Preliminary Study for Raicilla Authentication by PCA and Cluster on Some Physicochemical Properties
Abstract
1. Introduction
- Raicilla: The cooking of the heads is performed in shaft furnaces, masonry, or autoclave, while the grinding is performed in tahona, Egyptian, or Chilean mill, ripper trapiche, or mill train. Fermentation is performed in wooden containers, masonry pools, or stainless steel tanks. The distillation is carried out in alembics or continuous or discontinuous distillers of copper or stainless steel.
- Artisanal raicilla: In this case, the cooking of the heads is carried out in shaft furnaces or elevated masonry heated with gas or firewood. The grinding is performed with a mallet, bakery, Chilean or Egyptian mill, trapiche, or tearing machine. Fermentation is performed in stone, soil, or trunk masonry pools, wooden or clay containers, and animal skins, utilizing a process that includes the use of maguey fiber (bagasse). Distillation is performed with direct fire in copper alembics or clay pots and with a montera made of clay, wood, copper, or stainless steel capable of holding up to 500 L. In this process, the fiber of the maguey bagasse can be included.
- Ancestral tradition raicilla: The agave heads or maguey heads are cooked in shaft furnaces or masonry ovens. The grinding is performed with mallets on a tahona, a traditional Chilean or Egyptian mill, or a wooden mortar, such as a wooden pool, like a canoe. The fermentation, in this case, is carried out in the same way as in the case of the artisanal raicilla. The distillation is performed with a direct fire produced with firewood in a clay pot and a clay or wooden montera. The maguey fiber (bagasse) must be included in this process.
- Mistletoe Ethanol: the aromatic profile was examined, revealing that the sensory properties of citrus, earth, wood, and mint are attributed to terpenes and terpene alcohols [12].
- Local Alcoholic Beverages in the Regional State of Southern Nations, Nationalities, and Southern Towns (Ethiopia): this study focused on the physicochemical characterization of beverages, including pH, total dissolved solids, total suspended solids, total acidity, and alcohol content. The findings, based on physicochemical characterization, allowed for the identification of the most acidic beverage, as well as the one with the highest alcohol content, resulting in the final recommendation of their consumption levels for health protection [13].
- Alcoholic Beverages (Bangladesh, India, and Nepal): 10 alcoholic beverages, particularly beers and wines, were analyzed physicochemically and microbiologically. The analysis included pH, acidity, total solids, proteins, ash, humidity, alcohol content, and sensory evaluation. The author found that coliform concentrations ranged from 0.03/mL to 2.4/mL. Furthermore, the Nepalese beverage was the best product in terms of sensory analysis [14].
- Beer with Added Cashew Pepuncle and Orange Peel: this study aimed to innovate and improve the nutritional value of beer by adding cashew pepuncle and orange peel. Factors such as pH, total acidity, total sugar, total soluble solids, and humidity were analyzed. The study concluded that adding these ingredients offers new possibilities for innovation in the brewing sector and benefits the environment as the ingredients used are waste products that cause environmental issues [15].
2. Materials and Methods
2.1. pH Analysis
2.2. Conductivity Measurement
2.3. Alcoholic Strength
2.4. Refractive Index
2.5. Viscosity
2.6. Density and Sound Velocity Measurements
2.7. Total Solids
- = initial weight;
- = final weight.
2.8. Data Statistical Treatment
3. Results and Discussion
3.1. Analysis of Raicilla
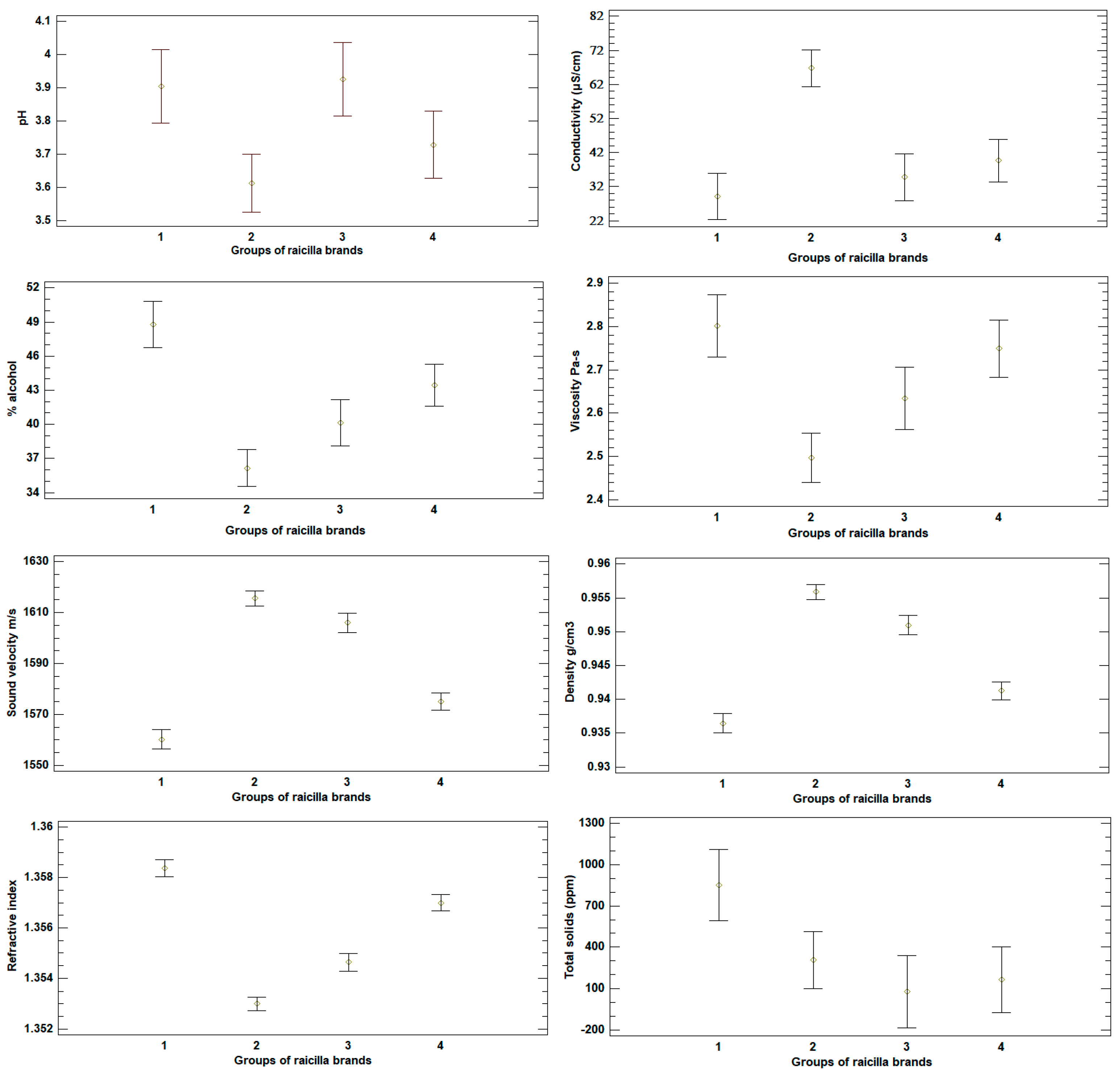
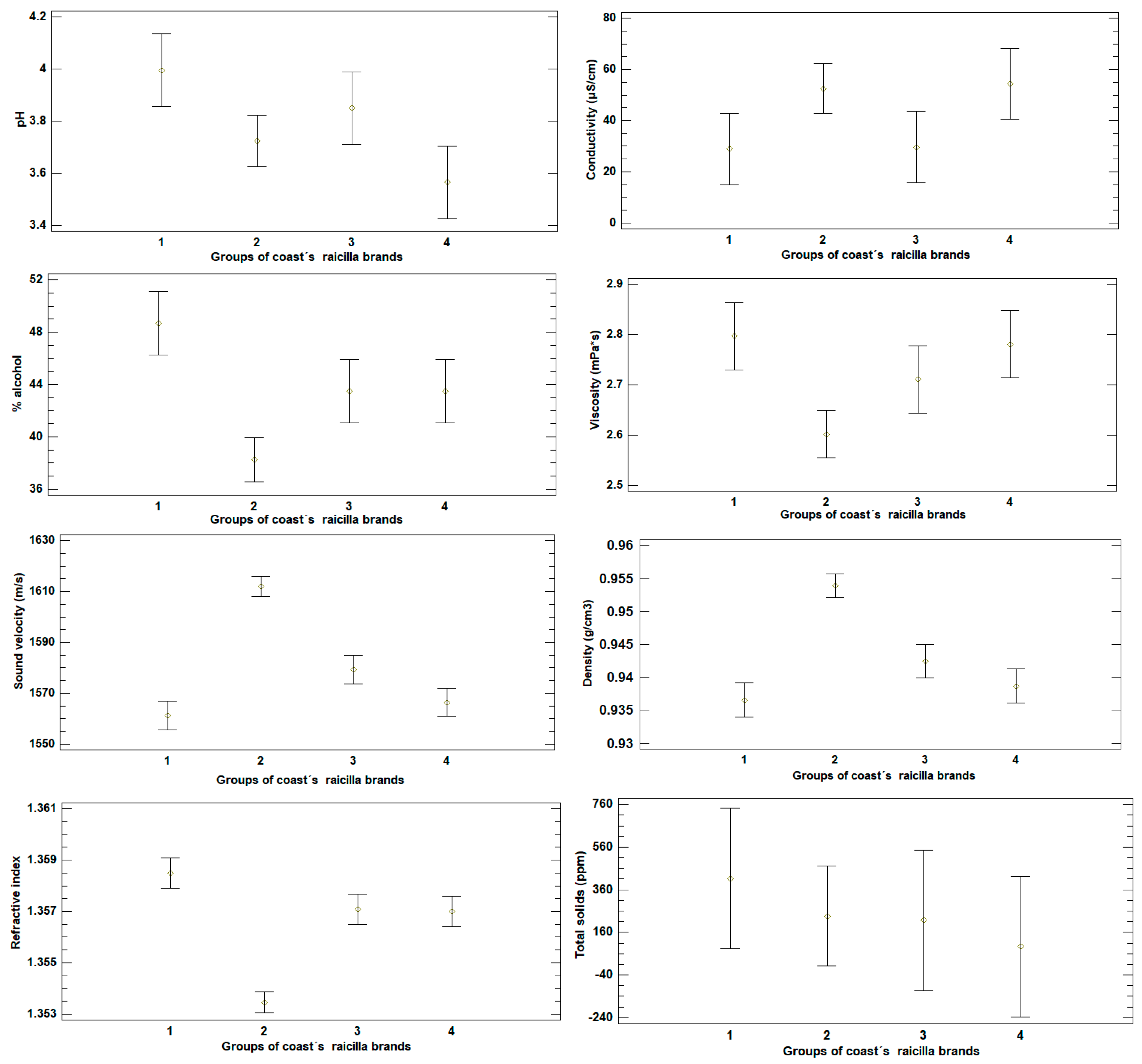
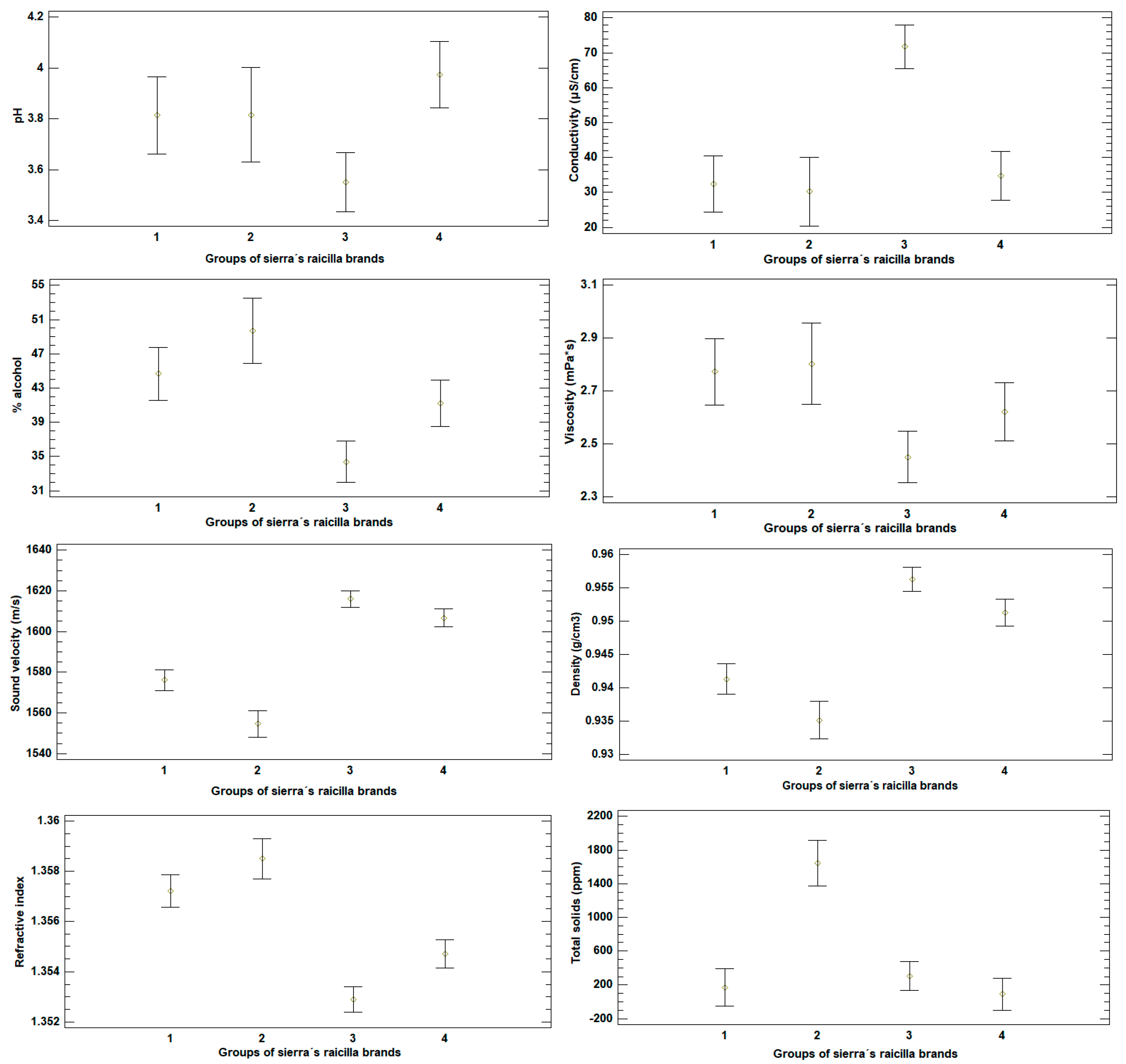
3.2. Analysis of Physicochemical Measurements in Raicilla
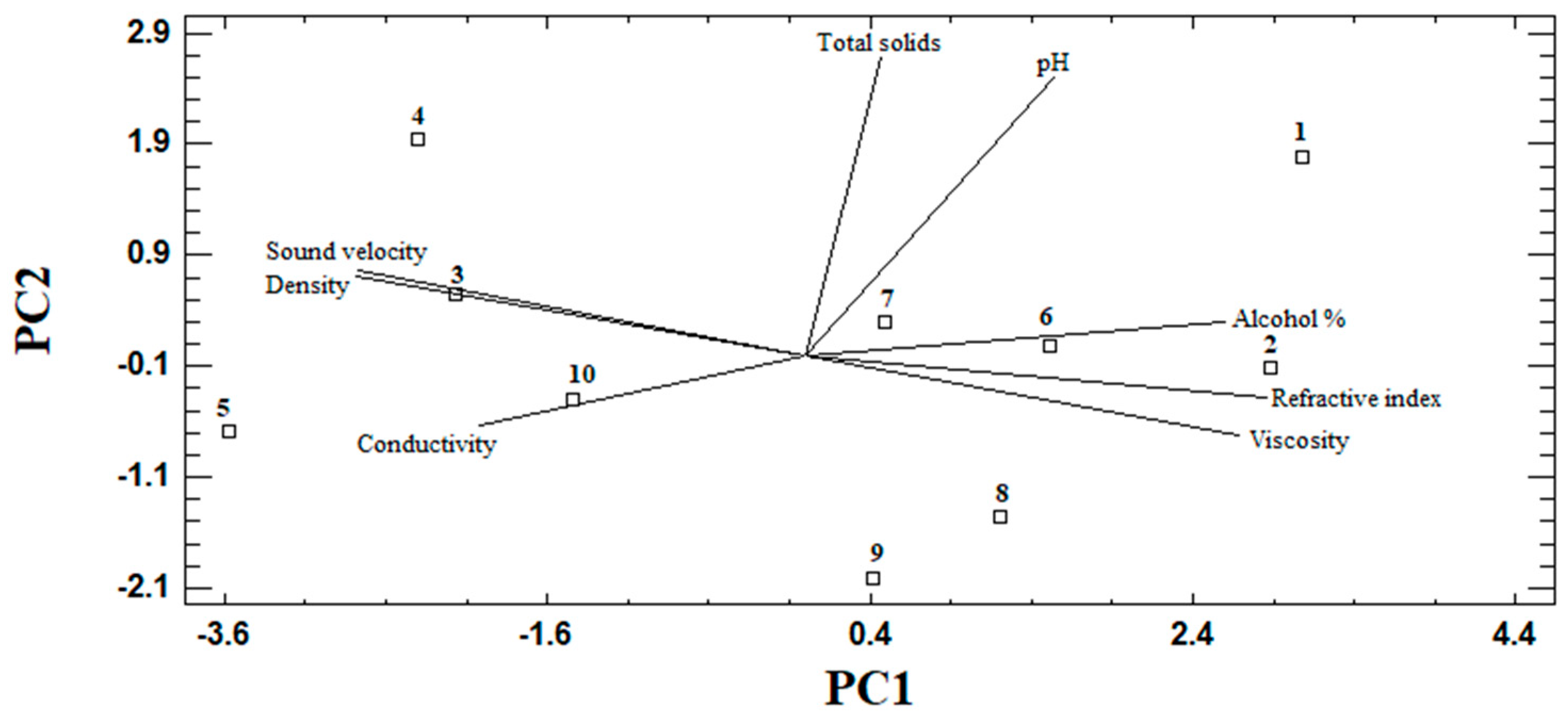
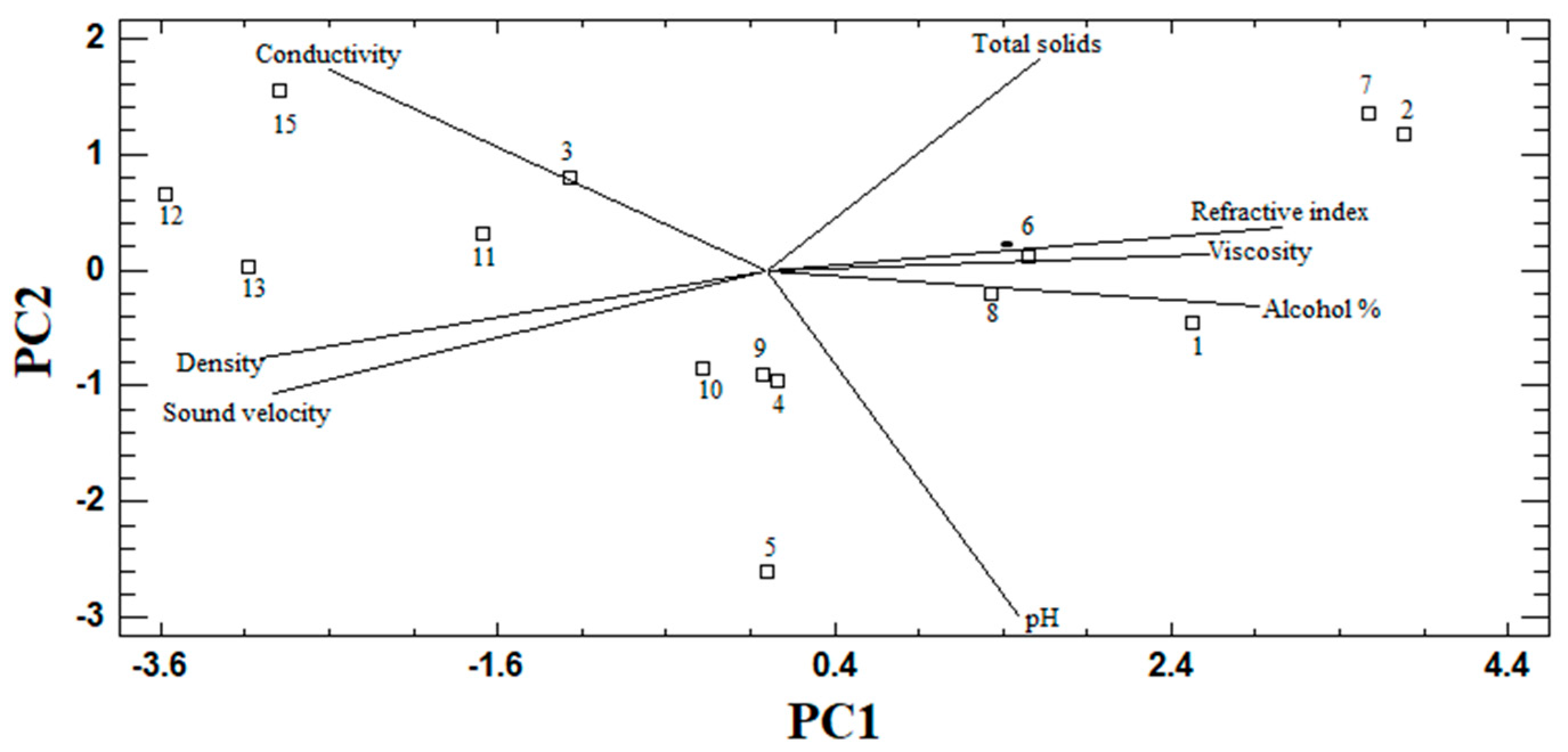
3.3. PCA in Raicilla
3.4. Analysis by Cluster in Raicilla
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PROYECTO de Norma Oficial Mexicana PROY-NOM-257-SE-2021, B. Alcohólicas-R.-D. Especificaciones, Información Comercial y Métodos de Prueba. DOF—Diario Oficial de La Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5650295&fecha=27/04/2022#gsc.tab=0 (accessed on 6 January 2025).
- Arrizón, J.; Arizaga, J.J.; Hernandez, R.E.; Estarrón, M.; Gschaedler, A. Production of Volatile Compounds in Tequila and Raicilla Musts by Different Yeasts Isolated from Mexican Agave Beverages. ACS Symp. Ser. 2007, 946, 167–177. [Google Scholar] [CrossRef]
- Franco Gordo, M.; Goyas Mejía, R.; Navarro Ochoa, A.; Nuño Gutiérrez, M.R.; Tulet, J.-C.; Carreón Álvarez, M.A.; Sánchez Huerta, A.I.; Trujillo Orozco, A.G.; Zamudio Ojeda, A.; Zurita Martínez, F. La Raicilla. Herencia y Patrimonio Cultural de Jalisco; Editorial Universidad de Guadalajara: Guadalajara, Spain, 2015. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Escalante-Minakata, P.; Jiménez-García, M.I.; Ordoñez-Acevedo, L.G.; Flores Flores, J.L.; Barba de la Rosa, A.P. Characterization of Volatile Compounds from Ethnic Agave Alcoholic Beverages by Gas Chromatography-Mass Spectrometry. Available online: https://www.ftb.com.hr/?view=article&id=256:characterization-of-volatile-compounds-from-ethnic-agave-alcoholic-beverages-by-gas-chromatography-mass-spectrometry&catid=67&highlight=WyJjaGFyYWN0ZXJpemF0aW9uIiwib2YiLCJ2b2xhdGlsZSIsImNvbXBvdW5kcyIsImZyb20iLCJldGhuaWMiLCJhZ2F2ZSIsImFsY29ob2xpYyIsImJldmVyYWdlcyIsImJ5IiwiZ2FzIiwiY2hyb21hdG9ncmFwaHktbWFzcyIsInNwZWN0cm9tZXRyeSJd (accessed on 25 December 2024).
- Trujillo-Orozco, A.; Gómez-Salazar, S.; Bárcena-Soto, M.; Carreon-Alvarez, A.; Estrada-Vargas, A.; Prado-Ramírez, R.; Casillas, N. Detection of Cu, Pb, Cd and Zn in Mexican Spirituous Beverages by Differential Pulse Polarography (DPP). ECS Trans. 2011, 36, 363–372. [Google Scholar] [CrossRef]
- Carreón Álvarez, M.A.; Sánchez Huerta, A.I.; Trujillo Orozco, A.G.; Zamudio Ojeda, A.; Zurita Martínez, F. Estudio de Parámetros Fisicoquímicos de La Raicilla En Diferentes Zonas Del Estado de Jalisco. In Herencia y Patrimonio Cultural de Jalisco; Editorial Universidad de Guadalajara: Guadalajara, Spain, 2015; pp. 142–162. [Google Scholar]
- Contreras-Loera, U.; Barbosa-García, O.; Ramos-Ortíz, G.; Pichardo-Molina, J.L.; Meneses-Nava, M.A.; Maldonado, J.L. Identificación y Discriminación de Tequilas Reposados in Situ Para La Protección de Marca. Nova Sci. 2009, 1, 22–32. [Google Scholar] [CrossRef][Green Version]
- Martelo-Vidal, M.J.; Vázquez, M. Determination of Polyphenolic Compounds of Red Wines by UV–VIS–NIR Spectroscopy and Chemometrics Tools. Food Chem. 2014, 158, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Martelo-Vidal, M.J.; Vázquez, M. Evaluation of Ultraviolet, Visible, and near Infrared Spectroscopy for the Analysis of Wine Compounds. Czech J. Food Sci. 2014, 32, 37–47. [Google Scholar] [CrossRef]
- Pontes, M.J.C.; Santos, S.R.B.; Araújo, M.C.U.; Almeida, L.F.; Lima, R.A.C.; Gaião, E.N.; Souto, U.T.C.P. Classification of Distilled Alcoholic Beverages and Verification of Adulteration by near Infrared Spectrometry. Food Res. Int. 2006, 39, 182–189. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Fu, M.; Li, G.; Lou, Z. Classification of Chinese Yellow Wines by ChemometricAnalysis of Cyclic Voltammogram of Copper Electrodes. Sensors 2005, 5, 529–536. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Lukin, P.; Derewiaka, D.; Mrvčić, J.; Stanzer, D. Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages 2022, 8, 46. [Google Scholar] [CrossRef]
- Alemayehu, H.G. Physico-Chemical Characterization of Commercial Local Alcohol Beverages Available in South Nations, Nationalities and People’s Regional State, Ethiopia. Int. J. Chemtech Res. 2018, 11, 227–231. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Yadav, B.; Sah, P.; Ara, R.; Alam, M.; Zzaman, W. Microbial, Physicochemical and Sensory Characteristics Analysis of Selected Alcoholic Beverages of from Bangladesh, India and Nepal. J Biosci. 2018, 23, 67–75. [Google Scholar] [CrossRef]
- Pereira, I.M.C.; Matos Neto, J.D.; Figueiredo, R.W.; Carvalho, J.D.G.; de Figueiredo, E.A.T.; de Menezes, N.V.S.; Gaban, S.V.F. Physicochemical Characterization, Antioxidant Activity, and Sensory Analysis of Beers Brewed with Cashew Peduncle (Anacardium occidentale) and Orange Peel (Citrus sinensis). Food Sci. Technol. 2020, 40, 749–755. [Google Scholar] [CrossRef]
- NOM-142-SSA1/SCFI-2014; Bebidas Alcohólicas. Especificaciones Sanitarias. Etiquetado Sanitario y Comercial. DOF—Diario Oficial de La Federación: Mexico City, Mexico, 2015. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5386313&fecha=23/03/2015#gsc.tab=0 (accessed on 5 January 2025).
- NOM-070-SCFI-2016; B. Alcohólicas-M.-Especificaciones. DOF—Diario Oficial de La Federación: Mexico City, Mexico, 2016.
- NOM-006-SCFI-2012; B. alcohólicas-T.-Especificaciones. DOF—Diario Oficial de La Federación: Mexico City, Mexico, 2012. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5282165&fecha=13/12/2012#gsc.tab=0 (accessed on 5 January 2025).
- Fernandes, W.J.; das Graças Cardoso, M.; Vilela, F.J.; de Morais, A.R.; de Fátima Silva, V.; Nelson, D.L. Physicochemical Quality of a Blend of Domestic Cachaças from the South of Minas Gerais. J. Food Compos. Anal. 2007, 20, 257–261. [Google Scholar] [CrossRef]
- Adeleke, R.O.; Abiodun, O.A. Physico-Chemical Properties of Commercial Local Beverages in Osun State, Nigeria. Pak. J. Nutr. 2010, 9, 853–855. [Google Scholar] [CrossRef]
- Carreon-Alvarez, A.; Suárez-Gómez, A.; Zurita, F.; Gómez-Salazar, S.; Soltero, J.F.A.; Barcena-Soto, M.; Casillas, N.; Porfirio-Gutierrez; Moreno-Medrano, E.D. Assessment of Physicochemical Properties of Tequila Brands: Authentication and Quality. J. Chem. 2016, 2016, 6254942. [Google Scholar] [CrossRef]
- NMX-V-013-NORMEX-2019; DECLARATORIA de Vigencia de la Norma Mexicana. DOF—Diario Oficial de La Federación: Mexico City, Mexico, 2019. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5594809&fecha=11/06/2020#gsc.tab=0 (accessed on 6 January 2025).
- Belay, A.; Assefa, G. Concentration, Wavelength and Temperature Dependent Refractive Index of Sugar Solutions and Methods of Determination Contents of Sugar in Soft Drink Beverages Using Laser Lights. J. Lasers Opt. Photonics 2018, 5, 1000187. [Google Scholar] [CrossRef]
- Gasinski, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gasior, J.; Pietrzak, W. Volatile Compounds Content, Physicochemical Parameters, and Antioxidant Activity of Beers with Addition of Mango Fruit (Mangifera indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, G.B.; Otunola, G.A.; Ajao, T.A. Physicochemical, Microbiological and Sensory Characteristics of Kunu Prepared from Millet, Maize and Guinea Corn and Stored at Selected Temperatures. Adv. J. Food Sci. Technol. 2010, 2, 41–46. [Google Scholar]
- Santos, M.J.; Correia, E.; Vilela, A. Exploring the Impact of α-Amylase Enzyme Activity and PH on Flavor Perception of Alcoholic Drinks. Foods 2023, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, X.; Yang, L.; Zhao, J.; Zhu, X. Effect of Deacidification Treatment on the Flavor Quality of Zaosu Pear–Kiwifruit Wine. Foods 2022, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, A.J.; Rosenblatt, M. PH in Distilled Alcoholic Beverages. J. AOAC Int. 1942, 25, 163–168. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Andrade-Sobrinho Luis, G.; Leite-Neto, A.F.; Reche, R.V.; Isique, W.D.; Ferreira, M.M.C.; Lima-Neto, B.S.; Franco, D.W. Comparison between Cachaça and Rum Using Pattern Recognition Methods-PubMed. J. Agric. Food Chem. 2004, 52, 3429–3433. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, A.; Chalarakis, E.; Zarangas, G. Influence of Raw Materials and Distillation Equipment on the Heavy Metal Content of Waste from an Alcoholic Anis-Type Beverage. J. Hazard. Mater. 2003, 96, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in Alcoholic Beverages: A Review of Sources, Effects, Concentrations, Removal, Speciation, and Analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Sha, S.; Yin, H.; Zhang, H.; Wang, Y.; Zhao, B.; Song, F. Analysis of the Tendency for the Electronic Conductivity to Change during Alcoholic Fermentation. Sci. Rep. 2019, 9, 5512. [Google Scholar] [CrossRef] [PubMed]
- Leveling, T. The Relationship Between PH and Conductivity in a Lithium Contaminated, De-Ionized Water System; Fermi National Accelerator Laboratory (FNAL): Batavia, IL, USA, 2002. [Google Scholar]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The Analysis of Vodka: A Review Paper. Food Anal. Methods 2015, 8, 2000–2010. [Google Scholar] [CrossRef]
- Resende Oliveira, É.; Caliari, M.; Soares Soares, M., Jr.; Ribeiro Oliveira, A.; Cristina Marques Duarte, R.; Valério de Barros Vilas Boas, E. Assessment of Chemical and Sensory Quality of Sugarcane Alcoholic Fermented Beverage. J. Food Sci. Technol. 2018, 55, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Castellan Gilbert, W. Fisicoquimica Segunda Edición; Addison Wesley Longman: Boston, MA, USA, 1987; Volume 2, ISBN 9789684443167. [Google Scholar]
- Jordão, A.M.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Spillman, P.J.; Pollnitz, A.P.; Liacopoulos, D.; Skouroumounis, G.K.; Sefton, M.A. Accumulation of Vanillin during Barrel-Aging of White, Red, and Model Wines. J. Agric. Food Chem. 1997, 45, 2584–2589. [Google Scholar] [CrossRef]
- Öztürk, I.; Karaman, S.; Törnük, F.; Saǧdiç, O. Physicochemical and Rheological Characteristics of Alcohol Free Probiotic Boza Produced Using Lactobacillus Casei Shirota: Estimation of Apparent Viscosity of Boza Using Non-Linear Modeling Techniques. Turk. J. Agric. For. 2013, 37, 475–487. [Google Scholar] [CrossRef]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.J. Properties of Sugar, Polyol, and Polysaccharide Water−Ethanol Solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]


| Number | Variety | Zone | pH | Conductivity (µS/cm) | Alcohol Content (%) | Total Solids (ppm) |
| 1 | White | Coast | 4.05 ± 0.00 | 29.67 ± 0.11 | 49.00 ± 0.00 | 680.00 ± 118.39 |
| 2 | White | Sierra | 3.90 ± 0.01 | 27.28 ± 0.14 | 47.33 ± 0.58 | 142.67 ± 15.14 |
| 3 | White | Coast | 3.94 ± 0.02 | 28.06 ± 0.13 | 48.33 ± 0.58 | 145.33 ± 18.04 |
| 4 | White | Sierra | 3.89 ± 0.01 | 24.69 ± 0.13 | 48.66 ± 0.58 | 2060.00 ± 42.14 |
| 5 | Rested | Sierra | 3.56 ± 0.01 | 69.57 ± 0.40 | 39.33 ± 0.58 | 302.67 ± 82.00 |
| 6 | White | Sierra | 3.84 ± 0.00 | 30.30 ± 0.10 | 41.00 ± 0.00 | 58.67 ± 15.14 |
| 7 | White | Sierra | 4.36 ± 0.02 | 34.47 ± 0.35 | 40.00 ± 0.00 | 112.00 ± 49.15 |
| 8 | White | Sierra | 3.74 ± 0.01 | 37.33 ± 0.15 | 45.66 ± 0.58 | 217.33 ± 28.38 |
| 9 | White | Sierra | 3.74 ± 0.01 | 35.73 ± 0.15 | 50.66 ± 0.58 | 1233.33 ± 44.96 |
| 10 | White | Sierra | 3.80 ± 0.01 | 32.60 ± 0.30 | 41.00 ± 0.00 | 156.00 ± 8.00 |
| 11 | White | Sierra | 3.85 ± 0.01 | 34.63 ± 0.15 | 41.33 ± 0.58 | 70.67 ± 10.07 |
| 12 | White | Sierra | 3.84 ± 0.01 | 39.57 ± 0.51 | 42.66 ± 0.58 | 121.33 ± 28.38 |
| 13 | White | Sierra | 3.59 ± 0.01 | 61.60 ± 1.25 | 38.33 ± 0.58 | 216.00 ± 39.39 |
| 14 | White | Sierra | 3.57 ± 0.01 | 84.30 ± 1.13 | 31.33 ± 0.58 | 224.00 ± 21.17 |
| 15 | White | Sierra | 3.67 ± 0.01 | 57.93 ± 0.25 | 35.00 ± 0.00 | 716.00 ± 47.16 |
| 16 | White | Coast | 3.80 ± 0.01 | 45.40 ± 0.72 | 39.66 ± 0.58 | 153.33 ± 65.16 |
| 17 | Rested | Coast | 3.84 ± 0.02 | 66.10 ± 1.00 | 39.00 ± 0.00 | 698.67 ± 114.29 |
| 18 | White | Coast | 3.51 ± 0.02 | 63.80 ± 0.61 | 38.66 ± 0.58 | 73.33 ± 8.33 |
| 19 | White | Coast | 3.83 ± 0.00 | 28.03 ± 0.17 | 46.00 ± 0.00 | 222.67 ± 19.73 |
| 20 | Rested | Coast | 3.87 ± 0.01 | 31.33 ± 0.64 | 41.00 ± 0.00 | 209.33 ± 46.36 |
| 21 | Abocado | Sierra | 3.72 ± 0.02 | 1909.67 ± 18.04 | ------------- | 166,984.0 ± 5988.99 |
| 22 | White | Coast | 3.62 ± 0.02 | 47.47 ± 0.07 | 44.33 ± 0.58 | 106.67 ± 20.53 |
| 23 | White | Coast | 3.51 ± 0.01 | 61.37 ± 0.32 | 42.66 ± 0.58 | 78.67 ± 12.22 |
| 24 | White | Coast | 3.74 ± 0.01 | 34.90 ± 0.0 | 35.66 ± 0.58 | 21.33 ± 6.11 |
| 25 | White | Sierra | 3.36 ± 0.01 | 85.37 ± 0.15 | 28.00 ± 0.00 | 58.67 ± 8.33 |
| Number | Variety | Zone | Viscosity (mPa × s) | Sound velocity (m/s) | Density (g/cm3) | Refractive Index |
| 1 | White | Coast | 2.79 ± 4.0 × 10−5 | 1561.69 ± 0.05 | 0.93665 ± 0.06 | 1.35833 ± 7.6 × 10−4 |
| 2 | White | Sierra | 2.80 ± 5.0 × 10−4 | 1569.39 ± 0.06 | 0.93879 ± 0.06 | 1.35783 ± 2.8 × 10−4 |
| 3 | White | Coast | 2.80 ± 5.0 × 10−5 | 1560.79 ± 0.05 | 0.93650 ± 0.06 | 1.35867 ± 2.8 × 10−4 |
| 4 | White | Sierra | 2.80 ± 1.0 × 10−5 | 1559.11 ± 0.05 | 0.93580 ± 0.06 | 1.35833 ± 2.8 × 10−4 |
| 5 | Rested | Sierra | 2.65 ± 2.3 × 10−4 | 1608.26 ± 0.08 | 0.95223 ± 0.05 | 1.35433 ± 7.6 × 10−4 |
| 6 | White | Sierra | 2.67 ± 8.9 × 10−4 | 1604.23 ± 0.08 | 0.95047 ± 0.05 | 1.35500 ± 5.0 × 10−4 |
| 7 | White | Sierra | 2.62 ± 7.1 × 10−4 | 1611.09 ± 0.09 | 0.95270 ± 0.05 | 1.35450 ± 0 |
| 8 | White | Sierra | 2.75 ± 6.2 × 10−4 | 1577.53 ± 0.06 | 0.94274 ± 0.06 | 1.35700 ± 5.0 × 10−4 |
| 9 | White | Sierra | 2.81 ± 5.8 × 10−4 | 1549.96 ± 0.04 | 0.93449 ± 0.06 | 1.35867 ± 2.8 × 10−4 |
| 10 | White | Sierra | 2.77 ± 2.4 × 10−4 | 1581.37 ± 0.07 | 0.94244 ± 0.06 | 1.35683 ± 2.8 × 10−4 |
| 11 | White | Sierra | 2.66 ± 3.0 × 10−4 | 1604.35 ± 0.08 | 0.95031 ± 0.05 | 1.35483 ± 2.8 × 10−4 |
| 12 | White | Sierra | 2.55 ± 5.4 × 10−4 | 1607.22 ± 0.08 | 0.95153 ± 0.05 | 1.35450 ± 0 |
| 13 | White | Sierra | 2.55 ± 6.9 × 10−4 | 1613.96 ± 0.09 | 0.95490 ± 0.04 | 1.35333 ± 2.8 × 10−4 |
| 14 | White | Sierra | 2.29 ± 2.3 × 10−4 | 1621.57 ± 0.09 | 0.95922 ± 0.04 | 1.35217 ± 1.04 × 10−3 |
| 15 | White | Sierra | 2.16 ± 1.4 × 10−3 | 1624.26 ± 0.09 | 0.96078 ± 0.04 | 1.35167 ± 2.8 × 10−4 |
| 16 | White | Coast | 2.60 ± 3.0 × 10−4 | 1613.38 ± 0.09 | 0.95469 ± 0.04 | 1.35333 ± 2.8 × 10−4 |
| 17 | Rested | Coast | 2.60 ± 4.0× 10−5 | 1613.60 ± 0.09 | 0.95450 ± 0.04 | 1.35333 ± 5.7 × 10−4 |
| 18 | White | Coast | 2.53 ± 1.60 × 10−4 | 1617.69 ± 0.09 | 0.95652 ± 0.04 | 1.35283 ± 2.8 × 10−4 |
| 19 | White | Coast | 2.76 ± 1.12 × 10−3 | 1577.61 ± 0.06 | 0.94249 ± 0.06 | 1.35733 ± 2.8 × 10−4 |
| 20 | Rested | Coast | 2.66 ± 6.70 × 10−4 | 1580.87 ± 0.06 | 0.94242 ± 0.06 | 1.35683 ± 2.8 × 10−4 |
| 21 | Abocado | Sierra | 2.44 ± 5.61 × 10−3 | 1596.50 ± 0.08 | 1.05085 ± 0.05 | 1.36283 ± 1.04 × 10−3 |
| 22 | White | Coast | 2.79 ± 1.80 × 10−4 | 1564.53 ± 0.05 | 0.93760 ± 0.06 | 1.35700 ± 0 |
| 23 | White | Coast | 2.78 ± 3.00 × 10−4 | 1568.15 ± 0.06 | 0.93977 ± 0.06 | 1.35700 ± 0 |
| 24 | White | Coast | 2.68 ± 4.20 × 10−4 | 1603.35 ± 0.08 | 0.94983 ± 0.05 | 1.35433 ± 2.8 × 10−4 |
| 25 | White | Sierra | 2.60 ± 1.60 × 10−4 | 1611.77 ± 0.09 | 0.95420 ± 0.04 | 1.35300 ± 0 |
| Component Number | Eigenvalue | Percentage of Variance | Accumulated Percentage |
|---|---|---|---|
| 1 | 5.3905 | 67.381 | 67.381 |
| 2 | 1.1513 | 14.391 | 81.772 |
| 3 | 0.9461 | 11.826 | 93.598 |
| 4 | 0.2060 | 2.574 | 96.173 |
| 5 | 0.1559 | 1.949 | 98.121 |
| 6 | 0.1388 | 1.735 | 99.856 |
| 7 | 0.0099 | 0.124 | 99.980 |
| 8 | 0.0016 | 0.020 | 100.00 |
| Component 1 | Component 2 | |
|---|---|---|
| pH | 0.215809 | −0.769737 |
| Conductivity (µS/cm) | −0.345717 | 0.459528 |
| Alcohol content (%) | 0.399783 | −0.056169 |
| Viscosity (mPa × s) | 0.369187 | 0.172723 |
| Sound velocity (m/s) | −0.40408 | −0.280002 |
| Density (g/cm3) | −0.412441 | −0.231211 |
| Refractive index | 0.421131 | 0.132307 |
| Total solids (ppm) | 0.164443 | 0.118264 |
| Component Number | Eigenvalue | Percentage of Variance | Accumulated Percentage |
|---|---|---|---|
| 1 | 5.2496 | 65.619 | 65.619 |
| 2 | 1.5799 | 19.748 | 85.368 |
| 3 | 0.8527 | 10.659 | 96.027 |
| 4 | 0.1953 | 2.441 | 98.468 |
| 5 | 0.0774 | 0.968 | 99.436 |
| 6 | 0.0345 | 0.432 | 99.868 |
| Component 1 | Component 2 | |
|---|---|---|
| pH | 0.2310 | 0.6326 |
| Conductivity (µS/cm) | −0.3048 | −0.1591 |
| Alcohol content (%) | 0.3915 | 0.0756 |
| Viscosity (mPa × s) | 0.4037 | −0.1815 |
| Sound velocity (m/s) | −0.4163 | 0.1945 |
| Density (g/cm3) | −0.4184 | 0.1792 |
| Refractive index | 0.4293 | −0.0981 |
| Total solids (ppm) | 0.0699 | 0.6755 |
| Component Number | Eigenvalue | Percentage of Variance | Accumulated Percentage |
|---|---|---|---|
| 1 | 5.5845 | 69.807 | 69.807 |
| 2 | 1.2438 | 15.547 | 85.354 |
| 3 | 0.7970 | 9.962 | 95.316 |
| 4 | 0.1987 | 2.484 | 97.800 |
| 5 | 0.0921 | 1.151 | 98.952 |
| 6 | 0.0799 | 0.999 | 99.951 |
| 7 | 0.0029 | 0.037 | 99.988 |
| 8 | 0.0009 | 0.012 | 100.00 |
| Component 1 | Component 2 | |
|---|---|---|
| pH | 0.2034 | −0.7191 |
| Conductivity (µS/cm) | −0.3535 | 0.4183 |
| Alcohol content (%) | 0.3970 | −0.0776 |
| Viscosity (mPa × s) | 0.3577 | 0.03475 |
| Sound velocity (m/s) | −0.3992 | −0.2592 |
| Density (g/cm3) | −0.4093 | −0.1846 |
| Refractive index | 0.4162 | 0.0899 |
| Total solids (ppm) | 0.2195 | 0.4375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreon-Alvarez, A.; Zurita, F.; Carreon-Alvarez, C.; Sanchez-Tizapa, M.; Huerta, H.; Tepale, N.; Morán-Lázaro, J.P. Preliminary Study for Raicilla Authentication by PCA and Cluster on Some Physicochemical Properties. Beverages 2025, 11, 107. https://doi.org/10.3390/beverages11040107
Carreon-Alvarez A, Zurita F, Carreon-Alvarez C, Sanchez-Tizapa M, Huerta H, Tepale N, Morán-Lázaro JP. Preliminary Study for Raicilla Authentication by PCA and Cluster on Some Physicochemical Properties. Beverages. 2025; 11(4):107. https://doi.org/10.3390/beverages11040107
Chicago/Turabian StyleCarreon-Alvarez, Alejandra, Florentina Zurita, Clara Carreon-Alvarez, Marciano Sanchez-Tizapa, Héctor Huerta, Nancy Tepale, and Juan Pablo Morán-Lázaro. 2025. "Preliminary Study for Raicilla Authentication by PCA and Cluster on Some Physicochemical Properties" Beverages 11, no. 4: 107. https://doi.org/10.3390/beverages11040107
APA StyleCarreon-Alvarez, A., Zurita, F., Carreon-Alvarez, C., Sanchez-Tizapa, M., Huerta, H., Tepale, N., & Morán-Lázaro, J. P. (2025). Preliminary Study for Raicilla Authentication by PCA and Cluster on Some Physicochemical Properties. Beverages, 11(4), 107. https://doi.org/10.3390/beverages11040107







