Green Tea: Antioxidant vs. Pro-Oxidant Activity
Abstract
1. Introduction
2. Methods
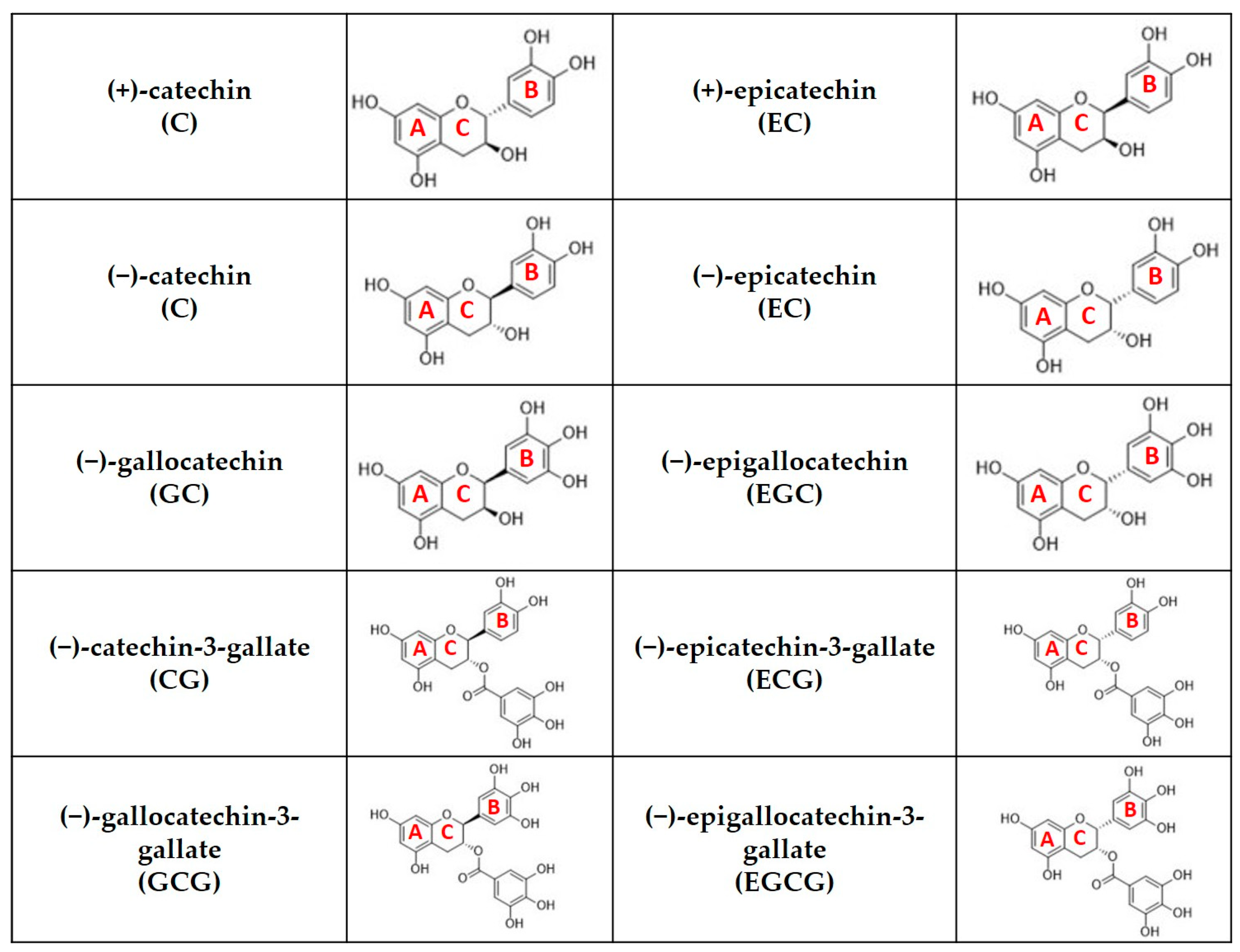
3. Antioxidant Activity of Green Tea Leaves
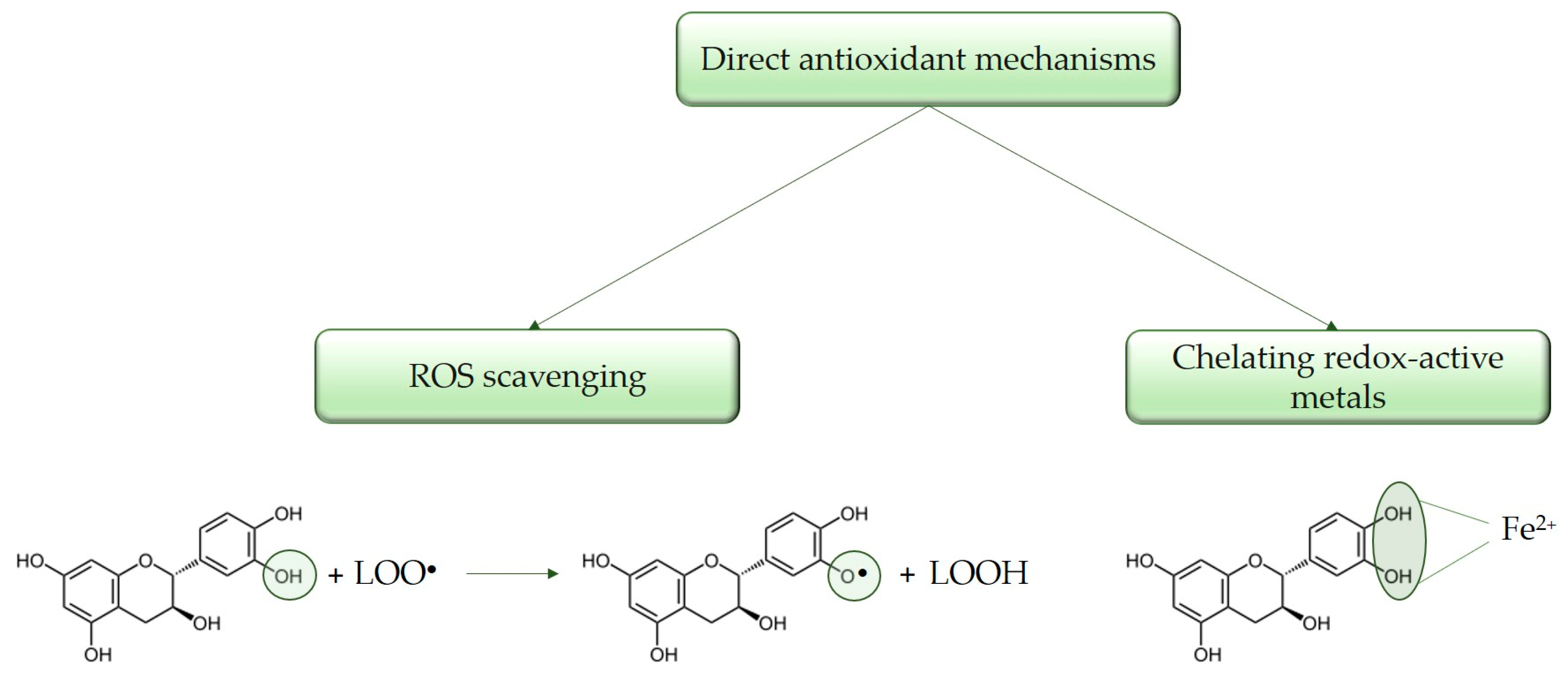
3.1. Direct Antioxidant Properties
3.1.1. Direct ROS Scavenging
3.1.2. Metal Ion Chelation
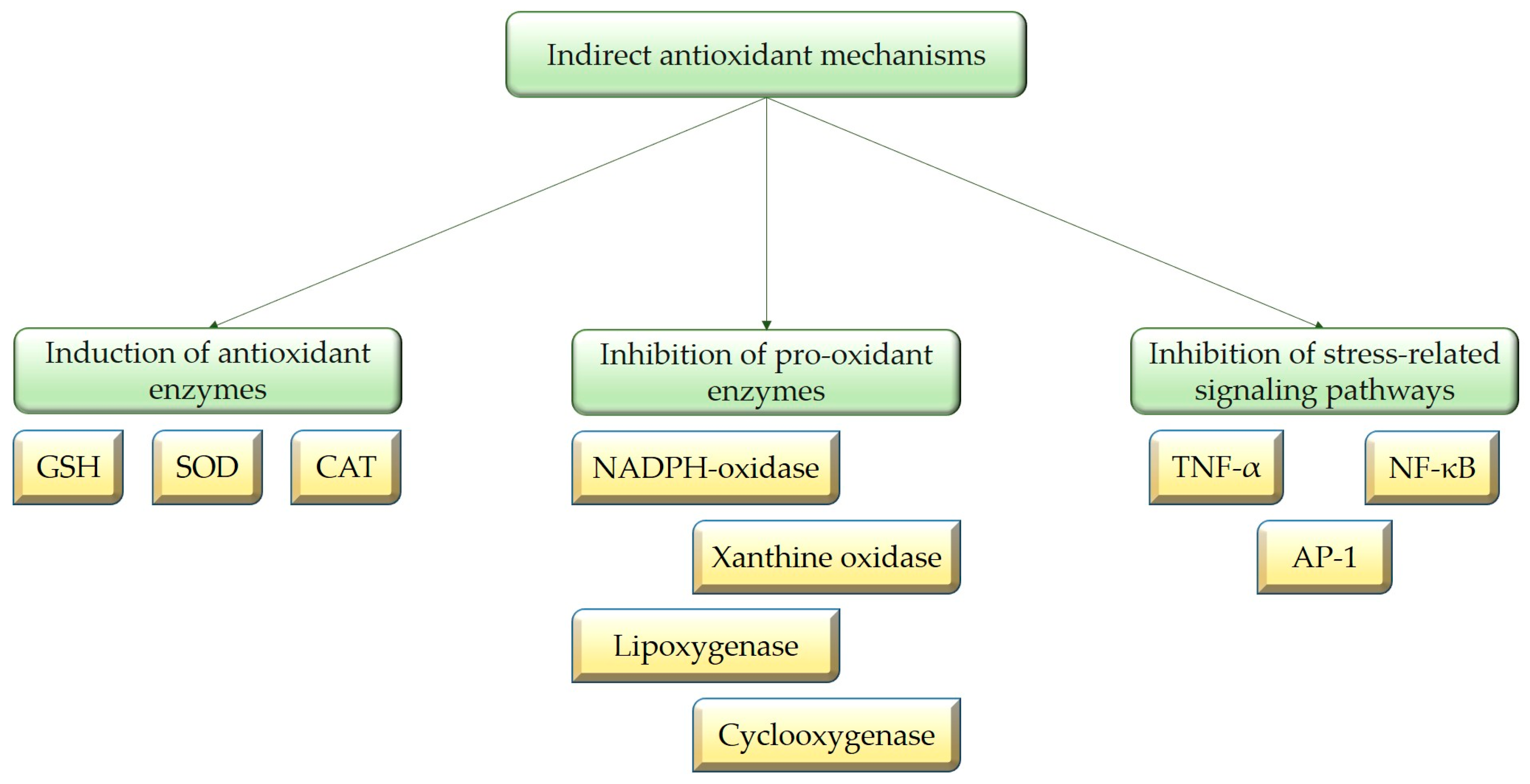
3.2. Indirect Antioxidant Properties
4. Pro-Oxidant Activity of Green Tea Leaves
5. Association Between the Pro-Oxidant Activity of Green Tea Catechins and Hepatotoxicity Manifestation
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGCG | (−)-epigallocatechin-3-gallate |
| EGC | (−)-epigallocatechin |
| ECG | (−)-epicatechin-3-gallate |
| EC | (−)-epicatechin |
| C | Catechin |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| NF-kB | Nuclear factor kappa B |
| MAPK | Mitogen-activated protein kinase |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| AP-1 | Activator protein 1 |
| STAT1 | Signal transducer and activator of transcription 1 |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| XO | Xanthine oxidase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| COX | Cyclooxygenase |
| PPARγ | Peroxisome proliferator activated factor gamma |
| TNF alpha | Tumor necrosis factor alpha |
| DNA | Deoxyribonucleic acid |
| GTE | Green tea extract |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| γH2AX | gamma H2A histone family member X |
| γGT | γ-glutamyltransferase |
| ALP | Alkaline phosphatase |
| NOAEL | No observed adverse effect level |
| HED | Human equivalent dose |
| EFSA | European Food Safety Authority |
References
- Truong, V.L.; Jeong, W.S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with Green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2021, 371, 131098. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crop. Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Epimerisation of catechins in green tea infusions. Food Chem. 2000, 70, 337–344. [Google Scholar] [CrossRef]
- Krahe, J.; Krahe, M.A.; Naumovski, N. The Implications of Post-Harvest Storage Time and Temperature on the Phytochemical Composition and Quality of Japanese-Styled Green Tea Grown in Australia: A Food Loss and Waste Recovery Opportunity. Beverages 2021, 7, 25. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Yamanouchi, H.; Sahara, H.; Iwanaga, T.; Kuroda, R.; Yamamoto, A.; Minami, Y.; Sekijima, M.; Yamada, K.; Kajiya, K. Catechin and caffeine contents in green tea at different harvest periods and their metabolism in miniature swine. Food Sci. Nutr. 2019, 7, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Paul, R.K.; Karak, T. Catechin and caffeine content of tea (Camellia sinensis L.) leaf significantly differ with seasonal variation: A study on popular cultivars in North East India. J. Food Compos. Anal. 2021, 96, 103684. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Zhang, X.-H.; Gao, H.-Q.; Huang, L.-Y.; Ye, J.-J.; Ye, J.-H.; Lu, J.-L.; Ma, S.-C.; Liang, Y.-R. Green Tea Catechins and Skin Health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef]
- Rojano-Ortega, D. Regular, but not acute, green tea supplementation increases total antioxidant status and reduces exercise-induced oxidative stress: A systematic review. Nutr. Res. 2021, 94, 34–43. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.-F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Maiti, S.; Nazmeen, A.; Medda, N.; Patra, R.; Ghosh, T.K. Flavonoids green tea against oxidant stress and inflammation with related human diseases. Clin. Nutr. Exp. 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fasipe, B.; Laher, I. Potential harms of supplementation with high doses of antioxidants in athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Zhang, Y.-C. Multiple free radical scavenging reactions of flavonoids. Dye. Pigment. 2022, 198, 109877. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 594386. [Google Scholar] [CrossRef]
- Naróg, D.; Sobkowiak, A. Electrochemistry of Flavonoids. Molecules 2023, 28, 7618. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Intra, J.; Kuo, S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal Caco-2 cells. Chem. Biol. Interact. 2007, 169, 91–99. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Peiró, S.; Fajarí, L.; Julià, L.; Almajano, M.P. Radical scavenging of white tea and its flavonoid constituents by electron paramagnetic resonance (EPR) spectroscopy. J. Agric. Food Chem. 2014, 62, 5743–5748. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Comparative study of the alkyl and peroxy radical scavenging activities of polyphenols. Chemosphere 2006, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–354. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Green tea catechins suppress xanthine oxidase activity in dairy products: An improved HPLC analysis. J. Food Compost. Anal. 2016, 48, 120–127. [Google Scholar] [CrossRef]
- Zuo, X.; Tian, C.; Zhao, N.; Ren, W.; Meng, Y.; Jin, X.; Zhang, Y.; Ding, S.; Ying, C.; Ye, X. Tea polyphenols alleviate high fat and high glucose-induced endothelial hyperpermeability by attenuating ROS production via NADPH oxidase pathway. BMC Res. Notes 2014, 7, 120. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Dingda, D.; Wang, L.; Gao, F. The primary studies of epigallocatechin-3-gallate in improving brain injury induced by chronic high-altitude natural environment in rats by 7.0T high-field MR imaging. Arch Biochem. Biophys. 2025, 764, 110224. [Google Scholar] [CrossRef]
- Ahmad, A.; Singhal, U.; Hossain, M.M.; Islam, N.; Rizvi, I. The role of the endogenous antioxidant enzymes and malondialdehyde in essential hypertension. J. Clin. Diagn. Res. JCDR 2013, 7, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef]
- Suhail, M.; Rehan, M.; Tarique, M.; Tabrez, S.; Husain, A.; Zughaibi, T.A. Targeting a transcription factor NF-κB by green tea catechins using in silico and in vitro studies in pancreatic cancer. Front. Nutr. 2023, 9, 1078642. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Negishi, H.; Xu, J.W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004, 134, 38–42. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Huang, Y.; Tsang, D.; Chen, Z.Y. Regeneration of alpha-tocopherol in human low-density lipoprotein by green tea catechin. J. Agric. Food Chem. 1999, 47, 2020–2025. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Chen, W.-K.; Tsai, C.-F. Senescence-Mediated Redox Imbalance in Liver and Kidney: Antioxidant Rejuvenating Potential of Green Tea Extract. Int. J. Environ. Res. Public Health 2022, 19, 260. [Google Scholar] [CrossRef]
- Xue, H.; Xu, M.; Gong, D.; Zhang, G. Mechanism of flavonoids inhibiting xanthine oxidase and alleviating hyperuricemia from structure–activity relationship and animal experiments: A review. Food Front. 2023, 4, 1643–1665. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol Supplementation as a Complementary Medicinal Approach to Treating Inflammatory Bowel Disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. PTR 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef]
- Caro, A.A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and pro-oxidant mechanisms of (+) catechin in microsomal CYP2E1-dependent oxidative stress. Toxicol. Vitr. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-Macromolecules Interactions in Human Diseases with Focus on Alzheimer, Atherosclerosis and Cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A.; American Heart Association Council on Basic Cardiovascular Sciences. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Mita, S.R.; Muhtar, N.I.; Kusuma, S.A.F.; Sriwidodo, S.; Hendrawan, R.P. Catechins as Antimicrobial Agents and Their Contribution to Cosmetics. Cosmetics 2025, 12, 11. [Google Scholar] [CrossRef]
- Acosta, L.; Byham-Gray, L.; Kurzer, M.; Samavat, H. Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5′-Diphospho-glucuronosyltransferase 1A4 Genotypes. J. Dietary Suppl. 2023, 20, 850–869. [Google Scholar] [CrossRef]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal Products and the Liver: A Review of Adverse Effects and Mechanisms. Gastroenterology 2015, 148, 517–532.e3. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from Green Tea: A Review of the Literature and Two Unpublished Cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Ballotin, V.R.; Bigarella, L.G.; Brandão, A.B.d.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef]
- Lin, J.K.S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Zheng, E.X.; Navarro, V.J. Liver Injury from Herbal, Dietary, and Weight Loss Supplements: A Review. J. Clin. Transl. Hepatol. 2015, 28, 93–98. [Google Scholar] [CrossRef]
- Chen, C.; Shen, G.; Hebbar, V.; Hu, R.; Owuor, E.D.; Kong, A.-N.T. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1369–1378. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef]
- Bun, S.S.; Bun, H.; Guédon, D.; Rosier, C.; Ollivier, E. Effect of green tea extracts on liver functions in Wistar rats. Food Chem. Toxicol. 2006, 44, 1108–1113. [Google Scholar] [CrossRef]
- Zuhra, K.; Petrosino, M.; Gupta, B.; Panagaki, T.; Cecconi, M.; Myrianthopoulos, V.; Schneiter, R.; Mikros, E.; Majtan, T.; Szabo, C. Epigallocatechin gallate is a potent inhibitor of cystathionine beta-synthase: Structure-activity relationship and mechanism of action. Nitric Oxide 2022, 128, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Hong, J.; Tian, S.; Lee, M.J.; Stark, R.E.; Ho, C.T.; Yang, C.S. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005, 18, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Kessebohm, K.; Weimann, R.; Seitz, H.K. Review of liver injury associated with dietary supplements. Liver Int. 2011, 31, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Vial, T.; Bernard, G.; Lewden, B.; Dumortier, J.; Descotes, J. Hépatite Aiguë Imputable à l’Exolise®(Camellia sinensis). Gastroenterol. Clin. Biol. 2003, 27, 1166–1167. [Google Scholar]
- Fong, T.L.; Klontz, K.C.; Canas-Coto, A.; Casper, S.J.; Durazo, F.A.; Davern, T.J.; Hayashi, P.; Lee, W.M.; Seeff, L.B. Hepatotoxicity due to hydroxycut: A case series. Am. J. Gastroenterol. 2010, 105, 1561–1566. [Google Scholar] [CrossRef]
- Stevens, T.; Qadri, A.; Zein, N.N. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann. Intern. Med. 2005, 142, 477–478. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez, M.C. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006, 44, 616–617. [Google Scholar] [CrossRef]
- Gloro, R.; Hourmand-Ollivier, I.; Mosquet, B.; Mosquet, L.; Rousselot, P.; Salamé, E.; Piquet, M.A.; Dao, T. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1135–1137. [Google Scholar] [CrossRef]
- Federico, A.; Tiso, A.; Loguercio, C. A case of hepatotoxicity caused by green tea. Free Radic. Biol. Med. 2007, 43, 474. [Google Scholar] [CrossRef]
- Arzenton, E.; Magro, L.; Paon, V.; Capra, F.; Apostoli, P.; Guzzo, F.; Conforti, A.; Leone, R. Acute epatitis caused by green tea infusion: A case report. Adv. Pharmacoepidemiol. Drug Saf. 2014, 3, 170. [Google Scholar] [CrossRef]
- Amariles, P.; Angulo, N.; Agudelo-Agudelo, J.; Gaviria, G. Hepatitis asociada a infusiones acuosas de té verde: A propósito de un caso [Hepatitis associated with aqueous green tea infusions: A case study]. Farm. Hosp. 2009, 33, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Bonkovsky, H.L. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): A growing concern. J. Hepatol. 2006, 45, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M. Acute liver failure induced by green tea extracts: Case report and review of the literature. Liver Transpl. 2006, 12, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Olsson, R. Serious adverse liver reactions associated with herbal weight-loss supplements. J. Hepatol. 2007, 47, 295–297. [Google Scholar] [CrossRef]
- Samavat, H. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: Study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015, 26, 1405–1419. [Google Scholar] [CrossRef]
- Yu, Z. Effect of green tea supplements on liver enzyme elevation: Results from a randomized intervention study in the United States. Cancer Prev. Res. Phila. 2017, 10, 571–579. [Google Scholar] [CrossRef]
- Dostal, A.M. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Dostal, A.M. Green tea extract and catechol-O-methyltransferase genotype modify the post-prandial serum insulin response in a randomised trial of overweight and obese post-menopausal women. J. Hum. Nutr. Diet. 2017, 30, 166–176. [Google Scholar] [CrossRef]
- Dostal, A.M. Green tea extract and Catechol-O-Methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Lovera, J. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015, 358, 46–52. [Google Scholar] [CrossRef]
- Radeva-Ilieva, M.; Stoeva, S.; Hvarchanova, N.; Georgiev, K.D. Green Tea: Current Knowledge and Issues. Foods 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). Toxicology studies of green tea extract in F344/NTac rats and B6C3F1/N mice and Toxicology and carcinogenesis studies of green tea extract in Wistar Han [Crl: Wi (Han)] rats and B6C3F1/N mice. Toxicol. Program Tech. Rep. Ser. 2016, 585, NTP-TR-585. [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the safety of green tea catechins. EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.; Maor, Y.; Neuman, M.G. Green Tea Consumption Is Increasing but There Are Significant Hepatic Side Effects. GastroHep 2022, 2307486, 5. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Jachimowicz-Rogowska, K.; Kwiecień, M.; Borsuk-Stanulewicz, M.; Tomczyk-Warunek, A.; Stamirowska-Krzaczek, E.; Purwin, C.; Stryjecka, M.; Tomaszewska, M. Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI). Nutrients 2024, 16, 2837. [Google Scholar] [CrossRef]
- Isomura, T.; Suzuki, S.; Origasa, H.; Hosono, A.; Suzuki, M.; Sawada, T.; Terao, S.; Muto, Y.; Koga, T. Liver-related safety assessment of green tea extracts in humans: A systematic review of randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1221–1229. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Schönthal, A.H. Adverse effects of concentrated green tea extracts. Mol. Nutr. Food Res. 2011, 55, 874–885. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, J.; Cui, H.; Ni, D.; Jiang, H. Dual effects of ascorbic acid on the stability of EGCG by the oxidation product dehydroascorbic acid promoting the oxidation and inhibiting the hydrolysis pathway. Food Chem. 2021, 337, 127639. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Int. Food Res. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res Int. 2021, 142, 110186. [Google Scholar] [CrossRef] [PubMed]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Isbrucker, R. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: Genotoxicity. Food Chem. Toxicol. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Isbruker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Kapetanovic, I.M. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology 2009, 260, 28–36. [Google Scholar] [CrossRef]
- Chow, H.H. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Salminen, W.F.; Yang, X.; Shi, Q.; Greenhaw, J.; Davis, K.; Ali, A.A. Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2012, 50, 1439–1446. [Google Scholar] [CrossRef]
- Yellapu, R.K.; Mittal, V.; Grewal, P.; Fiel, M.; Schiano, T. Acute liver failure caused by ‘fat burners’ and dietary supplements: A case report and literature review. Can. J. Gastroenterol. 2011, 25, 157–160. [Google Scholar] [CrossRef]
- Dela Cruz, A.C.; Patel, T.T.; Maynard, E.; Shah, M.; Lee, E.Y.; Angulo, P. Fulminant liver failure secondary to “Saba appetite control and energy” weight loss supplement. Gastroenterology 2014, 146, 1002. [Google Scholar] [CrossRef]
- Whitsett, M.; Halegoua-De Marzio, D.; Rossi, S. SlimQuick™-associated hepatotoxicity resulting in fulminant liver failure and orthotopic liver transplantation. ACG Case Rep. J. 2014, 1, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Petitpain, N.; Kalt, P.; Ancel, D.; Petit-Laurent, F.; Trechot, P.; Barraud, H.; Bronowicki, J.P. Probable hepatoxicity from epigallocatecol gallate used for phytotherapy. Gastroenterol. Clin. Biol. 2004, 28, 404–406. [Google Scholar] [CrossRef]
- Juneja, L.R.; Kapoor, M.P.; Okubo, T.; Rao, T. Green Tea: History, Processing Techniques, Principles, Traditions, Features and Attractions. In Green Tea Polyphenols, Nutraceuticals of Modern Life; Juneja, L.R., Kapoor, M.P., Okubo, T., Rao, T., Eds.; CRS Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2013; pp. 1–18. [Google Scholar]
- Vuong, Q.V.; Tan, S.P.; Stathopoulos, C.E.; Roach, P.D. Improved extraction of green tea components from teabags using the microwave oven. J. Food Compos. Anal. 2012, 27, 95–101. [Google Scholar] [CrossRef]
- Sano, M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999, 47, 1906–1910. [Google Scholar] [CrossRef]
- Hayward, D.G.; Wong, J.W.; Park, H.Y. Determinations for pesticides on Black, Green, Oolong, and White Teas by gas chromatography triple-quadrupole mass spectrometry. J. Agric. Food Chem. 2015, 63, 8116–8124. [Google Scholar] [CrossRef]
- Goodin, M.G.; Bray, B.J.; Rosengreen, R.J. Sex- and straindependent effects of epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) in the mouse. Food Chem. Toxicol. 2006, 44, 1496–1504. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez, C. Green tea extracts and acute liver failure: The need for caution in their use and diagnostic assessment. Liver Transpl. 2007, 13, 1067. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Lu, X.; Min, H.; Wu, Q.Q.; Shi, X.T.; Bian, K.Q.; Zou, X.P. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition 2016, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Bonkovsky, H.L.; Phillips, E.J.; Li, Y.J.; Ahmad, J.; Barnhart, H.; Durazo, F.; Fontana, R.J.; Gu, J.; Khan, I.; et al. Hla-b*35:01 and green tea-induced liver injury. Hepatology 2021, 73, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann. Intern. Med. 2006, 144, 68–71. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T. Suspected herb induced liver injury by green tea extracts: Critical review and case 2 analysis applying RUCAM for causality assessment. Jpn. J. Gastroenterol. Hepatol. 2019, 1, 1–16. [Google Scholar]
- Pascale, B. Dietary supplements: Knowledge and adverse event reporting among American Medical Society for Sports Medicine physicians. Clin. J. Sport. Med. 2016, 26, 139–144. [Google Scholar] [CrossRef]
- Hoofnagle, J.H. Drug induced liver injury network (DILIN). Hepatology 2004, 40, 773. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. Drug-Induced Liver Injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) Still Used 25 Years After Its Launch? Drug Saf. 2018, 41, 735–743. [Google Scholar] [CrossRef]
- Health Canada, Green Tea Extract-Containing Natural Health Products—Rare Risk of Serious Liver Injury. 2017. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/green-tea-extract-containing-natural-health-products-assessing-potential-risk-liver-injury.html (accessed on 9 February 2025).
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef]
| Study | Green Tea Product | Duration of Intake | Liver Injury | Other Supplements and Medications | Recovery After Discontinuation | References |
|---|---|---|---|---|---|---|
| Case report | Green tea infusion (6 cups/day) | 4 months | Acute hepatitis | No | Yes | [97] |
| Case report | Green tea extract (Exolise®) | Approximately 5 weeks | Hepatocellular insufficiency | Bronz’age® (dietary supplement), one dose; nicergoline, piribedil, prednisolone for less than a week; paracetamol, one dose | A liver transplant has been performed | [98] |
| Case report | Green tea infusion | At least 5 years | Elevated serum levels of liver enzymes | Estrogen and progestogen for 1 year, 5 years ago | Yes | [99] |
| Case report | Green tea infusion, two or three cups/day | 9 months | Elevated serum levels of liver enzymes | No | Yes | [100] |
| Case report | Green tea infusion | 8 months | Elevated serum levels of liver enzymes | No | Yes | [101] |
| Randomized, placebo-controlled, double-blinded trial | GTE (equal to 843 ± 44 mg EGCG daily) | 12 months | ALT or AST elevation | No | Yes | [105,106,107] |
| Single group futility study | Green tea extract, 50% EGCG (Polyphenon E®), two capsules twice daily | 6 months | Abnormal liver function tests | No | Yes | [110] |
| Parallel group randomized double-blind placebo-controlled study | Green tea extract, 50% EGCG (Polyphenon E®), two capsules twice daily | About 5 months; the study was terminated due to high incidence of liver toxicity | Abnormal liver function tests | No | Yes | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoeva, S.; Hvarchanova, N.; Georgiev, K.D.; Radeva-Ilieva, M. Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages 2025, 11, 64. https://doi.org/10.3390/beverages11030064
Stoeva S, Hvarchanova N, Georgiev KD, Radeva-Ilieva M. Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages. 2025; 11(3):64. https://doi.org/10.3390/beverages11030064
Chicago/Turabian StyleStoeva, Stanila, Nadezhda Hvarchanova, Kaloyan D. Georgiev, and Maya Radeva-Ilieva. 2025. "Green Tea: Antioxidant vs. Pro-Oxidant Activity" Beverages 11, no. 3: 64. https://doi.org/10.3390/beverages11030064
APA StyleStoeva, S., Hvarchanova, N., Georgiev, K. D., & Radeva-Ilieva, M. (2025). Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages, 11(3), 64. https://doi.org/10.3390/beverages11030064







