The Effects of Pasteurization and Beer Type on the Functional Compounds and Flavor Substances in Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Beer
2.2. Determination of Amino Acid Derivatives (GABA and GSH)
2.2.1. Detection of γ-Aminobutyric Acid (GABA)
2.2.2. Detection of Glutathione (GSH)
2.3. Determination of β-Glucan
2.4. Determination of Phenolic Acids and Vitamin B
2.4.1. Detection of Phenolic Acids
2.4.2. Detection of B Vitamins
2.5. Determination of Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
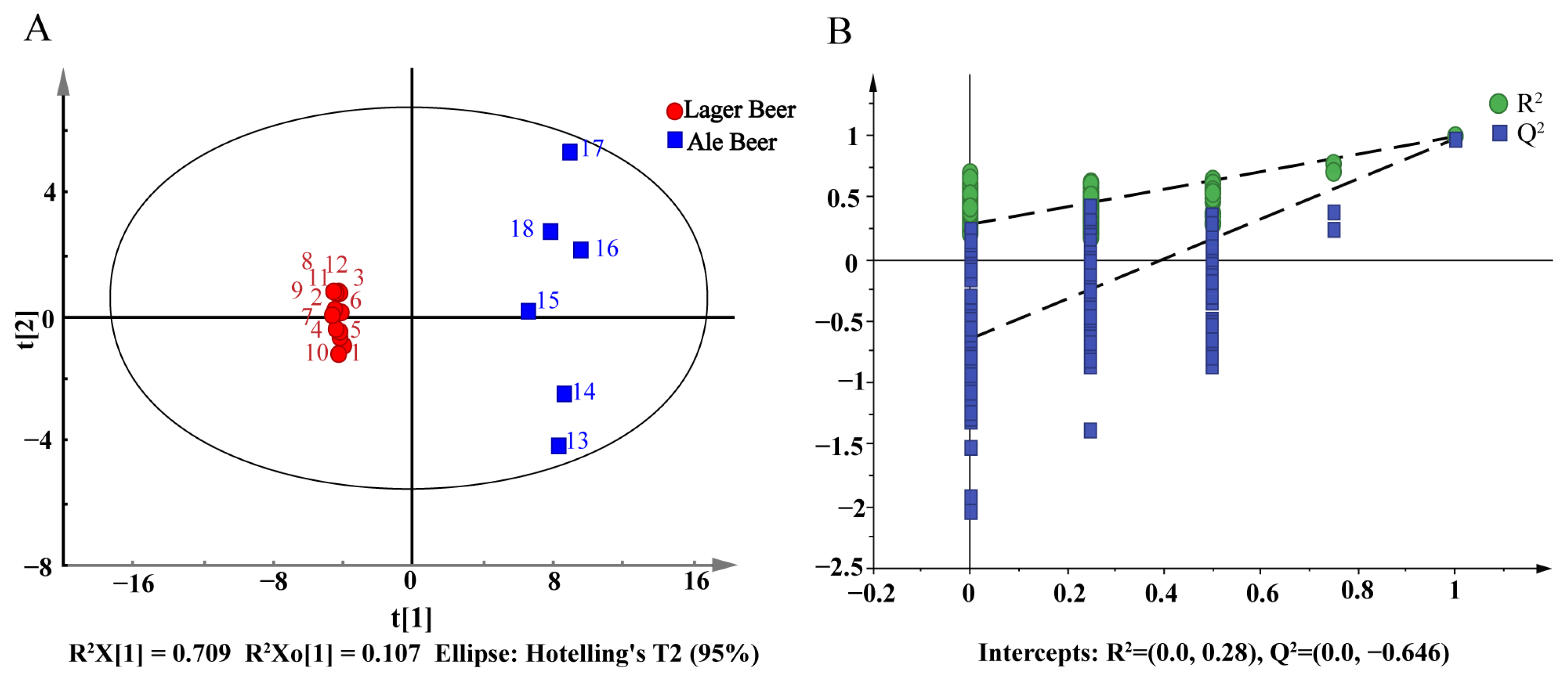
3.1. Establishment of Screening Scheme for Functional Compounds in Beer
3.2. Effects of Different Types on Amino Acid Derivatives in Beers (GABA and GSH)
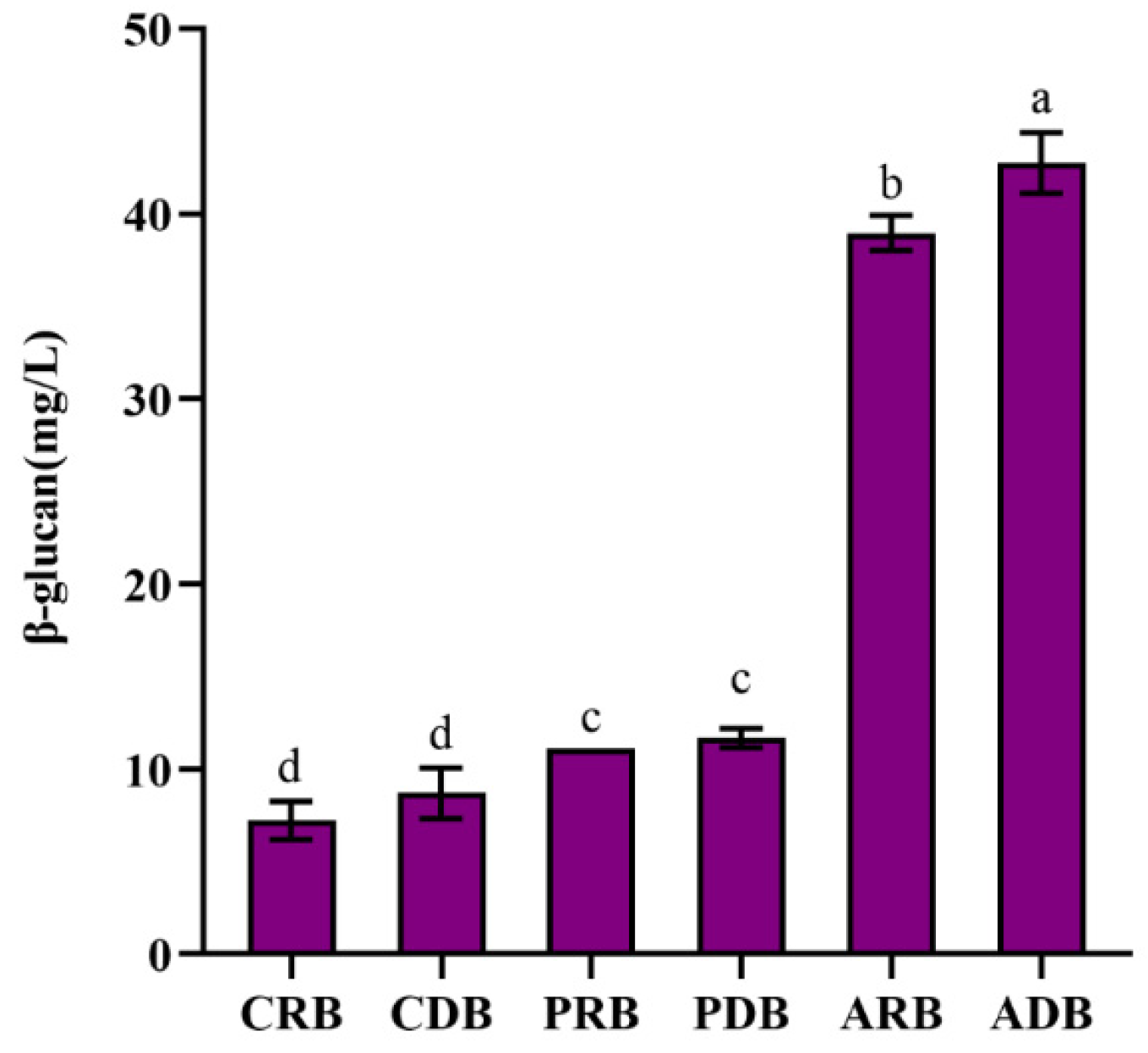
3.3. Effect of Different Types on the Content of β-Glucan in Beers
3.4. Effect of Different Types on the Contents of B Vitamins and Phenolic Acids in Beers
3.5. Effect of Different Types on Volatile Compounds in Beers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Ceccaroni, D.; Marconi, O.; Sileoni, V.; Perretti, G.; Fantozzi, P. Development of an all rice malt beer: A gluten free alternative. LWT-Food Sci. Technol. 2016, 67, 67–73. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Gagula, G.; Đurđević-Milošević, D.; Ncube, T.; Magdić, D. The effect of pasteurisation and storage on aroma compounds in lager. J. Inst. Brew. 2024, 130, 83–92. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Advanced analytical methods for the analysis of chemical and microbiological properties of beer. J. Food Drug Anal. 2020, 19, 1. [Google Scholar] [CrossRef]
- Iimure, T.; Kihara, M.; Hirota, N.; Zhou, T.; Hayashi, K.; Ito, K. A method for production of γ-amino butyric acid (GABA) using barley bran supplemented with glutamate. Food Res. Int. 2009, 42, 319–323. [Google Scholar] [CrossRef]
- Fracassetti, D.; Lawrence, N.; Tredoux, A.G.J.; Tirelli, A.; Nieuwoudt, H.H.; Du Toit, W.J. Quantification of glutathione, catechin and caffeic acid in grape juice and wine by a novel ultra-performance liquid chromatography method. Food Chem. 2011, 128, 1136–1142. [Google Scholar] [CrossRef]
- Garofalo, C.; Sabbatini, R.; Zamporlini, F.; Minazzato, G.; Ferrocino, I.; Aquilanti, L.; Raffaelli, N.; Osimani, A. Exploratory study on the occurrence and dynamics of yeast-mediated nicotinamide riboside production in craft beers. LWT-Food Sci. Technol. 2021, 147, 111605. [Google Scholar] [CrossRef]
- Jin, Y.L.; Speers, A.; Paulson, A.T.; Stewart, R.J. Effects of β-glucans and environmental factors on the viscosities of wort and beer. J. Inst. Brew. 2004, 110, 104–116. [Google Scholar] [CrossRef]
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J.P. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Schneider, J.K.; Leal, I.L.; de Abreu Barreto, G.; Batista, T.; Machado, B.A.S.; Druzian, J.I.; Krause, L.C.; da Costa Mendonça, M.; et al. Physicochemical and sensory profile of Beauregard sweet potato beer. Food Chem. 2020, 312, 126087. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, B.; Zhou, Y.; Chen, J.; Tu, H. HPLC determination of γ-aminobutyric acid in Chinese rice wine using pre-column derivatization. J. Inst. Brew. 2015, 121, 163–166. [Google Scholar] [CrossRef]
- Phuong Hong, L.; Verscheure, L.; Thien Trung, L.; Verheust, Y.; Raes, K. Implementation of HPLC Analysis for γ-Aminobutyric Acid (GABA) in Fermented Food Matrices. Food Anal. Methods 2020, 13, 1190–1201. [Google Scholar]
- Andujar-Ortiz, I.; Angeles Pozo-Bayon, M.; Victoria Moreno-Arribas, M.; Martin-Alvarez, P.J.; Rodriguez-Bencomo, J.J. Reversed-Phase High-Performance Liquid Chromatography-Fluorescence Detection for the Analysis of Glutathione and Its Precursor γ-Glutamyl Cysteine in Wines and Model Wines Supplemented with Oenological Inactive Dry Yeast Preparations. Food Anal. Methods 2012, 5, 154–161. [Google Scholar] [CrossRef]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Rahman, M.J.; Liang, J.; Eskin, N.A.M.; Eck, P.; Thiyam-Hollander, U. Identification of hydroxycinnamic acid derivatives of selected canadian and foreign commercial beer extracts and determination of their antioxidant properties. LWT-Food Sci. Technol. 2020, 122, 109021. [Google Scholar] [CrossRef]
- Kupetz, M.; Aumer, J.; Harms, D.; Zarnkow, M.; Sacher, B.; Becker, T. High-throughput β-glucan analyses and their relationship with beer filterability. Eur. Food Res. Technol. 2017, 243, 341–351. [Google Scholar] [CrossRef]
- Chen, Y.T.; Ling, Y.C. Detection of water-soluble vitamins by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using porphyrin matrices. J. Mass Spectrom. 2002, 37, 716–730. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Rossi, F. LC-MS/MS Determination of Mono-Glutamate Folates and Folic Acid in Beer. Food Anal. Methods 2019, 12, 722–728. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Termopoli, V. Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario. Chemistry 2022, 4, 1679–1695. [Google Scholar] [CrossRef]
- Wei, A.; Mura, K.; Shibamoto, T. Antioxidative activity of volatile chemicals extracted from beer. J. Agric. Food Chem. 2001, 49, 4097–4101. [Google Scholar] [CrossRef] [PubMed]
- Marsili, R.T.; Laskonis, C.R. Evaluation of Sequential-SBSE and TF-SPME Extraction Techniques Prior to GC-TOFMS for the Analysis of Flavor Volatiles in Beer. J. Am. Soc. Brew. Chem. 2019, 77, 113–118. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction. J. Inst. Brew. 2015, 121, 197–203. [Google Scholar] [CrossRef]
- Niu, C.; Han, Y.; Wang, J.; Zheng, F.; Liu, C.; Li, Y.; Li, Q. Comparative analysis of the effect of protein Z4 from barley malt and recombinant Pichia pastoris on beer foam stability: Role of N-glycosylation and glycation. Int. J. Biol. Macromol. 2018, 106, 241–247. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.d.A.F.; Cruz, A.G. Characterization of Brazilian lager and brown ale beers based on color, phenolic compounds, and antioxidant activity using chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef]
- Hoff, S.; Lund, M.N.; Petersen, M.A.; Frank, W.; Andersen, M.L. Storage stability of pasteurized non-filtered beer. J. Inst. Brew. 2013, 119, 172–181. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Mulazzi, A.; Rastelli, S.; Donadini, G.; Rossi, F.; Spigno, G. Targeted healthy compounds in small and large-scale brewed beers. Food Chem. 2020, 310, 125935. [Google Scholar] [CrossRef]
- Hlangwani, E.; Adebiyi, J.A.; Adebo, O.A. Nutritional Compositions of Optimally Processed Umqombothi (a South African Indigenous Beer). Fermentation 2021, 7, 225. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, Z.; Jiang, X.; Zhu, Q.; Chen, R.; Wang, W.; Liu, A.; Wu, C.; Ma, C.; Zhang, J. Metabolomics analysis of flavor differences in Shuixian (Camellia sinensis) tea from different production regions and their microbial associations. Food Chem. 2024, 443, 138542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Muhammad Aqeel, S.; Wang, Y.; Zhang, Q.; Ma, J.; Zhou, J.; Li, J.; Du, G.; Liu, S. Enhanced expression of xylanase in Aspergillus niger enabling a two-step enzymatic pathway for extracting β-glucan from oat bran. Bioresour. Technol. 2023, 377, 128962. [Google Scholar] [CrossRef] [PubMed]
- Aith Barbará, J.; Primieri Nicolli, K.; Souza-Silva, É.A.; Camarão Telles Biasoto, A.; Welke, J.E.; Alcaraz Zini, C. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; Miranda, M.Z.d.; Zavareze, E.d.R.; Dias, A.R.G. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT-Food Sci. Technol. 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, K.; Xie, X.; Liu, X.; Jia, M.; Nie, L.; Li, H.; Wang, S. Disassociation of glutamate from γ-aminobutyric acid by zinc acetate-assisted differential precipitation/dissolution: Application to the quantification of γ-aminobutyric acid. J. Chromatogr. A 2019, 1590, 19–26. [Google Scholar] [CrossRef]
- Lamberts, L.; Rombouts, I.; Delcour, J.A. Study of nonenzymic browning in α-amino acid and γ-aminobutyric acid/sugar model systems. Food Chem. 2008, 111, 738–744. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Comparative evaluation of the formations of gamma-aminobutyric acid and other bioactive amines during unhopped wort fermentation. J. Food Process. Preserv. 2018, 42, e13405. [Google Scholar] [CrossRef]
- Gong, J.; Huang, J.; Xiao, G.; You, Y.; Yuan, H.; Chen, F.; Liu, S.; Mao, J.; Li, B. Determination of γ-aminobutyric acid in Chinese rice wines and its evolution during fermentation. J. Inst. Brew. 2017, 123, 417–422. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Rupa, P.; Matsui, T.; Tanaka, M.; Konishi, T.; Sauchi, Y.; Sato, K.; Ono, S.; Mine, Y. In Vitro and ex Vivo Uptake of Glutathione (GSH) across the Intestinal Epithelium and Fate of Oral GSH after in Vivo Supplementation. J. Agric. Food Chem. 2014, 62, 9499–9506. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Influence of yeast strain, extended lees contact and nitrogen supplementation on glutathione concentration in wine. Aust. J. Grape Wine Res. 2013, 19, 161–170. [Google Scholar] [CrossRef]
- Lu, S.Y.; Cui, H.P.; Zhan, H.; Hayat, K.; Jia, C.S.; Hussain, S.; Tahir, M.U.; Zhang, X.M.; Ho, C.T. Timely Addition of Glutathione for Its Interaction with Deoxypentosone To Inhibit the Aqueous Maillard Reaction and Browning of Glycylglycine-Arabinose System. J. Agric. Food Chem. 2019, 67, 6585–6593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Reis, S.F.; Messias, S.; Bastos, R.; Martins, V.J.; Correia, V.G.; Pinheiro, B.A.; Silva, L.M.; Palma, A.S.; Coimbra, M.A.; Coelho, E. Structural differences on cell wall polysaccharides of brewer’s spent Saccharomyces and microarray binding profiles with immune receptors. Carbohydr. Polym. 2023, 301, 120325. [Google Scholar] [CrossRef] [PubMed]
- Pferdmenges, L.E.; Schröter, A.; Lohmayer, R.; Striegel, L.; Rychlik, M.; Müller, A.; Meinhardt, A.-K.; Trierweiler, B.; Hartmann, B.M.; Frommherz, L. Characterization of the nutrient composition of German beer styles for the German nutrient database. J. Food Compos. Anal. 2022, 105, 104181. [Google Scholar] [CrossRef]
- Padonou, S.W.; Houngbedji, M.; Hounhouigan, M.H.; Chadare, F.J.; Hounhouigan, D.J. B-vitamins and heat processed fermented starchy and vegetable foods in sub-Saharan Africa: A review. J. Food Sci. 2023, 88, 3155–3188. [Google Scholar] [CrossRef]
- Hucker, B.; Vriesekoop, F.; Vriesekoop-Beswick, A.; Wakeling, L.; Vriesekoop-Beswick, H.; Hucker, A. Vitamins in brewing: Effects of post-fermentation treatments and exposure and maturation on the thiamine and riboflavin vitamer content of beer. J. Inst. Brew. 2016, 122, 278–288. [Google Scholar] [CrossRef]
- Araki, S.; Kimura, T.; Shimizu, C.; Furusho, S.; Takashio, M.; Shinotsuka, K. Estimation of Antioxidative Activity and its Relationship to Beer Flavor Stability. J. Am. Soc. Brew. Chem. 2018, 57, 34–37. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, F.; Yun, Y.; Yi, J.; Cai, S.; Zhou, L. Characterization of the flavor, sensory quality and in vitro bioaccessibility in cloudy pomegranate juice treated by high pressure and thermal processing. J. Sci. Food Agric. 2022, 103, 666–679. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, J.J.; Yin, H.; Zhao, Y.X.; Chen, R.; Wan, X.J.; Chen, P.; Hou, X.P.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, G.Q.; Guo, P.; Li, Y.C. Influence of Pasteurising Intensity on Beer Flavour Stability. J. Inst. Brew. 2011, 117, 587–592. [Google Scholar] [CrossRef]
- Castro, R.; Díaz, A.B.; Durán-Guerrero, E.; Lasanta, C. Influence of different fermentation conditions on the analytical and sensory properties of craft beers: Hopping, fermentation temperature and yeast strain. J. Food Compos. Anal. 2022, 106, 104278. [Google Scholar] [CrossRef]
- Duncan, R.E.; Archer, M.C. Farnesol decreases serum triglycerides in rats: Identification of mechanisms including up-regulation of PPARα and down-regulation of fatty acid synthase in hepatocytes. Lipids 2008, 43, 619–627. [Google Scholar] [CrossRef]
- Ku, C.M.; Lin, J.Y. Farnesol, a sesquiterpene alcohol in essential oils, ameliorates serum allergic antibody titres and lipid profiles in ovalbumin-challenged mice. Allergol. Immunopathol. 2016, 44, 149–159. [Google Scholar] [CrossRef]
- Lemarie, F.; Beauchamp, E.; Drouin, G.; Legrand, P.; Rioux, V. Dietary caprylic acid and ghrelin O-acyltransferase activity to modulate octanoylated ghrelin functions: What is new in this nutritional field? Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 121–127. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.Y.; Zhang, J.; Zhou, Y.J.J.; Wang, F.; Wang, Z.G.; Li, X. Advances in microbial production of geraniol: From metabolic engineering to potential industrial applications. Crit. Rev. Biotechnol. 2024, 45, 727–742. [Google Scholar] [CrossRef] [PubMed]



| Beer Type | Beer Sample | Pasteurization Treatment | Raw Wort Concentration | Alcoholic Strength | Raw Materials |
|---|---|---|---|---|---|
| Classic beer | CRB | Pasteurized | 8°P | 3.10%vol | 69.99% malt 1, 29.99% rice, 0.2‰ hops 1, 107 CFU/mL lager yeast |
| CDB | Unpasteurized | 8°P | 3.10%vol | ||
| Pure beer | PRB | Pasteurized | 8°P | 3.10%vol | 69.99% malt 2, 29.99% rice, 0.2‰ hops 2, 107 CFU/mL lager yeast |
| PDB | Unpasteurized | 8°P | 3.10%vol | ||
| Ale beer | ARB | Pasteurized | 14°P | 5.20%vol | 99.99% malt 3, 0.2‰ hops 1, 107 CFU/mL ale yeast |
| ADB | Unpasteurized | 14°P | 5.20%vol |
| Q1 | Q3 | RT | ID | DP | CE |
|---|---|---|---|---|---|
| 218 | 88 | 3.95 | VB5_1 | −54 | −19 |
| 218 | 146 | 3.95 | VB5_2 | −54 | −22 |
| 204 | 168 | 2.40 | VB6_1 | −52 | −15 |
| 204 | 150 | 2.40 | VB6_2 | −52 | −25 |
| 124 | 80 | 1.66 | VB3_1 | 80 | 30 |
| 124 | 80 | 1.66 | VB3_2 | 80 | 31 |
| 124 | 53 | 1.66 | VB3_3 | 80 | 39 |
| 265 | 122 | 1.74 | VB1_1 | 90 | 22 |
| 265 | 144 | 1.74 | VB1_2 | 90 | 24 |
| 377 | 243 | 4.80 | VB2_1 | 29 | 34 |
| 377 | 172 | 4.80 | VB2_3 | 33 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Hou, X.; Li, J.; Zhao, X.; Hu, S. The Effects of Pasteurization and Beer Type on the Functional Compounds and Flavor Substances in Beer. Beverages 2025, 11, 63. https://doi.org/10.3390/beverages11030063
Ding J, Hou X, Li J, Zhao X, Hu S. The Effects of Pasteurization and Beer Type on the Functional Compounds and Flavor Substances in Beer. Beverages. 2025; 11(3):63. https://doi.org/10.3390/beverages11030063
Chicago/Turabian StyleDing, Jiahui, Xiaoping Hou, Jianghua Li, Xinrui Zhao, and Shumin Hu. 2025. "The Effects of Pasteurization and Beer Type on the Functional Compounds and Flavor Substances in Beer" Beverages 11, no. 3: 63. https://doi.org/10.3390/beverages11030063
APA StyleDing, J., Hou, X., Li, J., Zhao, X., & Hu, S. (2025). The Effects of Pasteurization and Beer Type on the Functional Compounds and Flavor Substances in Beer. Beverages, 11(3), 63. https://doi.org/10.3390/beverages11030063





