Highlights
- Underwater aging for 12 months preserved anthocyanins and colour stability.
- Blue–green light and sea vibrations contributed to aroma complexity.
- Underwater conditions increased furanone and pyranone levels, enhancing caramel notes.
- An electronic nose classified samples with 96% accuracy for authentication.
Abstract
Underwater aging of alcoholic beverages has gained growing interest in recent years as a novel strategy for product differentiation. This study investigated the effects of 12 months of underwater aging at 13 m depth on the chemical, volatile, and phenolic profiles of wine-based liqueurs, compared to traditional cellar aging. Individual bottles were analysed using an E-nose, achieving 96% correct classification in the cross-validated confusion matrix. Chemical analysis revealed no significant differences in pH, ethanol content, total and volatile acidity. Although total phenolic content did not differ significantly, underwater-aged liquors exhibited higher levels of anthocyanins, suggesting reduced degradation of phenolic compounds in the anaerobic underwater environment. This was supported by higher levels of free alpha-amino nitrogen and total proteins, suggesting slower oxidation. As a result, underwater-aged liquors showed a lower b* index (yellowness), likely due to the reduced oxidation of red colour compounds. Underwater aging induced some changes in the volatile profile, with a significant increase in certain furanones and pyranones, such as 5-hydroxymethylfurfural, 4-hydroxydihydro-2-(3H)-furanone and 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, responsible for strawberry, toasted, and caramel notes. This increased production could be attributed to the unique underwater environment, characterised by oscillating vibrations, blue-green light, lower and more constant temperatures and reduced oxygen levels.
1. Introduction
Aging is a key process in the production of alcoholic beverages, driving the maturation of aroma and colour through complex chemical reactions that enhance their organoleptic quality [1]. Among the various aging techniques, bottle maturation is well-established, particularly for wines and liqueurs and is characterised by reactions affecting both volatile and non-volatile compounds, leading to transformations in the flavour and colour of the beverages [2]. The bottle aging process has been reported to involve three distinct stages. In the initial stage, maturation enhances the wine’s characteristics, while the peak sensory quality is achieved during the second phase. However, extending the bottle aging period beyond this optimal window (third stage) can lead to a decline in quality [3,4].
Volatile compounds play a crucial role in defining the wine’s aroma. Numerous chemical reactions occur among these volatile compounds during bottle aging, modifying their composition and concentration and hence affecting the sensory attributes of wine [5]. Oxygen significantly influences these transformations, particularly through oxidation reactions, which affect both volatile and non-volatile components in aged wines [6,7,8]. For example, the oxidation of phenolic compounds during bottle aging contributes to sensory changes in wine, affecting its colour, taste and trigeminal sensations [9]. Moderate oxidation during bottle aging can lead to the conversion of ethanol into acetaldehyde, which further reacts with tannins or anthocyanins in the wine to form complex ethyl-linked compounds [10]. Furthermore, fruity aroma can diminish over time due to ester and acetate hydrolysis during aging, while oxidative aroma begins to emerge [11]. In contrast, long-chain esters, such as ethyl succinate, increase in concentration after the first year of aging and may gradually decrease in subsequent years [12]. Generally, a decrease in alcohols is also reported during bottle aging, likely due to their reaction with acids in a condensation process, leading to the formation of esters [13].
Other non-volatile substrates that play a critical role in the development of volatile compounds during aging are carotenoids. Over time, carotenoids can degrade to form norisoprenoids, such as TDN, α-ionone and β-damascenone, which are key contributors to the characteristic aroma of long-aged wines, like Riesling, imparting distinctive kerosene-like notes [14].
Increased oxygen permeation into the bottle during aging can accelerate oxidative reactions, leading to the oxidation of ethanol through acetaldehyde into acetic acid, which can deteriorate the wine’s quality after bottling [15].
Recently, sea underwater aging has emerged as a new aging method for wines and liqueurs. This approach takes advantage of the unique underwater conditions, including more stable temperature, gentle oscillatory movement, and moderate exposure to light, to influence the maturation processes. Preliminary results have shown that during underwater aging, Merlot wine maintains more stable levels of total phenolic compounds, which decrease at a slower rate compared to cellar aging [16]. Moreover, wine aged under the sea appears to better preserve the total anthocyanin content over the aging period. On the other hand, in Malvasia white wines, the total antioxidant capacity (ABTS and DPPH) and total phenol content (Folin–Ciocalteu) did not show significant differences between underwater and cellar aging, despite LC–MS/MS quantitative analysis revealed that wines aged in underwater springs exhibited significantly higher concentrations of naringenin and myricetin [17]. These studies were characterized by a short aging time, such as one week. Another recent study investigated a longer aging period, with wines aged for 6 months underwater followed by an additional 6 months in a cellar, compared to wines aged entirely in a cellar for 12 months [18]. No significant differences in acidity, ethanol content, or pH were observed after 6 months, but underwater aging affected the colour (lower b* index) and resulted in minimal changes in volatile compounds, like higher levels of alcohols (2-methyl-1-propanol and 3-methyl-1-butanol) in the underwater-aged samples.
The aim of this study was to investigate the effect of 12 months underwater ageing on the chemical composition of Elixir Falernum liqueur. Produced in Mondragone, in the Campania region of southern Italy, Elixir Falernum is a wine-based liqueur made with carefully selected ingredients, including Falerno del Massico Primitivo wine, 3-year-aged brandy, glucose syrup, alcohol, berry fruit juices and natural aroma.
In the first phase, an electronic nose was used as a portable and rapid method to discriminate the headspace of the individual bottles of the two types of liqueurs. The E-nose was employed for the first time to distinguish liqueur samples aged underwater from those matured in a cellar, offering a possible authentication tool for underwater-aged alcoholic products.
In the second phase, the chemical properties, colour, phenolic, anthocyanin, and α-amino nitrogen, protein content and volatile compounds content were analysed.
2. Materials and Methods
2.1. Liquor Samples
The product under investigation, Elixir Falernum 2023, bottled in 70 cL glass bottles, is a wine-based liqueur produced by Antica Distilleria Petrone, located in Mondragone, in the Campania region of southern Italy. The production process includes barrel aging and its ingredients comprise Falerno del Massico Primitivo red wine, water, sugar, 3-year-aged brandy, glucose syrup, alcohol, berry fruit juices (blackberry, sour cherry and strawberry) and flavouring agents.
Bottles of Falernum 2023, originating from the same batch, were sealed with anti-tamper screw caps (29 × 13 mm, 19 mm diameter, polypropylene head), a shellac capsule and wrapped with a protective PVC film around the neck and cap. The film was further sealed with a marine-grade silicone adhesive.
All bottles, whether stored in the cellar or in the underwater cage, were kept horizontally throughout the 12-month aging period. The bottles were stored in the underwater cage (107 × 107 × 98 cm) at a depth of 13 m below sea level (latitude 41°07′21.9′′, longitude 13°50′48.541′′) (Figure 1).

Figure 1.
Cage used for underwater aging of liquors.
All the samples were collected after the 12 months of aging. 17 bottles aged underwater and 17 control bottles aged in a cellar were sampled using a cross-shaped pattern. This method involved selecting bottles along the diagonals, including those positioned at the sides and centre of the cage, to guarantee a representative sampling. At a depth of 13 m below sea level, the bottles were exposed to blue light, whereas in the cellar, they were stored under shaded conditions.
To monitor the temperature conditions during the 12-month aging period, both underwater and cellar-aged liqueur bottles were equipped with temperature probes (Sensorify, Y Digital Firm, Mondragone, Italy). The probes were inserted into the bottle caps to record the internal temperature throughout the aging process.
17 bottles of liquor S, aged underwater, and 17 bottles of liquor C, aged in the cellar, were individually analysed, in duplicate, using an electronic nose (E-nose PEN2, WMA Airsense Analytics GmbH, Schwerin, Germany), equipped with 10 MOS sensors.
Then, two 2 L bottles were prepared by mixing equal aliquots from the 17 underwater-aged bottles (S) and the 17 cellar-aged bottles (C), resulting in two composite batches. These batches were used for chemical, colour and GC/MS analyses. All samples were stored in the dark at a temperature of 24 °C ± 1 for one week before analyses.
2.2. Electronic Nose Analysis
A volume of 5 mL from each bottle was transferred into a 20 mL glass vial and sealed with a screw cap equipped with a Teflon/silicone septum to analyse the odour fraction in the headspace [19]. The analyses were conducted using a portable Electronic Nose PEN2 (WMA Airsense Analytics GmbH, Schwerin, Germany), which is equipped with 10 metal oxide semiconductor (MOS) sensors. Table 1 shows the characteristics of the 10 MOS sensors.

Table 1.
Characteristics of the 10-metal oxide semiconductor (MOS) sensors of E-nose (PEN 2, Airsense Analytics, Germany).
Before data acquisition, the vials were placed in a thermostatic bath at 25 °C for 15 min to allow equilibrium of the volatile compounds in the headspace [19]. The headspace odour sampling system included an aspiration needle that extracted and transferred the volatile fraction to the E-nose sensors at a constant flow rate of 400 mL/min. Data acquisition was performed at 1-s intervals for a duration of 90 s. A recovery time of 120 s was employed for sensor cleaning using reference air.
The E-nose was operated at 24 °C ± 1. The average G/G0 values of the responses from the 10 MOS sensors were calculated from measurements taken between 75 and 80 s, corresponding to the stabilized sensor response. Data analysis was carried out using WinMuster v.1.6 software (Airsense Analytics GmbH, Schwerin, Germany). All individual bottles were analysed in duplicate.
2.3. Chemical Analyises
Ethanol content (% v/v) was measured in triplicate using a Malligand ebulliometer (Ing. Bullio Castore, Milan, Italy). Total and volatile acidity were determined by titration with 0.1 N sodium hydroxide (VWR Chemicals, Radnor, PA, USA) and expressed as g/L of tartaric acid and acetic acid, respectively, while pH was measured using a PH50+ DHS pH meter (Turin, Italy).
2.4. Determination of Total Polyphenol, Anthocyanin, and α-Amino Nitrogen and Protein Content
The determinations were performed using an iMagic AutoAnalyzer (R-Biopharm Srl, Melegnano, Italy), following the manufacturer’s instructions. The total polyphenol content was quantified using the Polyphenols kit (Code: E2530—red wine calibration, R-Biopharm Srl, Melegnano, Italy), and results were expressed as mg gallic acid equivalents (GAE) per liter of sample. The anthocyanin content was determined according to the procedure specified in the Polyphenol kit (Code: E2510, R-Biopharm Srl, Melegnano, Italy), measuring the concentration of a chromogenic complex formed under acidic conditions by the reaction of anthocyanins with the kit reagent at 520 nm. This method does not quantify polymerized anthocyanins, tannins, or anthocyanins complexed with tannic acid. The α-amino nitrogen content was determined using the o-phthalaldehyde (OPA) test (Code: E2500). Protein content was assessed using the Total Protein kit (Code: E2620, R-Biopharm Srl, Melegnano, Italy), based on the pyrogallol red molybdate method. Samples were filtered through a 0.45 μm syringe filter before conducting any analytical determinations.
2.5. Colour Analysis
Colour measurements were conducted in six replicates using the Portable Color Meter FRU® WR-10QC (Shenzhen Wave Optoelectronics Technology Co Ltd., Shenzhen, China). The device is equipped with a photodiode array sensor and an 8 mm diameter sensor head. During the measurements, the sensor head was immersed in 50 mL of sample placed in a glass vessel. The measurements were performed under dark conditions to avoid interference from external light sources. The L*, a* and b* values of the CIELAB colour scale were recorded, where L* indicates lightness (100)/darkness (0), a* represents redness (+values)/greenness (−values), and b* corresponds to yellowness (+values)/blueness (−values). Colour difference (ΔE) was calculated as the Euclidean distance between two points (1 and 2) in three-dimensional (L*, a*, b*) space, using the following equation:
2.6. Extraction and Analysis of Volatile Organic Compounds by SPME-GC/MS
The extraction of volatile organic compounds (VOCs) was performed using Headspace-SPME technique according to the procedure described by Ziółkowska et al. [20]. In brief, 10 mL of sample were transferred into a 20 mL glass vial, and 50 µL of 2-octanol (purity 99%, Sigma-Aldrich, St. Louis, MO, USA) were added as an internal standard (178 mg/L in ethanol solution). The vial was conditioned at 50 °C for 5 min to allow the sample to equilibrate and to favour the partitioning of VOCs between the matrix and the headspace. VOCs were adsorbed by inserting a 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 2 cm fibre (Supelco, Bellefonte, Pennsylvania, USA) into the headspace of the vial, exposing the polymer for 15 min at the same temperature.
VOCs were desorbed directly in the injector port of GC kept at a temperature of 250 °C in split mode with a 4:1 split ratio, for 10 min. Volatile compound analysis was performed on an Agilent 7890A GC System gas chromatograph coupled to an Agilent 5975C VL MSD with Triple-Axis-Detector mass spectrometer (Agilent Technologies, Inc., Palo Alto, CA, USA). GC was equipped with a Zebron ZB-WAX capillary column (60 m × 0.25 mm i.d. × 0.25 μm film thickness 100% polyethylene glycol; Phenomenex, Torrance, CA, USA). The carrier gas was helium with a flow of 1.3 mL/min. The temperature program was as follows: 40 °C for 5 min, then ramped at 2 °C/min to 220 °C, and held at the maximum temperature for 20 min [21]. Mass spectra were recorded at 70 eV. The source temperature was 230 °C, the quadrupole temperature was 150 °C and the interface temperature was 250 °C.
The identification of VOCs was performed by comparing retention times and mass spectra obtained by analysing pure reference standard compounds (Sigma-Aldrich, St. Louis, MO, USA) in the same conditions. The identification was confirmed by comparing mass spectra with those of the National Institute of Standards and Technology (NIST) database. The fibre was conditioned at 270 °C for 1.5 h before the analysis. A blank test was performed before each analysis. The quantitative data of the volatile compounds were obtained by normalising the peak areas of each compound with respect to the peak area of the internal standard. Peak area data were processed by MSD ChemStation 5975 TAD Data Analysis software v.E.02.00.493 (Agilent Technologies, Palo Alto, CA, USA). Analyses were conducted in triplicate.
2.7. Statistical Analysis
The resulting 10-dimensional data vectors were calculated from the responses of the E-nose sensors during the 75–80 s time interval and were used to construct the data pattern. This pattern was then processed using Principal Component Analysis (PCA) and Linear Discriminant Analysis (LDA). The PCA was visualized as a biplot to explore the contribution of the 10 MOS sensors in the discrimination of the samples. The number of variables processed by LDA in this experiment was reduced through PCA, performed on the original patterns, with the aim of retaining those variables that contributed most significantly to the variance explained by the first two principal components [22].
Comparisons were performed using analysis of variance (ANOVA) followed by Tukey’s HSD test, with a significance level set at p < 0.05. Statistical analyses were carried out using Winmuster v.1.6 software (Airsense Analytics GmbH, Schwerin, Germany), SPSS software package (version 29.0.1.0) and XLStat (Version 2019 v.2.2), an add-in software package for Microsoft Excel v.2404 (Addinsoft Corp., Paris, France).
3. Results and Discussion
3.1. Electronic Nose Analysis of Individual Bottles
Figure 2 shows the temperature values recorded over the aging period using a data logger. The data suggest that the underwater samples had more stable temperatures, whereas greater variability was observed for the cellar-aged samples. The most notable temperature variations between the two aging methods occurred over a five-month period: from August to September 2023, with an average temperature difference (ΔTμ) of about 5.2 °C, and from May to July 2024, with an increased ΔTμ of about 7.4 °C.
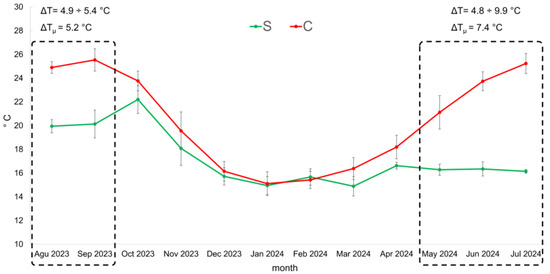
Figure 2.
Temperature evolution measured with a probe inserted in the bottle cap during the 12 months of underwater aging (S) and cellar aging (C). ΔTμ represents the average temperature variation during the months with the highest temperature fluctuation (August–September 2023; May–July 2024).
The E-nose responses, expressed as the conductance ratio G/G0 (where G represents the sensor conductivity when exposed to the sample gas and G0 represents the conductivity when exposed to the zero gas), were analysed for all 34 bottles (17 underwater-aged, S, and 17 cellar-aged, C). To ensure consistent data, the time range of 75–80 s, during which the ten MOS sensors provided a stable signal response, was selected to construct the data pattern for the liquor samples.
In recent years, numerous attempts have been made to apply electronic noses for wine classification [23,24,25,26]. To evaluate the ability of the E-nose to differentiate between the two aging treatments, the constructed data pattern was subjected to statistical analysis using Principal Component Analysis (PCA) and Linear Discriminant Analysis (LDA). The PCA biplot of the E-nose data pattern is shown in Figure 3.
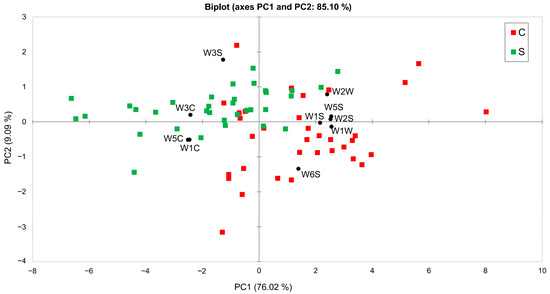
Figure 3.
PCA biplot of the E-nose data pattern illustrating the separation of underwater-aged (S) and cellar-aged (C) samples, with the contribution of the responses of the10 E-nose MOS sensors (black circle).
The results indicate a partial separation between S and C samples, with the first two principal components explaining 76% and 9% of the total variance, respectively. However, a partial overlap is observed between the two groups along the PC1 axis, suggesting that some samples share similar characteristics in relation to the variables that contribute most to this axis. The PC2 axis also contributes to the separation, although to a lesser extent.
Specifically, a higher response was observed from the sensors W1C, W3C, and W5C for the S samples. These sensors are particularly sensitive to aromatic and aroma-aliphatic compounds [27], suggesting an increased presence of these compounds during underwater aging. The combination of PC1 and PC2 provides an appreciable separation between the two groups, indicating the potential of the electronic nose to discriminate between different aging methods.
Based on the PCA results, we examined the factor loadings of the selected principal components to evaluate the contribution of each sensor to the explained variance [22]. In particular, W6S was excluded from further analysis due to its loadings being distributed across multiple factors and its low contribution to the discrimination along the first two principal components (F1: 0.53, F2: −0.51; p > 0.05). Additionally, to reduce multicollinearity, W5C was excluded, as it showed high similarity to W1C in terms of component loadings, which was also evident from their overlap in the PCA plot (Figure 3), indicating redundancy [28]. LDA was then performed on the modified dataset to assess the classification accuracy of the E-nose in distinguishing between the two aging methods. The analysis assumed equal within-class covariance matrices and prior probabilities, with a significance level of 5%. The cross-validated confusion matrix achieved an overall classification accuracy of 96%, confirming the ability of the E-nose to effectively discriminate between underwater-aged and cellar-aged bottles (Table 2).

Table 2.
Confusion matrix from discriminant analysis of E-nose data, showing correct and incorrect classifications for 34 cellar-aged (C) and 34 underwater-aged (S) liquor samples in both training and cross-validation.
Misclassifications were minimal, with two S samples incorrectly identified as C, and one C sample misclassified as S. Figure 4 illustrates the distribution of samples in the LDA space, highlighting a clear separation between the two groups of samples along the two primary discriminant axes. The first axis, which explains 47% of the variance, captures the majority of the inter-group separation, with S samples clustering on the left and C samples clustering on the right. The second axis, accounting for 13% of the variance, contributes minor differentiation within groups but does not significantly enhance their separation.
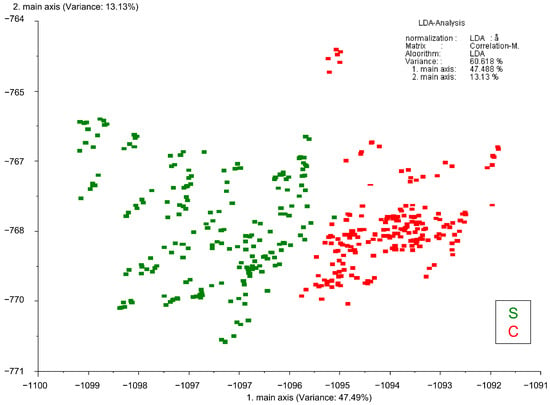
Figure 4.
LDA plot of the E-nose data pattern obtained using WinMuster v.1.6 software (Airsense Analytics GmbH, Schwerin, Germany), illustrating the classification of underwater-aged (S) and cellar-aged (C) samples based on the responses of 10 MOS sensors.
3.2. Chemical and Colour Properties
The effect of underwater aging on the chemical parameters of the liqueur, including total acidity, volatile acidity, ethanol content, and pH, was assessed by analysing two composite samples. These two bulk samples were made by equally mixing the individual bottles from both the cellar-aged (C) and underwater-aged (S) groups. The results are presented in Table 3.

Table 3.
Ethanol content, total acidity, volatile acidity, pH and instrumental colour parameters of liquor samples aged underwater (S) and in the cellar (C) for 12 months.
Although no significant differences were observed, a trend towards a higher ethanol content was noted in the underwater-aged sample, suggesting a reduced oxidation process during the one-year aging period. The results are in agreement with recent preliminary studies on bottle-aged wines stored underwater and in cellars, although these studies investigated a shorter aging period compared to the present study [16,18].
The CIELab colour analysis revealed no statistically significant differences in the L* (lightness) or a* (red-green) values between the S and C samples (Table 3).
However, the b* (yellow-blue) value was significantly higher in the C samples compared to the S samples, suggesting an intensification of yellow tones during cellar aging. The calculated ΔE value, which represents the colour difference between C and S samples, was 1.90. This value indicates minimal overall colour differences between the two aging methods. While this difference is below the threshold typically perceptible to an untrained observer, it may be noticeable to a trained assessor.
3.3. Total Polyphenol, Anthocyanin, and α-Amino Nitrogen and Protein Content
Total polyphenols analysis showed no statistically significant differences between the investigated samples (Figure 5). Reported values were 4617 ± 664 and 4301 ± 494 mg GAE/L for C and S samples, respectively. The results were 44.6% and 34.7% higher than the median of the maximum values reported in literature for red wines (3192 mg GAE/L) [29]. This observation highlights differences in the nature and composition of the liqueurs, which, beside red wine, also contain other ingredients like red fruits juice, which could have increased the polyphenols value. In a previous study, the total phenolic compounds content of red fruit juices, ranged from 1234.27 mg GAE/L to 6361.89 mg GAE/L for red raspberry and elderberry, respectively [30]. The high internal variability observed in the cellar-aged liqueur sample could indeed be attributed to oxidative degradation affecting sample homogeneity.
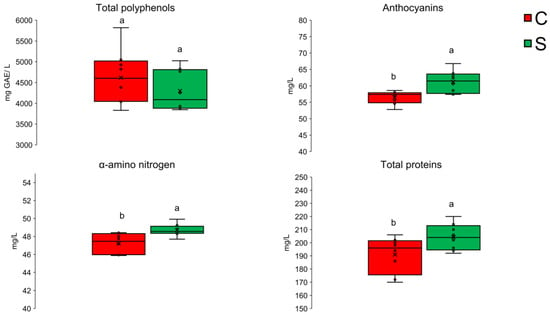
Figure 5.
Concentrations of total polyphenols, anthocyanins, free alpha-amino nitrogen, and total proteins in underwater-aged and cellar-aged liquors. Different letters indicate statistically significant differences (p ≤ 0.05).
Oxidation is a common issue in wine and liqueur storage, leading to the degradation of phenolic compounds such as anthocyanins, which are responsible for colour and stability. A study on the stability of anthocyanins highlights that their degradation is influenced by factors such as pH, light, temperature, and oxygen exposure [31]. The presence of oxygen, in particular in the cellar-aged sample, could have led to a more pronounced oxidative degradation, affecting the colour and quality of the beverage. This degradation could have resulted in uneven distribution of these oxidated compounds, causing variability within the cellar-aged subsamples. Therefore, the observed variability in the cellar-aged liqueur sample is likely due to oxidative degradation processes affecting the homogeneity of anthocyanins and other phenolic compounds. The anthocyanin content of the liqueurs differed significantly (p < 0.01) between the two treatments (Figure 5), with the undersea sample being the richer (61.3 ± 3.4 mg/L), indicating a lower degradation of these chemical species in the undersea-aged sample compared to the cellar-aged sample, which had a lower value (56.5. ± 2.0 mg/L). According to He, et al. [32] the typical concentrations of free anthocyanins in young red wines range from 500 mg/L to 2000 mg/L.
However, another study reported that the anthocyanin concentration in red wines can range between 90 and 400 mg/L [33]. Additionally, other literature data report that the total anthocyanin content in berry juice ranged from 205.98 mg/L to 4188.63 mg/L, depending on the type of red fruits [30]. Although the measured anthocyanin content in our study is lower than these reported ranges, this discrepancy likely reflects the specific liqueur recipe and the composition of the raw materials, aligning more closely with the findings of Gutiérrez-Escobar et al. [33].
However, the quinones derived from the oxidation of anthocyanins and phenolic compounds present in the liqueurs could have immediately reacted both reversibly and irreversibly with the free amino acids and proteins in the wine, leading to their precipitation [34]. It is well established that the formation and activity of quinone-amino acid/protein conjugates can influence the colour, taste, and aroma of food products, particularly liqueurs [35]. Therefore, although the difference between the two samples remains negligible, the statistically significant higher content of free alpha amino nitrogen (i.e., amino acids and peptides resulting from the yeast fermentation), and total proteins in the undersea-aged sample, might represent an indirect clue supporting the better preservation of phenolic compounds in this sample compared to the control one (Figure 5). The average content of soluble proteins determined in the investigated samples is about double than the maximum level reported in literature for red wines (105 mg/L), [36,37]. This can be attributed to the addition of other ingredients in the liqueur’s recipe, which could have contributed to the total protein amount. On the other hand, the alpha amino nitrogen content is in the range reported for wine musts [38].
The oxidation of phenolic compounds leads to the formation of quinones and yellow to brown pigments [39], which may explain the observed increase in the b* value for the C samples (Table 3). Such oxidative reactions are known to occur during aging and may be facilitated by the presence of oxygen and higher temperature variability in cellar conditions. Additionally, interactions between anthocyanins and tannins, or other phenolic compounds, could result in the formation of polymeric complexes, which contribute to a reduction in red hues and a shift toward yellow tones [40]. In contrast, the underwater-aged samples exhibited lower b* values, aligning with findings reported by Maioli et al. [18] for wines aged in an underwater environment.
3.4. Volatile Organic Compounds
A total of 77 volatile organic compounds were identified in the samples, with no qualitative differences observed between the S and C aging methods. These compounds included 16 ethyl esters, 10 alcohols, 7 furans, 8 furanones and pyranones, 4 ketones and lactones, 16 aromatics, 4 terpenes, 4 volatile acids, 3 acetates and 2 norisoprenoids (Table 4).

Table 4.
Effect of underwater aging (S) on the volatile compound composition of wine-based liqueurs aged for one year in bottles.
Overall, furans, furanones, and pyranones were more abundant in the underwater-aged samples (S), whereas terpene compounds were higher in the cellar-aged samples (C).
Significant differences (p < 0.05) were observed for few VOCs. The S liqueurs exhibited higher concentrations of 5-hydroxymethylfurfural (5-HMF), 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, and 4-hydroxydihydro-2-(3H)-furanone, which are known to contribute to strawberry, caramel, and roasted odours. Previous studies have reported greater increases in 5-HMF under oxygen-free conditions compared to oxygen-exposed conditions over six months in sweet red wines and over 12 months in sweet white wines [45]. Furans, such as furfural and 5-HMF, tend to increase during the first 12 months of aging, although their concentrations gradually decline as aging progresses [46]. Typically, these compounds are present in relatively low concentrations in wine. However, in the samples analysed in this study, the presence of sugars in the formulation may have contributed to the development of higher amounts of these VOCs, as sugars are known precursors in the formation of furans during bottle ageing, even when stored at relatively low temperatures (e.g., 20 °C), as previously observed in Port and Madeira wines [47,48].
Interestingly, environmental factors such as light exposure, positioning, and vibrations, in addition to temperature conditions and oxygen availability, can influence the formation of these compounds. Particularly, light and vibrations have been associated with increased levels of these compounds [49,50], which could plausibly be linked to the underwater environment at a depth of 13 m. At these depths, the underwater environment is dominated by blue-green light due to the selective absorption of longer wavelengths (e.g., red, orange, and yellow) by water, while shorter wavelengths, such as blue and green, penetrate more deeply, defining the spectral quality of light at this depth [51]. In grape berries, exposure to different light wavelengths has been shown to affect the production of certain VOCs. For instance, blue light, compared to red, white, or standard light, has been associated with higher concentrations of furans and phenolic aldehydes, such as furfural, benzene acetaldehyde, and 2,4-dihydroxybenzaldehyde [52]. Therefore, among other conditions, it is possible that the effect of light transmission at these depths potentially influenced the amount of specific VOCs in the liquor. However, further investigations are needed to fully understand this effect. S liquors also had a higher amount of anethol, consistent with a higher response from the W5C, W3C and W1C sensors, as observed in the E-nose analysis, which are sensitive to aromatic compounds [27].
In contrast, the cellar-aged samples (C) contained higher levels of 4-terpineol and ethyl-3-hydroxybutanoate. Compounds such as D-limonene and linalool during aging can undergo hydration of double bonds, dehydration, and cyclization, leading to the formation of 4-terpineol and other terpenoids [53], a process that tends to intensify at elevated temperatures [54]. Ethyl 3-hydroxybutanoate has been previously reported to increase gradually during aging [55], and it could potentially indicate early aging in the cellar-aged liqueur. Moreover, compounds with phenolic groups, such as anethol, tend to decrease during bottle aging [56], supporting that the cellar-aged sample underwent faster aging compared to the underwater-aged sample. This behaviour is also corroborated by the analysis of anthocyanins and free amino acids (Figure 5) and the higher b* colour parameter (Table 3), suggesting a potential increased rate of oxidation reactions in the C samples over the 12-month aging period.
4. Conclusions
At a depth of 13 m, the underwater environmental conditions seem to have significantly slowed down certain oxidation reactions in the aged liquors. This is shown by a lower yellow index (b*) and lower degradation of anthocyanins. Furthermore, cellar-aged samples exhibited higher levels of 4-terpineol and ethyl 3-hydroxybutanoate, along with lower amounts of anethol, suggesting a faster aging process. Consistently, the W5C, W3C, and W1C sensors of the electronic nose, sensitive to molecules with aromatic groups such as anethol, showed a higher response for the underwater-aged liqueurs. The statistical analysis of the E-nose data pattern revealed a 96% correct classification rate, demonstrating the E-nose’s ability to serve as an objective method for quality control and distinguishing between underwater-aged and cellar-aged wine-based liqueurs.
The presence of blue-green light and sea vibrations may have contributed to an increased concentration of furanones and furans, which are known to impart toasted, caramel and strawberry aroma notes, in addition to almond notes (with benzaldehyde being one of the most abundant VOCs identified).
Although this study investigated a longer aging period compared to recent research on underwater aging, further studies are essential to thoroughly examine the effects of underwater parameters. Sensory analysis with trained panels should be included to assess its impact on colour, aroma and taste, providing a clearer understanding of how this method affects the sensory properties. Further investigations would also be necessary to evaluate the economic feasibility of underwater aging.
Author Contributions
Conceptualization, A.G., S.V. and P.F.; methodology, A.G. and P.F.; formal analysis, A.B. and G.D.; investigation, A.B., G.D. and A.C.; resources, R.S., A.G. and P.F.; data curation, A.B., G.D. and A.G.; writing—original draft preparation, A.B. and G.D.; writing—review and editing, A.G., R.S., P.F. and S.V.; visualization, A.B. and G.D.; supervision, A.G. and P.F.; project administration P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out under the research agreement between Antica Distilleria Petrone (Mondragone, Italy) and the Department of Agricultural Sciences, University of Naples Federico II, titled “Analisi metabolomica ed aromatica di liquori durante l’invecchiamento su fondale marino” (Project Code: E77H23003010007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that this study received funding from Antica Distilleria Petrone (Mondragone, Italy). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| E-nose | Electronic nose |
| MOS | Metal oxide semiconductor |
| SPME | Solid phase microextraction |
| GC/MS | Gas chromatography mass spectrometry |
| VOCs | Volatile organic compounds |
| LDA | Linear discriminant analysis |
| PCA | Principal component analysis |
| S | Sea underwater aged |
| C | Cellar aged |
References
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle aging and storage of wines: A review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Julien, M.; Jourdes, M.; Teissedre, P.-L. Impact of closures on wine post-bottling development: A review. Eur. Food Res. Technol. 2011, 233, 905–914. [Google Scholar] [CrossRef]
- Liu, D.; Xing, R.-R.; Li, Z.; Yang, D.-M.; Pan, Q.-H. Evolution of volatile compounds, aroma attributes, and sensory perception in bottle-aged red wines and their correlation. Eur. Food Res. Technol. 2016, 242, 1937–1948. [Google Scholar] [CrossRef]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.r.; Saucier, C.; Darriet, P.; Teissedre, P.-L.; Dubourdieu, D. Impact of oxygen dissolved at bottling and transmitted through closures on the composition and sensory properties of a Sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef]
- Coetzee, C.; Van Wyngaard, E.; Suklje, K.; Silva Ferreira, A.C.; Du Toit, W.J. Chemical and sensory study on the evolution of aromatic and nonaromatic compounds during the progressive oxidative storage of a Sauvignon blanc wine. J. Agric. Food Chem. 2016, 64, 7979–7993. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Bueno, M.; Franco-Luesma, E.; Cullere, L.; Fernandez-Zurbano, P. Key changes in wine aroma active compounds during bottle storage of Spanish red wines under different oxygen levels. J. Agric. Food Chem. 2014, 62, 10015–10027. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.; Morel-Salmi, C.; Souquet, J.M.; Dieval, J.; Aagaard, O.; Vidal, S.; Fulcrand, H.; Cheynier, V. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010, 123, 107–116. [Google Scholar] [CrossRef]
- Gambuti, A.; Siani, T.; Picariello, L.; Rinaldi, A.; Lisanti, M.T.; Ugliano, M.; Dieval, J.; Moio, L. Oxygen exposure of tannins-rich red wines during bottle aging. Influence on phenolics and color, astringency markers and sensory attributes. Eur. Food Res. Technol. 2017, 243, 669–680. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef]
- Ugliano, M.; Dieval, J.-B.; Siebert, T.E.; Kwiatkowski, M.; Aagaard, O.; Vidal, S.; Waters, E.J. Oxygen consumption and development of volatile sulfur compounds during bottle aging of two Shiraz wines. Influence of pre- and post bottling controlled oxygen exposure. J. Agric. Food Chem. 2012, 60, 8561–8570. [Google Scholar] [CrossRef]
- Xu, M.; Yu, Y.; Ramaswamy, H.; Zhu, S. Characterization of Chinese liquor aroma components during aging process and liquor age discrimination using gas chromatography combined with multivariable statistics. Sci. Rep. 2017, 7, 39671. [Google Scholar] [CrossRef]
- Qian, X.; Jia, F.; Cai, J.; Shi, Y.; Duan, C.; Lan, Y. Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging. Foods 2022, 11, 74. [Google Scholar] [CrossRef]
- Ziegler, M.; Wegmann-Herr, P.; Schmarr, H.-G.; Gök, R.; Winterhalter, P.; Fischer, U. Impact of rootstock, clonal selection, and berry size of Vitis vinifera sp. Riesling on the formation of TDN, Vitispiranes, and other volatile compounds. J. Agric. Food Chem. 2020, 68, 3834–3849. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar]
- Mercanti, N.; Pieracci, Y.; Macaluso, M.; Fedel, M.; Brazzarola, F.; Palla, F.; Verdini, P.G.; Zinnai, A. Exploring Red Wine Aging: Comparative Analysis of Cellar and Sea Underwater Aging on Chemical Composition and Quality. Foods 2024, 13, 1812. [Google Scholar] [CrossRef]
- Birkić, N.; Ožbolt, E.; Reynolds, C.A.; Pavlešić, T.; Lučin, I.; Andabaka, Ž.; Saftić Martinović, L. Maturation of wine in underwater springs as a novel wine production process. Eur. Food Res. Technol. 2024, 250, 615–622. [Google Scholar] [CrossRef]
- Maioli, F.; Picchi, M.; Bandinelli, A.; Colavolpe, G.; Kottakhs, E.; Canuti, V. Effect of underwater aging treatment on wine quality: A preliminary study. Eur. Food Res. Technol. 2024, 251, 391–404. [Google Scholar] [CrossRef]
- Wei, Y.J.; Yang, L.L.; Liang, Y.P.; Li, J.M. Application of electronic nose for detection of wine-aging methods. Adv. Mater. Res. 2014, 875, 2206–2213. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef]
- Genovese, A.; Moio, L.; Sacchi, R.; Piombino, P. Sip volume affects oral release of wine volatiles. Food Res. Int. 2015, 77, 426–431. [Google Scholar] [CrossRef]
- Falchero, L.; Sala, G.; Gorlier, A.; Lombardi, G.; Lonati, M.; Masoero, G. Electronic Nose analysis of milk from cows grazing on two different Alpine vegetation types. J. Dairy Res. 2009, 76, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Arroyo, T.; Santos, J.; Cabellos, J.; Horrillo, M. Electronic nose for wine ageing detection. Sens. Actuators B Chem. 2008, 133, 180–186. [Google Scholar] [CrossRef]
- García, M.; Aleixandre, M.; Gutiérrez, J.; Horrillo, M. Electronic nose for wine discrimination. Sens. Actuators B Chem. 2006, 113, 911–916. [Google Scholar] [CrossRef]
- Stevan, S., Jr. Measurement of quality indicators of wine: From bottle to aging. Anal. Chim. Acta 2018, 1040, 62–68. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Modesti, M.; Moreno-García, J.; Rodríguez-Moreno, M.; Catini, A.; Capuano, R.; Di Natale, C.; Bellincontro, A.; Moreno, J. Differentiation through E-nose and GC-FID data modeling of rosé sparkling wines elaborated via traditional and Charmat methods. J. Sci. Food Agric. 2024, 105, 1439–1447. [Google Scholar] [CrossRef]
- Balivo, A.; Sacchi, R.; Di Francia, A.; Masucci, F.; Genovese, A. E-nose analysis of milk to detect the inclusion of hydroponic barley forage in the buffalo diet. J. Food Comp. Anal. 2024, 131, 106230. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R.; Taylor, J. Linear regression. In An Introduction to Statistical Learning: With Applications in Python, 2023; Springer International Publishing: Cham, Switzerland, 2023; pp. 69–134. [Google Scholar]
- Visioli, F.; Panaite, S.-A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Medvidovic-Kosanovic, M.; Novak, I. Anthocyanin content and antioxidant activity of various red fruit juices. Deut. Lebensm. Rundsch. 2007, 103, 58. [Google Scholar]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Smith, M.R.; Penner, M.H.; Bennett, S.E.; Bakalinsky, A.T. Quantitative colorimetric assay for total protein applied to the red wine pinot noir. J. Agric. Food Chem. 2011, 59, 6871–6876. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Yokotsuka, K. Content and origin of protein in white and red wines: Changes during fermentation and maturation. Am. J. Enol. Vitic. 2003, 54, 178–188. [Google Scholar] [CrossRef]
- Crowell, E.; Ough, C.; Bakalinsky, A. Determination of alpha amino nitrogen in musts and wines by TNBS method. Am. J. Enol. Vitic. 1985, 36, 175–177. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; del Alamo-Sanza, M.; Martínez-Gil, A.M.; Nevares, I. Red Wine Aging by Different Micro-Oxygenation Systems and Oak Wood—Effects on Anthocyanins, Copigmentation and Color Evolution. Processes 2020, 8, 1250. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Genovese, A.; Dimaggio, R.; Lisanti, M.T.; Piombino, P.; Moio, L. Aroma Composition of Red Wines by Different Extraction Methods and Gas Chromatography-SIM/Mass Spectrometry Analysis. Ann. Di Chim. J. Anal. Environ. Cult. Herit. Chem. 2005, 95, 383–394. [Google Scholar] [CrossRef]
- Zhao, P.; Qian, Y.; He, F.; Li, H.; Qian, M. Comparative Characterization of Aroma Compounds in Merlot Wine by Lichrolut-EN-Based Aroma Extract Dilution Analysis and Odor Activity Value. Chemosens. Percept. 2017, 10, 149–160. [Google Scholar]
- Yang, Y.; Ai, L.; Mu, Z.; Liu, H.; Yan, X.; Ni, L.; Xia, Y. Flavor Compounds with High Odor Activity Values (OAV > 1) Dominate the Aroma of Aged Chinese Rice Wine (Huangjiu) by Molecular Association. Food Chem. 2022, 383, 132370. [Google Scholar] [CrossRef]
- Lan, Y.B.; Xiang, X.F.; Qian, X.; Wang, J.M.; Ling, M.Q.; Zhu, B.Q.; Duan, C.Q. Characterization and Differentiation of Key Odor-Active Compounds of ‘Beibinghong’ Icewine and Dry Wine by Gas Chromatography-Olfactometry and Aroma Reconstitution. Food Chem. 2019, 287, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Cerdán, T.G.; Goñi, D.T.; Azpilicueta, C.A. Accumulation of volatile compounds during ageing of two red wines with different composition. J. Food Eng. 2004, 65, 349–356. [Google Scholar] [CrossRef]
- Câmara, J.S.; Marques, J.C.; Alves, M.A.; Silva Ferreira, A.C. 3-Hydroxy-4, 5-dimethyl-2 (5 H)-furanone levels in fortified Madeira wines: Relationship to sugar content. J. Agric. Food Chem. 2004, 52, 6765–6769. [Google Scholar] [CrossRef]
- Williams, A.A.; Lewis, M.J.; May, H.V. The volatile flavour components of commercial port wines. J. Sci. Food Agric. 1983, 34, 311–319. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of phenolic and volatile compounds during bottle storage of a white wine without added sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef]
- Antoine, D.; Babin, M.; Berthon, J.-F.; Bricaud, A.; Gentili, B.; Loisel, H.; Maritorena, S.; Stramski, D. Shedding light on the sea: André Morel’s legacy to optical oceanography. Annu. Rev. Mar. Sci. 2014, 6, 1–21. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Li, W.; Zhang, P.; Zhang, X.; Wang, J.; Wang, L.; Chen, K.; Fang, Y.; Zhang, K. Effect of Supplementary Light with Different Wavelengths on Anthocyanin Composition, Sugar Accumulation and Volatile Compound Profiles of Grapes. Foods 2023, 12, 4165. [Google Scholar] [CrossRef]
- Clark, B., Jr.; Chamblee, T. Acid-catalyzed reactions of citrus oils and other terpene-containing flavors. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1992; Volume 28, pp. 229–285. [Google Scholar]
- Marais, J.; Van Wyk, C.J.; Rapp, A. Effect of Storage Time, Temperature and Region on the Levels of 1,1,6-Trimethyl-1,2-dihydronaphthalene and Other Volatiles, and on Quality of Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 1992, 13, 33–44. [Google Scholar] [CrossRef][Green Version]
- Lytra, G.; Cameleyre, M.; Tempere, S.; Barbe, J.-C. Distribution and organoleptic impact of ethyl 3-hydroxybutanoate enantiomers in wine. J. Agric. Food Chem. 2015, 63, 10484–10491. [Google Scholar] [CrossRef]
- de Esteban, M.L.G.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).