2022, 46, 101373. [Google Scholar] [CrossRef]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Volti, G.L.; Galvano, F.; Grosso, G. Coffee Components and Cardiovascular Risk: Beneficial and Detrimental Effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; de Toledo Benassi, M. Caffeine, Trigonelline, Chlorogenic Acids, Melanoidins, and Diterpenes Contents of Coffea Canephora Coffees Produced in the Amazon. J. Food Compos. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Tsukui, A.; Santos Júnior, H.M.; Oigman, S.S.; de Souza, R.O.M.A.; Bizzo, H.R.; Rezende, C.M. Microwave-Assisted Extraction of Green Coffee Oil and Quantification of Diterpenes by HPLC. Food Chem. 2014, 164, 266–271. [Google Scholar] [CrossRef]
- Moeenfard, M.; Silva, J.A.; Borges, N.; Santos, A.; Alves, A. Diterpenes in Espresso Coffee: Impact of Preparation Parameters. Eur. Food Res. Technol. 2015, 240, 763–773. [Google Scholar] [CrossRef]
- Silva, J.A.; Borges, N.; Santos, A. Method Validation for Cafestol and Kahweol Quantification in Coffee Brews by Method Validation for Cafestol and Kahweol Quantification in Coffee Brews by HPLC-DAD. Food Anal. Methods 2012, 5, 1404–1410. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Peng, X.; Hu, G.; Qiu, M. Identification of New Diterpene Esters from Green Arabica Coffee Beans, and Their Platelet Aggregation Accelerating Activities. Food Chem. 2018, 263, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mafu, S.; Zerbe, P. Plant Diterpenoid Metabolism for Manufacturing the Biopharmaceuticals of Tomorrow: Prospects and Challenges. Phytochem. Rev. 2018, 17, 113–130. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 3 August 2024).
- Dias, R.C.E.; De Faria, A.F.; Mercadante, A.Z.; Bragagnolo, N.; De Benassi, M.T. Comparison of Extraction Methods for Kahweol and Cafestol Analysis in Roasted Coffee. J. Braz. Chem. Soc. 2013, 24, 492–499. [Google Scholar] [CrossRef]
- Kölling-Speer, I.; Strohschneider, S.; Speer, K. Determination of Free Diterpenes in Green and Roasted Coffees. HRC J. High. Resol. Chromatogr. 1999, 22, 43–46. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Scholz, M.B.D.S.; Pereira, L.F.P.; Vieira, L.G.E.; Sera, T.; Silva, J.B.G.D.; de Toledo Benassi, M. Diterpenes in Green and Roasted Coffee of Coffea Arabica Cultivars Growing in the Same Edapho-Climatic Conditions. J. Food Compos. Anal. 2013, 30, 52–57. [Google Scholar] [CrossRef]
- De Roos, B.; Van Der Weg, G.; Urgert, R.; Van De Bovenkamp, P.; Charrier, A.; Katan, M.B. Levels of Cafestol, Kahweol, and Related Diterpenoids in Wild Species of the Coffee Plant Coffea. J. Agric. Food Chem. 1997, 45, 3065–3069. [Google Scholar] [CrossRef]
- Francisco, J.S.; Dias, R.C.E.; Alves, E.A.; Rocha, R.B.; Dalazen, J.R.; Mori, A.L.B.; Benassi, M.T. Natural Intervarietal Hybrids of Coffea Canephora Have a High Content of Diterpenes. Beverages 2021, 7, 77. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Hovenier, R.; Beynen, A.; Urgert, R.; Weusten-Van Der Wouw, M.; Hovenier, R.; Meyboom, S.; Beynen, A.C.; Katan, M.B. Diterpenes from Coffee Beans Decrease Serum Levels of Lipoprotein(a) in Humans: Results from Four Randomized, Controlled Trials. Eur. J. Clin. Nutr. 1997, 51, 431–436. [Google Scholar] [CrossRef]
- Wuerges, K.L.; dos Santos, A.C.F.; Mori, A.L.B.; Benassi, M.T. Contents of Diterpenes in Espresso Coffee Brews Prepared from Commercial Capsules. Coffee Sci. 2016, 11, 276–284. [Google Scholar]
- Weusten-Van der Wouw, M.P.M.E.; Katan, M.B.; Viani, R.; Huggett, A.C.; Liardon, R.; Lund-Larsen, P.G.; Thelle, D.S.; Ahola, I.; Aro, A.; Meyhoom, S.; et al. Identity of the Cholesterol-Raising Factor from Boiled Coffee and Its Effects on Liver Function Enzymes. J. Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The Cholesterol-Raising Factor from Coffee Beans. J. R. Soc. Med. 1996, 89, 618–623. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, N.J.; Song, J.K.; Chun, K.H. Kahweol Inhibits Lipid Accumulation and Induces Glucose-Uptake through Activation of AMP-Activated Protein Kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.D.; Nguyen, M.D.; Hermansen, K.; Jeppesen, P.B.; Al-Mashhadi, Z.K.; Ringgaard, S.; Gregersen, S. Effects of 12-Week Supplementation with Coffee Diterpene Cafestol in Healthy Subjects with Increased Waist Circumference: A Randomized, Placebo-Controlled Trial. Nutrients 2024, 16, 3232. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Oparil, S. Coffee and Arterial Hypertension. Curr. Hypertens. Rep. 2021, 23, 38. [Google Scholar] [CrossRef]
- Lee, K.-A.; Chae, J.-I.; Shim, J.-H. Natural Diterpenes from Coffee, Cafestol and Kahweol Induce Apoptosis through Regulation of Specificity Protein 1 Expression in Human Malignant Pleural Mesothelioma. J. Biomed. Sci. 2012, 19, 60. [Google Scholar] [CrossRef]
- Shen, T.; Lee, J.; Lee, E.; Kim, S.H.; Kim, T.W.; Cho, J.Y. Cafestol, a Coffee-Specific Diterpene, Is a Novel Extracellular Signal-Regulated Kinase Inhibitor with AP-1-Targeted Inhibition of Prostaglandin E2 Production in Lipopolysaccharide-Activated Macrophages. Biol. Pharm. Bull. 2010, 33, 128–132. [Google Scholar] [CrossRef]
- Lee, H.F.; Lin, J.S.; Chang, C.F. Acute Kahweol Treatment Attenuates Traumatic Brain Injury Neuroinflammation and Functional Deficits. Nutrients 2019, 11, 2301. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Campanha, F.G.; Vieira, L.G.E.; Ferreira, L.P.; David, P.O.T.; Marraccini, P.; De Benassi, M.T. Evaluation of Kahweol and Cafestol in Coffee Tissues and Roasted Coffee by a New High-Performance Liquid Chromatography Methodology. J. Agric. Food Chem. 2010, 58, 88–93. [Google Scholar] [CrossRef]
- De Souza, R.M.N.; Benassi, M.T. Discrimination of Commercial Roasted and Ground Coffees According to Chemical Composition. J. Braz. Chem. Soc. 2012, 23, 1347–1354. [Google Scholar] [CrossRef]
- Available online: http://www.Prisma-Statement.Org/ (accessed on 13 November 2023).
- Pacetti, D.; Boselli, E.; Balzano, M.; Frega, N.G. Authentication of Italian Espresso Coffee Blends through the GC Peak Ratio between Kahweol and 16-O-Methylcafestol. Food Chem. 2012, 135, 1569–1574. [Google Scholar] [CrossRef]
- Guercia, E.; Berti, F.; Navarini, L.; Demitri, N.; Forzato, C. Isolation and Characterization of Major Diterpenes from C. Canephora Roasted Coffee Oil. Tetrahedron Asymmetry 2016, 27, 649–656. [Google Scholar] [CrossRef]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-Methylcafestol Is Present in Ground Roast Arabica Coffees: Implications for Authenticity Testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Girma, B.; Wale, K. Analytical Methods, Influencing Factors, and Health Benefits of Kahweol and Cafestol in Coffee: A Review. Int. J. Food Sci. Biotechnol. 2023, 8, 26–32. [Google Scholar] [CrossRef]
- Rendón, M.Y.; dos Santos Scholz, M.B.; Bragagnolo, N. Is Cafestol Retained on the Paper Filter in the Preparation of Filter Coffee? Food Res. Int. 2017, 100, 798–803. [Google Scholar] [CrossRef]
- Rendón, M.Y.; Dos Santos Scholz, M.B.; Bragagnolo, N. Physical Characteristics of the Paper Filter and Low Cafestol Content Filter Coffee Brews. Food Res. Int. 2018, 108, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Campanha, F.G.; Dias, R.C.E.; De Toledo Benassi, M. Discrimination of Coffee Species Using Kahweol and Cafestol: Effects of Roasting and of Defects. Coffee Sci. 2010, 5, 87–96. [Google Scholar]
- Kitzberger, C.S.G.; dos Santos Scholz, M.B.; de Toledo Benassi, M. Bioactive Compounds Content in Roasted Coffee from Traditional and Modern Coffea arabica Cultivars Grown under the Same Edapho-Climatic Conditions. Food Res. Int. 2014, 61, 61–66. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; dos Santos Scholz, M.B.; Pereira, L.F.P.; da Silva, J.B.G.D.; de Toledo Benassi, M. Profile of the Diterpenes, Lipid and Protein Content of Different Coffee Cultivars of Three Consecutive Harvests. AIMS Agric. Food 2016, 1, 254–264. [Google Scholar] [CrossRef]
- Hanifah, D.; Herawati, D.; Andarwulan, N. Effects of Roasting on Profiles of Non-Volatile and Volatile Compounds in Liberica Coffee from Jambi, Indonesia. Int. Food Res. J. 2025, 32, 165–185. [Google Scholar] [CrossRef]
- Sridevi, V.; Giridhar, P.; Ravishankar, G.A. Evaluation of Roasting and Brewing Effect on Antinutritional Diterpenes-Cafestol and Kahweol in Coffee. Glob. J. Med. Res. 2011, 11, 17–22. [Google Scholar]
- Dias, R.C.E.; de Faria-Machado, A.F.; Mercadante, A.Z.; Bragagnolo, N.; de Toledo Benassi, M. Roasting Process Affects the Profile of Diterpenes in Coffee. Eur. Food Res. Technol. 2014, 239, 961–970. [Google Scholar] [CrossRef]
- Araújo, J.M.A.; Sandi, D. Extraction of Coffee Diterpenes and Coffee Oil Using Supercritical Carbon Dioxide. Food Chem. 2006, 101, 1087–1094. [Google Scholar] [CrossRef]
- Bianchin, M.; Lima, H.H.C.D.; Monteiro, A.M.; Benassi, M.D.T. Optimization of Ultrasonic-Assisted Extraction of Kahweol and Cafestol from Roasted Coffee Using Response Surface Methodology. LWT 2020, 117, 108593. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Pagiatto, N.F.; Kitzberger, C.S.G.; Pereira, L.F.P.; Davrieux, F.; Charmetant, P.; Leroy, T. Validation of Near-Infrared Spectroscopy for the Quantification of Cafestol and Kahweol in Green Coffee. Food Res. Int. 2014, 61, 176–182. [Google Scholar] [CrossRef]
- Zhang, C.; Linforth, R.; Fisk, I.D. Cafestol Extraction Yield from Different Coffee Brew Mechanisms. FRIN 2012, 49, 27–31. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; da Silva, M.A.E.; Silva, D.C.; Aquino Neto, F.R.D.; Rezende, C.M. Extraction of Diterpene-Phytochemicals in Raw and Roasted Coffee Beans and Beverage Preparations and Their Relationship. Plants 2023, 12, 1580. [Google Scholar] [CrossRef]
- Liguori, C.; Giriwono, P.E.; Herawati, D. Kadar Bioaktif Dan Aktivitas Antioksidan Seduhan Kopi Arabika Dengan Variasi Metode Penyeduhan. J. Mutu Pangan Indones. J. Food Qual. 2024, 11, 11–18. [Google Scholar] [CrossRef]
- Wuerges, K.L.; Dias, R.C.E.; Viegas, M.C.; de Toledo Benassi, M. Kahweol and Cafestol in Coffee Brews: Comparison of Preparation Methods. Rev. Cienc. Agron. 2020, 51, e20186553. [Google Scholar] [CrossRef]
- D’Amelio, N.; De Angelis, E.; Navarini, L.; Schievano, E.; Mammi, S. Green Coffee Oil Analysis by High-Resolution Nuclear Magnetic Resonance Spectroscopy. Talanta 2013, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.M.A.; de Almeida, R.H.; de Oliveira, N.A.; Bostyn, S.; Gonçalves, C.B.; de Oliveira, A.L. Enrichment of Diterpenes in Green Coffee Oil Using Supercritical Fluid Extraction—Characterization and Comparison with Green Coffee Oil from Pressing. J. Supercrit. Fluids 2014, 95, 137–145. [Google Scholar] [CrossRef]
- Belandria, V.; Aparecida de Oliveira, P.M.; Chartier, A.; Rabi, J.A.; de Oliveira, A.L.; Bostyn, S. Pressurized-Fluid Extraction of Cafestol and Kahweol Diterpenes from Green Coffee. Innov. Food Sci. Emerg. Technol. 2016, 37, 145–152. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Teixeira, R.S.S.; de Rezende, C.M. Extrusion Pretreatment of Green Arabica Coffee Beans for Lipid Enhance Extraction. Ind. Crops Prod. 2024, 221, 119318. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Schievano, E.; Menegazzo, I.; Fioretti, L.; Caprioli, G.; Vittori, S.; Sagratini, G.; Alessandroni, L. Cafestol and Kahweol Content in Different Specialty Coffee Brews: Exploration by NMR Analysis and Evaluation of Brewing Parameters. J. Food Compos. Anal. 2025, 137, 106929. [Google Scholar] [CrossRef]
- Zanin, R.C.; Kitzberger, C.S.G.; de Toledo Benassi, M. Characterization of Roasted Coffea arabica Species by the Relationship between Caffeine and Diterpenes Contents. Braz. Arch. Biol. Technol. 2020, 63, e20180752. [Google Scholar] [CrossRef]
- Barbosa, M.D.S.G.; Scholz, M.B.D.S.; Kitzberger, C.S.G.; Benassi, M.D.T. Correlation between the Composition of Green Arabica Coffee Beans and the Sensory Quality of Coffee Brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Pagiatto, N.F.; Pereira, L.F.P.; Davrieux, F.; Pot, D.; Charmetant, P.; Leroy, T. Chemical composition in wild Ethiopian Arabica coffee accessions. Euphytica 2016, 209, 429. [Google Scholar] [CrossRef]
- Del Castillo, M.L.R.; Herraiz, M.; Blanch, G.P. Rapid Analysis of Cholesterol-Elevating Compounds in Coffee Brews by Off-Line High Performance Liquid Chromatography/High-Resolution Gas Chromatography. J. Agric. Food Chem. 1999, 47, 1525. [Google Scholar] [CrossRef]
- Urgert, R.; van der Weg, G.; Kosmeijer-Schuil, T.G.; van de Bovenkamp, P.; Hovenier, R.; Katan, M.B. Levels of the Cholesterol-Elevating Diterpenes Cafestol and Kahweol in Various Coffee Brews. J. Agric. Food Chem. 1995, 43, 2167–2172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).